Key Points
In patients with previously untreated CLL/small lymphocytic lymphoma, ibrutinib plus venetoclax resulted in immune restoration.
CLL cells were eradicated, normal B cells recovered, and counts of T-cell subtypes, monocytes, and dendritic cells were normalized.
Abstract
We evaluated immune cell subsets in patients with chronic lymphocytic leukemia (CLL) who received first-line therapy with 3 cycles of ibrutinib then 13 cycles of ibrutinib plus venetoclax in the minimal residual disease (MRD) cohort of the CAPTIVATE study (NCT02910583). Patients with Confirmed undetectable MRD (uMRD) were randomly assigned to placebo or ibrutinib groups; patients without Confirmed uMRD were randomly assigned to ibrutinib or ibrutinib plus venetoclax groups. We compared immune cell subsets in samples collected at 7 time points with age-matched healthy donors. CLL cells decreased within 3 cycles after venetoclax initiation; from cycle 16 onward, levels were similar to healthy donor levels (HDL; ≤0.8 cells per μL) in patients with Confirmed uMRD and slightly above HDL in patients without Confirmed uMRD. By 4 months after cycle 16, normal B cells had recovered to HDL in patients randomly assigned to placebo. Regardless of randomized treatment, abnormal counts of T cells, classical monocytes, and conventional dendritic cells recovered to HDL within 6 months (median change from baseline −49%, +101%, and +91%, respectively); plasmacytoid dendritic cells recovered by cycle 20 (+598%). Infections generally decreased over time regardless of randomized treatment and were numerically lowest in patients randomly assigned to placebo within 12 months after cycle 16. Sustained elimination of CLL cells and recovery of normal B cells were confirmed in samples from patients treated with fixed-duration ibrutinib plus venetoclax in the GLOW study (NCT03462719). These results demonstrate promising evidence of restoration of normal blood immune composition with ibrutinib plus venetoclax.
Introduction
Ibrutinib, a once daily dosage of Bruton tyrosine kinase (BTK) inhibitor, in combination with venetoclax, a B-cell lymphoma 2 (BCL-2) inhibitor, is approved as a fixed-duration (FD) combination therapy for the first-line treatment of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) in 48 countries across the European Union, United Kingdom, Middle East, and South America, as well as Hong Kong and Canada. The approval was based on results from the phase 2 CAPTIVATE study and the phase 3 GLOW study, which demonstrated high rates of undetectable minimal residual disease (uMRD), high rates of progression-free survival, and durable treatment-free remission.1-3 Ibrutinib and venetoclax have distinct and complementary modes of action that work synergistically to eliminate distinct CLL cell populations. CLL cells rely on the overexpression of antiapoptotic proteins (BCL-2, BCL extralarge [XL], and myeloid cell leukemia-1 [MCL-1]) for survival.4,5 Single-agent ibrutinib decreases levels of BCL-XL and MCL-1, but not BCL-2, in highly proliferative lymph node emigrant B cells (CD5hi CXCR4dim), mobilizes CLL cells from lymph nodes and lymphoid niches and into the peripheral blood, and enhances their susceptibility to venetoclax-induced apoptosis.4-7
Single-agent ibrutinib has been shown to reverse some of the profound immune dysregulation characteristic of CLL.8,9 This dysregulation includes changes in the balance of T-cell subsets, with chronic T-cell activation and exhaustion, changes in the expression of inhibitory molecules and cytokine secretion, and induction of myeloid-derived suppressor cells (MDSCs).10-20 Consequently, CLL is characterized by an immunosuppressive microenvironment that allows CLL cells to escape immune surveillance.11 Infections are frequently observed in patients with CLL, and this increased risk is thought to be linked to the impairment of innate and adaptive immunity associated with pathophysiological changes in the underlying disease.11 With increasing time from diagnosis, immune dysfunction in CLL becomes more severe and is exacerbated with chemoimmunotherapy.10,11 In contrast, treatment with single-agent ibrutinib results in a significant, progressively positive impact on adaptive and innate immune cell subsets in patients with CLL.8,9 In addition to inhibiting BTK, ibrutinib inhibits other Tec family kinases, including interleukin 2–inducible T-cell kinase (ITK). It has been hypothesized that inhibition of ITK by ibrutinib plays a role in improved T-cell immunity by shifting CD4+ T cells toward a tumor-suppressive type 1 T-helper (Th1) phenotype.21 Indeed, mounting evidence suggests that ibrutinib plays a role in reversing the immunosuppressive environment in CLL, with activation of T cells and reduction of T-cell exhaustion,22 increased T-cell receptor diversity,23 a shift toward a Th1 phenotype,21 and repaired immune synapse formation.24 These changes, leading to an overall improvement of T-cell functions, have been observed in patients with previously untreated and relapsed/refractory CLL under long-term ibrutinib treatment.8,9 Another hallmark of CLL is the high number of circulating leukemic B cells. Treatment with ibrutinib causes a steady reduction of total B cells, with a median reduction of 85% per year during the first 2 years and a preferential reduction of CLL cells over normal B cells.9 However, CLL cells are not eliminated with single-agent ibrutinib; B-cell counts remain abnormally high even after 4 years of continued treatment, with CLL cells representing more than 90% of total B cells.9
The impact of combined ibrutinib plus venetoclax on immune cells has not been previously evaluated. To address this, we monitored dynamic changes in the cellular immune profile of patients with previously untreated CLL/SLL treated with ibrutinib plus venetoclax in the MRD cohort of the CAPTIVATE study. Changes in circulating B-cell counts were confirmed using data from a separate cohort of patients in the GLOW study who were treated with FD ibrutinib plus venetoclax. We also confirmed BCL-2 sensitization after single-agent ibrutinib treatment by evaluating antiapoptotic proteins in patients with previously untreated CLL. Finally, we sought to determine whether changes in immune restoration were reflected in the safety profile observed in patients treated with ibrutinib plus venetoclax in the CAPTIVATE study.
Materials and methods
Study design and treatment
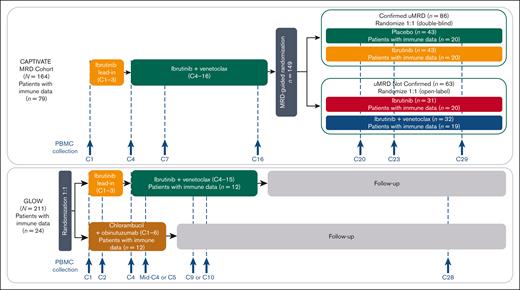
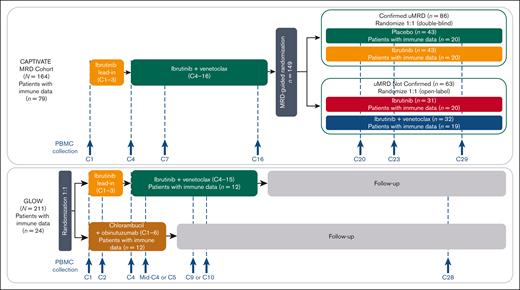
CAPTIVATE (NCT02910583) is an international, multicenter phase 2 study evaluating first-line ibrutinib plus venetoclax in patients with previously untreated CLL/SLL that includes an FD cohort and an MRD cohort. Detailed methods for the MRD cohort of CAPTIVATE were published previously.1 In the MRD cohort, patients received 3 cycles of ibrutinib lead-in followed by 12 cycles of ibrutinib plus venetoclax. Treatment was given in 28-day cycles. Patients were assessed for MRD during an additional cycle of combination treatment (cycle 16); patients with Confirmed undetectable MRD (uMRD; defined as uMRD serially over ≥3 cycles, in both peripheral blood and bone marrow) were randomly assigned 1:1 to double-blind treatment with placebo (ie, an FD regimen) or continued ibrutinib. Patients not meeting criteria for Confirmed uMRD (uMRD Not Confirmed) were randomly assigned 1:1 to open-label treatment with continued ibrutinib or ibrutinib plus venetoclax (Figure 1).
CAPTIVATE MRD cohort study design and disposition of patients included in the immunophenotyping study. C, cycle.
CAPTIVATE MRD cohort study design and disposition of patients included in the immunophenotyping study. C, cycle.
GLOW (NCT03462719) is a multicenter, randomized, open-label, phase 3 study evaluating ibrutinib plus venetoclax compared with chlorambucil plus obinutuzumab in patients with previously untreated CLL/SLL (Figure 1). Patients with del(17p) or TP53 mutations were excluded. Detailed methods for GLOW have been described previously.3 Briefly, patients were randomly assigned in a 1:1 ratio to receive ibrutinib plus venetoclax (3 cycles of ibrutinib lead-in followed by 12 cycles of combined ibrutinib plus venetoclax) or 6 cycles of chlorambucil plus obinutuzumab.
Both studies were approved by the institutional review boards of participating institutions and were conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All patients provided written informed consent.
Immunophenotyping
For analysis in CAPTIVATE, a deep immune profiling using high-dimensional cytometry to characterize numerous immune cell subsets was conducted using cryopreserved peripheral blood mononuclear cells (PBMCs) from 79 patients in the MRD cohort. Samples were collected at 7 time points: baseline (day 1 of cycle 1) and predose on day 1 of cycles 4, 7, 16, 20, 23, and 29. PBMCs were thawed, washed, treated with Benzonase Nuclease (Sigma-Aldrich, St Louis, MO), and stained with antibodies, following the manufacturer’s recommendations (supplemental Table 1). A 34-color flow cytometry panel was developed to determine the frequencies of subsets of B cells, T cells, natural killer (NK) cells, innate lymphoid cells, monocytes, dendritic cells (DCs), and MDSCs (supplemental Table 2).
For confirmatory analysis in GLOW, immunophenotyping was conducted using cryopreserved PBMCs from 24 patients treated with ibrutinib plus venetoclax or chlorambucil plus obinutuzumab. Samples were collected at 6 time points: baseline (day 1 of cycle 1), day 1 of cycle 2, days 1 and 15 of cycle 4, day 1 of cycle 9 or 10, and day 1 of cycle 28. PBMCs were thawed, washed, treated with Benzonase Nuclease, and stained with antibodies, following the manufacturer’s recommendations (supplemental Table 3). A 23-color flow cytometry panel was developed to determine the frequencies of subsets of B cells, T cells, NK cells, and monocytes and the expression of BCL-2 family proteins, assessing both surface markers and intracellular markers (supplemental Table 4).
For both panels, cells were acquired on a Cytek Aurora flow cytometer (Cytek Biosciences, Fremont, CA). Analyses were performed using OMIQ software (Dotmatics, Boston, MA). Absolute counts of cells in circulation were calculated by multiplying the frequency of each population among total lymphocytes by the absolute lymphocyte count obtained from whole-blood assessment on the day of sample collection. Healthy donor cell ranges were obtained from peripheral blood samples from 20 untreated, age-matched healthy donors, which were processed identically to patients’ samples.
BCL-2 sensitization
Sensitization to BCL-2 by ibrutinib was assessed using PBMCs collected at baseline and before dosing on day 1 of cycle 2 from patients with previously untreated CLL/SLL in the RESONATE-2 study (NCT01722487) who received oral ibrutinib 420 mg once daily in 28-day cycles and in PBMCs collected from patients treated with ibrutinib plus venetoclax vs chlorambucil plus obinutuzumab in the GLOW study. Detailed clinical methods for RESONATE-2 have been described previously.25 Treatment effects on antiapoptotic proteins were evaluated with intracellular markers (BCL-2, BCL-XL, MCL-1, and Ki-67) included in the 23-color flow cytometry panel described in “Immunophenotyping” (supplemental Tables 3-4).
Safety analysis
The incidence and prevalence of infections over time and complete resolution of infections were evaluated based on the randomized treatment arm in all patients from the CAPTIVATE MRD cohort who received at least 1 dose of study treatment. Adverse events were monitored throughout treatment until 30 days after the last dose of study treatment and were graded using the National Cancer Institute Common Terminology Criteria for adverse events, version 4.03. Infections were analyzed as combined terms for infections and infestations (identified using system organ class), opportunistic infection (identified using narrow and broad standardized MedDRA query), upper respiratory tract infection (including preferred terms for upper respiratory tract infection, viral upper respiratory tract infection, bronchitis, bronchitis bacterial, pharyngitis, pharyngitis streptococcal, nasopharyngitis, and sinusitis), urinary tract infection (including preferred terms for urinary tract infection and urinary tract infection bacterial), and infective pneumonia (identified using narrow standardized MedDRA query).
Statistical analysis
Median with interquartile range is reported. For normalization, data are reported as the median percentage change from baseline. The Wilcoxon matched-pairs signed rank test was used to determine statistically significant changes (at α = 0.05) at a time point of interest vs baseline (pretreatment). The Mann-Whitney 2-tailed test was performed to compare different treatment arms. Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Baseline characteristics and patient disposition
Key baseline characteristics of patients in the CAPTIVATE MRD cohort used to evaluate immune restoration (n = 79) are shown in Table 1 and were generally similar to those of the overall population of patients in the CAPTIVATE MRD cohort (supplemental Table 5), with some imbalances (≥10% difference) noted in the distribution of unmutated immunoglobulin heavy-chain variable region gene, bulky disease, hemoglobin ≤11 g/dL, and absolute lymphocyte count ≥25 × 109/L. Overall, 48 of 79 patients (61%) with immune data were male, and the median age was 59 years (range, 28-69 years). The median age of age-matched healthy donors was 64 years (range, 51-78 years). Key baseline characteristics for patients with immune data in the GLOW study are shown in supplemental Table 6.
Ibrutinib treatment sensitizes CLL cells to venetoclax
To confirm previous observations, expression levels of antiapoptotic proteins BCL-2, BCL-XL, and MCL-1 were assessed in lymph node emigrant CLL cells (CD5hi CXCR4dim) in PBMC samples from 3 patients treated with single-agent ibrutinib in the RESONATE-2 study and in 12 patients during single-agent ibrutinib lead-in in the GLOW study. After 1 cycle (28 days) of single-agent ibrutinib treatment in the RESONATE-2 study, expression of BCL-2, BCL-XL, and MCL-1 decreased from baseline by 10%, 95%, and 74%, respectively (supplemental Figure 1A), with a net result of predominant BCL-2 cellular dependence, the target for venetoclax inhibitory activity. In samples collected on day 1 of cycle 2 during single-agent ibrutinib lead-in in the GLOW study, expression of BCL-2, BCL-XL, and MCL-1 decreased from baseline by 26%, 35%, and 58%, respectively (supplemental Figure 1B).
Addition of venetoclax to ibrutinib drives a rapid decrease of circulating CLL cells
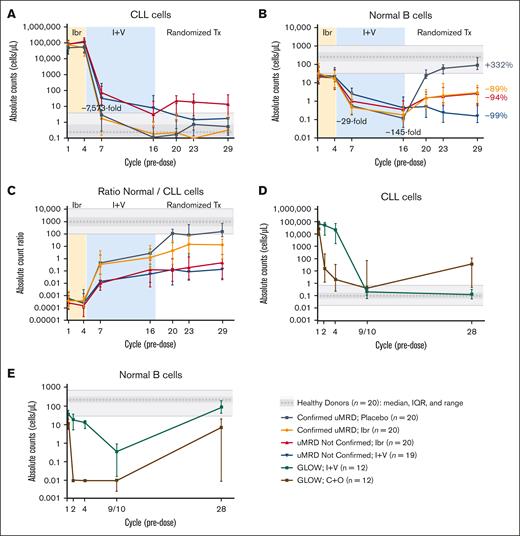
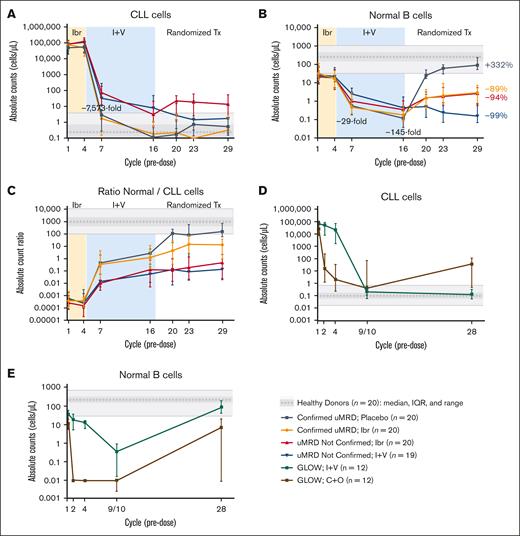
A rapid and significant decrease in circulating CLL cells occurred within the first 3 cycles after initiation of venetoclax in patients in the CAPTIVATE MRD cohort (Figure 2A, cycle 7). Patients with Confirmed uMRD (n = 40) had a significantly greater decrease in circulating CLL cell count compared with patients with uMRD Not Confirmed (n = 39), both during ibrutinib lead-in (decrease at cycle 4; P = .0073) and with combined ibrutinib plus venetoclax as assessed at cycles 7 and 16 (P < .0001 at both time points). From cycle 16 onward, patients with Confirmed uMRD randomly assigned to placebo or ibrutinib had CLL cell counts similar to those of healthy donors (≤0.8 cells per μL), whereas median CLL cell counts in patients with uMRD Not Confirmed ranged from 1.5 to 22.9 CLL cells per μL, with lower levels in patients randomly assigned to ibrutinib plus venetoclax compared with ibrutinib alone at cycle 23 (P = .0329) and at cycle 29 (P = .0454).
Ibrutinib plus venetoclax rapidly eradicates CLL cells. (A) Absolute counts of circulating CLL cells in CAPTIVATE. (B) Absolute counts of normal B cells in CAPTIVATE. (C) Ratio of absolute counts of normal B cells to CLL cells in CAPTIVATE. (D) Absolute counts of circulating CLL cells in GLOW. (E) Absolute counts of normal B cells in GLOW. Data points represent median values, and error bars represent the interquartile range. For GLOW data, samples at cycle 28 were available only for patients with a best overall response of complete response (n = 6 per arm). C+O, chlorambucil plus obinutuzumab; I+V, ibrutinib plus venetoclax; Ibr, ibrutinib; IQR, interquartile range; Tx, treatment.
Ibrutinib plus venetoclax rapidly eradicates CLL cells. (A) Absolute counts of circulating CLL cells in CAPTIVATE. (B) Absolute counts of normal B cells in CAPTIVATE. (C) Ratio of absolute counts of normal B cells to CLL cells in CAPTIVATE. (D) Absolute counts of circulating CLL cells in GLOW. (E) Absolute counts of normal B cells in GLOW. Data points represent median values, and error bars represent the interquartile range. For GLOW data, samples at cycle 28 were available only for patients with a best overall response of complete response (n = 6 per arm). C+O, chlorambucil plus obinutuzumab; I+V, ibrutinib plus venetoclax; Ibr, ibrutinib; IQR, interquartile range; Tx, treatment.
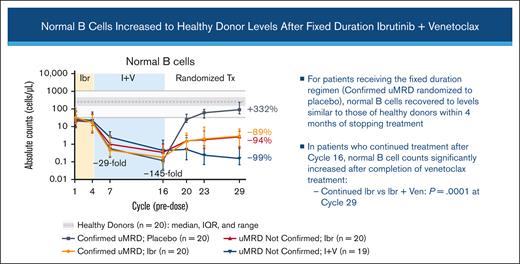
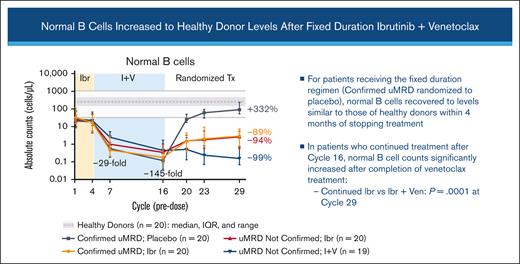
B-cell count recovery after cycle 16 varied based on the randomized treatment arm
Normal B-cell counts progressively decreased during the treatment with ibrutinib plus venetoclax. Among patients who discontinued all treatment (those with Confirmed uMRD who were randomly assigned to placebo), normal B-cell counts recovered to levels similar to those observed in healthy donors (median, 90.6 cells per μL at cycle 29, +332% vs baseline; Figure 2B). Patients receiving continued single-agent ibrutinib had moderate recovery of normal B-cell counts from cycle 16 regardless of Confirmed uMRD status, but counts remained below healthy donor levels at cycle 29 (−89% vs baseline in patients with Confirmed uMRD and −94% vs baseline in patients with uMRD Not Confirmed). Patients with uMRD Not Confirmed receiving continued ibrutinib plus venetoclax had minimal recovery of normal B-cell counts at cycle 29 (−99% vs baseline). At cycle 29, normal B-cell counts were significantly higher in patients receiving continued ibrutinib than in those receiving continued ibrutinib plus venetoclax (P < .0001; Figure 2B). The ratio of normal B cells to CLL cells increased continuously with ibrutinib plus venetoclax and with postrandomization treatment through cycle 29, with more pronounced improvements observed in patients with Confirmed uMRD (Figure 2C).
Abnormal counts of T cells normalized to healthy donor levels
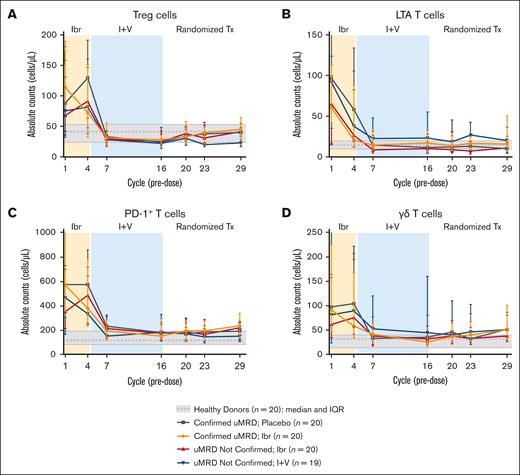
Across all randomized treatment arms in the CAPTIVATE MRD cohort, normalization of overall CD3+ T-cell counts occurred within the first 6 months of treatment, with a median decrease of 49% from baseline; these levels were maintained thereafter, regardless of randomized treatment. Abnormally high pretreatment counts of T-cell subsets, including regulatory T cells, long-term activated (LTA) T cells, programmed cell death 1–positive (PD-1+) exhausted T cells, and γδ T cells, were normalized to healthy donor levels (Figure 3). Normalization was also observed in other T-cell subsets, including CD8+, CD4+, follicular helper, Th1, Th2, Th17, and Th22 cells (supplemental Figure 2), and mucosal-associated invariant T cells (supplemental Figure 3). In addition, the ratio of CD4+ to CD8+ T cells, which is usually low in patients with CLL, increased to healthy donor levels by cycle 7 and was maintained thereafter (supplemental Figure 2).
Ibrutinib plus venetoclax normalizes abnormal T-cell counts to healthy donor levels within the first 6 months of treatment. (A) Absolute counts of regulatory T cells. (B) Absolute counts of LTA T cells. (C) Absolute counts of PD-1+ T cells. (D) Absolute counts of γδ T cells. Data points represent median values, and error bars represent the IQR.
Ibrutinib plus venetoclax normalizes abnormal T-cell counts to healthy donor levels within the first 6 months of treatment. (A) Absolute counts of regulatory T cells. (B) Absolute counts of LTA T cells. (C) Absolute counts of PD-1+ T cells. (D) Absolute counts of γδ T cells. Data points represent median values, and error bars represent the IQR.
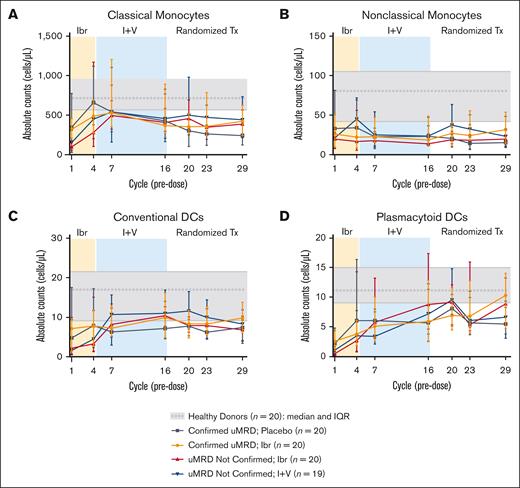
Ibrutinib plus venetoclax drives recovery of classical monocytes and DCs
Treatment with ibrutinib plus venetoclax favored the recovery of classical monocytes (twofold increase at cycle 7; Figure 4A) over nonclassic monocytes (Figure 4B). Conventional DC counts were largely restored by cycle 7, with a median increase of 284% from baseline in patients with uMRD Not Confirmed and a median 2% increase in patients with Confirmed uMRD; these levels were maintained in subsequent cycles regardless of randomized treatment (Figure 4C). Across randomized treatment arms, plasmacytoid DCs progressively increased to levels similar to healthy donors by cycle 20 (+598% vs baseline; Figure 4D). Monocytic MDSC levels decreased and were detected at levels of ≤5 cells per μL from cycle 7 onward (supplemental Figure 3). NK cells and innate lymphoid cells increased during ibrutinib lead-in, then decreased after venetoclax initiation (supplemental Figure 3), with a trend toward a greater reduction in overall NK cell counts for patients with Confirmed uMRD (−64%) than for those with uMRD Not Confirmed (−39%) (P = .05). In both patients with Confirmed uMRD and uMRD Not Confirmed, counts of immature (CD16–) NK cells were affected to a greater extent than those of mature (CD16+) NK cells (supplemental Figure 2). At cycle 29, immature NK cell counts decreased by 65% from baseline in patients with Confirmed uMRD and by 62% in patients with uMRD Not Confirmed, whereas mature NK cell counts decreased by 41% from baseline in patients with Confirmed uMRD and by 22% in patients with uMRD Not Confirmed. From cycle 7 onward, counts of both NK and innate lymphoid cells remained in the lower range of those observed in healthy donors across all randomized treatment arms (supplemental Figure 3).
Ibrutinib plus venetoclax drives recovery of classic monocytes and conventional DCs. (A) Absolute counts of classic monocytes. (B) Absolute counts of nonclassic monocytes. (C) Absolute counts of conventional DCs. (D) Absolute counts of plasmacytoid DCs. Data points represent median values, and error bars represent the IQR.
Ibrutinib plus venetoclax drives recovery of classic monocytes and conventional DCs. (A) Absolute counts of classic monocytes. (B) Absolute counts of nonclassic monocytes. (C) Absolute counts of conventional DCs. (D) Absolute counts of plasmacytoid DCs. Data points represent median values, and error bars represent the IQR.
Analysis from GLOW confirms the changes in immune cell subsets observed in CAPTIVATE
In patients treated with FD ibrutinib plus venetoclax in the GLOW study, a robust reduction of circulating CLL cells to counts within the range typical of healthy donors was observed after addition of venetoclax in cycle 4 (Figure 2D, cycles 9 and 10). Elimination of CLL cells was sustained long after completion of FD treatment, and CLL cell counts remained within healthy donor levels at cycle 28 (Figure 2D, cycle 28). In patients treated with chlorambucil plus obinutuzumab, a rapid elimination of CLL cells occurred in the first treatment cycle (Figure 2D, cycle 2); however, after the end of treatment, CLL cell counts increased to levels several fold above the healthy donor range (Figure 2D, cycle 28).
A reduction of normal B-cell counts was observed during treatment with ibrutinib plus venetoclax (Figure 2E, from cycle 4 to cycle 9 or 10), followed by an improvement in normal B-cell counts after completion of treatment that reached healthy donor levels at cycle 28 (Figure 2E). By contrast, patients treated with chlorambucil plus obinutuzumab showed rapid elimination of normal B cells in the first treatment cycle (Figure 2E, cycle 2); although some improvement in normal B cells was observed after the end of treatment, normal B-cell counts were variable at cycle 28 and did not reach the healthy donor range (Figure 2E, cycle 28). Changes in T-cell subsets (CD4+, CD8+, LTA, and PD-1+ T cells), NK cells, and monocytes in patients treated with ibrutinib plus venetoclax in the GLOW study were similar to those observed in the CAPTIVATE study (supplemental Figure 4). Reductions in T-cell counts were also observed with chlorambucil plus obinutuzumab.
Treatment-emergent infections decrease over time regardless of randomized treatment
Complete safety data for the CAPTIVATE MRD cohort were published previously.1 Both the prevalence (supplemental Table 7) and incidence (supplemental Table 8) of infection of any grade generally decreased over time across all randomized treatment arms. The extent of these decreases was variable between the arms, but overall prevalence was lowest numerically in the patients in the Confirmed uMRD placebo arm, particularly through month 24 (supplemental Tables 7-8). Complete resolution was observed for almost all (93% to 100%) treatment-emergent infections (supplemental Table 9). Similar patterns were observed when looking at specific types of infections (opportunistic infections, upper respiratory tract infections, urinary tract infections, and infective pneumonia).
Discussion
Results from the phase 2 CAPTIVATE study and the phase 3 GLOW study demonstrated that first-line treatment with ibrutinib plus venetoclax provides deep, durable responses and sustained progression-free survival in patients with CLL/SLL.1,3 In this analysis, we evaluated dynamic changes in circulating immune cell counts in patients enrolled in the MRD cohort of the CAPTIVATE study to understand the impact of both initial treatment with ibrutinib plus venetoclax and MRD-guided subsequent treatment. Confirmatory data were obtained from the phase 3 GLOW study. Our findings suggest that ibrutinib plus venetoclax treatment restores a healthier immune profile in patients with CLL, with a totality of data demonstrating eradication of CLL cells, recovery of normal B cells, and normalization of critical immune cells, including T-cell subsets, classical monocytes, and DC counts.
We evaluated the expression of antiapoptotic BCL-2 family proteins on circulating lymph node emigrant CLL cells present after single-agent ibrutinib treatment in RESONATE-2 and GLOW to confirm anti–BCL-2 sensitization with resultant venetoclax sensitivity. Consistent with previous findings observed in both cryopreserved and fresh samples,26,27 results demonstrated that MCL-1 and BCL-XL levels decreased after 1 cycle of treatment with single-agent ibrutinib, suggesting increased BCL-2 dependence for survival and corresponding BCL-2 inhibitor sensitization. Although differences were observed between RESONATE-2 and GLOW, the trend toward a greater decrease in BCL-XL and MCL-1 expression relative to BCL-2 was consistent between studies. These results confirm that single-agent ibrutinib lead-in primes CLL cells for BCL-2 inhibition by venetoclax and support the synergistic antitumor activity observed with the combination of ibrutinib plus venetoclax in preclinical models of CLL.4,5,28,29
Immunophenotyping data from patients in the CAPTIVATE MRD cohort confirmed that combined ibrutinib plus venetoclax led to a rapid eradication of circulating CLL cells after initiation of venetoclax, with a more pronounced reduction in patients who achieved Confirmed uMRD after a total of 13 cycles of ibrutinib plus venetoclax, as expected. CLL cell counts as assessed by flow cytometry criteria in patients with Confirmed uMRD remained within healthy donor levels thereafter, including for patients receiving an FD regimen of ibrutinib plus venetoclax (placebo arm). These findings are consistent with the previously reported lack of statistically significant differences in disease-free survival rates after randomization in patients with Confirmed uMRD during randomized treatment with placebo vs continued ibrutinib.1 In line with previously reported improvements in uMRD rates after randomization,1 CLL counts continued to decline in patients with uMRD Not Confirmed receiving continued ibrutinib plus venetoclax treatment but not in those receiving continued single-agent ibrutinib. Normal B-cell counts progressively recovered after the completion of venetoclax treatment in all randomized treatment arms, particularly on discontinuation of ibrutinib plus venetoclax in the Confirmed uMRD placebo arm (ie, an FD regimen), which resulted in rapid and sustained recovery of normal B-cell counts to healthy donor levels in contrast to patients receiving single-agent ibrutinib (with moderate recovery) or ibrutinib plus venetoclax (with minimal recovery). Sustained elimination of CLL cells and recovery of normal B cells were confirmed in samples from patients treated with FD ibrutinib plus venetoclax in the phase 3 GLOW study.
Changes in other immune cell subsets were generally similar to those previously observed with single-agent ibrutinib8,9 but with much faster kinetics. Normalization was achieved within 6 months after initiation of treatment for most immune cell subsets and was maintained thereafter regardless of randomized treatment in the CAPTIVATE MRD cohort. Ibrutinib plus venetoclax reduced and normalized counts of abnormally elevated immunosuppressive T-cell subsets that can contribute to tumor escape from immunosurveillance (regulatory T cells, LTA T cells, and PD-1+ T cells)11 and normalized counts of other T-cell subsets. Concomitantly, ibrutinib plus venetoclax treatment preferentially restored abnormally low counts of myeloid cell subsets that may contribute to antitumor activity, such as phagocytic classical monocytes and DCs, whereas proinflammatory nonclassical monocytes and protumor MDSCs were maintained below healthy donor levels. Although previous studies demonstrated increased plasmacytoid DCs but decreased conventional DCs with single-agent ibrutinib treatment,8,9 ibrutinib plus venetoclax facilitated the recovery of both plasmacytoid and conventional DCs toward healthy donor levels. NK cells were maintained within the lower range of healthy donor levels throughout treatment with ibrutinib plus venetoclax and postrandomization treatment. Collectively, this immunophenotyping analysis showed that ibrutinib plus venetoclax treatment had a positive impact on circulating innate and adaptive immune cells in patients with CLL that may result in the restoration of healthy immune function. Importantly, these positive effects were maintained after the completion of ibrutinib plus venetoclax treatment in patients with Confirmed uMRD who were randomly assigned to placebo, supporting an FD regimen.
A reduction in CLL disease burden and the restoration of a healthier immune profile may reduce susceptibility to infections in patients with CLL. In the CAPTIVATE MRD cohort, rates of infections generally decreased over time. There appeared to be a trend toward lower rates of infections in patients who achieved Confirmed uMRD than in those who did not, particularly for patients randomly assigned to the placebo arm. However, with relatively small numbers of patients in each treatment arm, random imbalances in infection rates were observed at the conclusion of prerandomization treatment with ibrutinib plus venetoclax. In addition, there was no stratification for comorbid conditions that could affect the risk of infection in these patient populations. Larger sample sizes and longer follow-up may be required to fully assess the clinical impact of immune restoration with ibrutinib plus venetoclax on the long-term risk of infections in patients with CLL/SLL.
In conclusion, these findings represent promising evidence for the restoration of important circulating adaptive and innate immune cells with ibrutinib plus venetoclax, particularly in patients receiving an FD regimen (randomly assigned to placebo) with previously untreated CLL/SLL, and support the observed clinical efficacy of this regimen.1-3
Acknowledgments
The authors thank the patients who participated in this trial and their families. The authors also thank Melanie Sweetlove for contributing to the medical writing and editing of the manuscript.
This study was supported by Pharmacyclics LLC, an AbbVie Company.
Authorship
Contribution: C.M. and C.S.T. contributed to investigation, drafted the manuscript, and critically revised the manuscript for intellectual content; R.S.M., C.Z., and E.S.-G analyzed the data; I.G.S. contributed to the study design, methodology, supervision, and investigation, provided resources, performed formal analysis, validation, and visualization of the data, drafted the manuscript, and critically revised the manuscript for intellectual content; A.G., L.S., T.J.K., S.S., R.S.M., and M.C. contributed to investigation and critically revised the manuscript for intellectual content; C.Z. performed validation of the data, contributed to the methodology, and critically revised the manuscript for intellectual content; J.P.D. and E.S.-G. provided supervision, validation and visualization of the data, contributed to the methodology, and critically revised the manuscript for intellectual content; and all authors had access to primary clinical data.
Conflict-of-interest disclosure: C.M. reports a consulting/advisory role with AbbVie, AstraZeneca, Janssen, and Sunesis; research funding from AbbVie and Janssen; and speakers’ bureau for Janssen. I.G.S., R.S.M., C.Z., J.P.D., and E.S.-G. report employment with Pharmacyclics LLC, an AbbVie Company, and stock or other ownership in AbbVie. C.S.T. reports honoraria from AbbVie, BeiGene, Janssen, and Loxo, and institutional research funding from AbbVie, BeiGene, and Janssen. A.G. reports a consulting/advisory role for Janssen and Novartis. L.S. reports honoraria from and a consulting/advisory role for AbbVie, AstraZeneca, BeiGene, Lilly, and Janssen; travel/accommodations/expenses from BeiGene and Janssen; and a speakers’ bureau role for Octapharma. T.J.K. reports a consulting/advisory role for AbbVie, Celgene, Genentech-Roche, Gilead, and Pharmacyclics LLC, an AbbVie Company, and research funding from AbbVie, Genentech-Roche, Oncternal, and Pharmacyclics LLC, an AbbVie Company. S.S. reports employment with Janssen. M.C. reports a consulting/advisory role for Genentech, and institutional research funding from AbbVie, TG Therapeutics, and Pharmacyclics LLC, an AbbVie Company.
Correspondence: Carol Moreno, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Carrer de Sant Quintí, 89, 08041 Barcelona, Spain; e-mail: cmorenoa@santpau.cat.
References
Author notes
∗C.M. and I.G.S. contributed equally to this study.
Individual participant data from clinical studies conducted by Pharmacyclics LLC, an AbbVie Company, are available on request from the Yale Open Data Access project site at http://yoda.yale.edu.
The full-text version of this article contains a data supplement.