Patients with blastoid and pleomorphic variant MCL have suboptimal outcomes.
Receipt of auto-HCT, MIPI score, and complete response to induction were associated with PFS; auto-HCT was not associated with OS.
Visual Abstract
Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin lymphoma; data indicate that blastoid and pleomorphic variants have a poor prognosis. We report characteristics and outcomes of patients with blastoid/pleomorphic variants of MCL. We retrospectively studied adults with newly diagnosed MCL treated from 2000 to 2015. Primary objectives were to describe progression-free survival (PFS) and overall survival (OS). Secondary objectives included characterization of patient characteristics and treatments. Of the 1029 patients with MCL studied, a total of 207 neoplasms were blastoid or pleomorphic variants. Median follow-up period was 82 months (range, 0.1-174 months); median PFS was 38 months (95% confidence interval [CI], 28-66) and OS was 68 months (95% CI, 45-96). Factors associated with PFS were receipt of consolidative autologous hematopoietic transplantation (auto-HCT; hazard ratio [HR], 0.52; 95% CI, 0.31-0.80; P < .05), MCL International Prognostic Index (MIPI) intermediate (HR, 2.3; 95% CI, 1.2-4.3; P < .02) and high (HR, 3.8; 95% CI, 2.0-7.4; P < .01) scores, and complete response to induction (HR, 0.29 (95% CI, 0.17-0.51). Receipt of auto-HCT was not associated with OS (HR, 0.69; 95% CI, 0.41-1.16; P = .16) but was associated with MIPI intermediate (HR, 5.7; 95% CI, 2.5-13.2; P < .01) and high (HR, 10.8; 95% CI, 4.7-24.9; P < .01) scores. We report outcomes in a large cohort of patients with blastoid/pleomorphic variant MCL. For eligible patients, receipt of auto-HCT after induction was associated with improved PFS but not OS. Higher MIPI score and auto-HCT ineligibility were associated with worse survival.
Introduction
Mantle cell lymphoma (MCL) accounts for ∼3% to 10% of all non-Hodgkin lymphomas.1-4 It is characterized by the t(11,14)(q13;q32)/CCND1:IGH translocation leading to overexpression of cyclin D1.5-8 Several morphologic variants of MCL, including small cell, diffuse, blastoid, and pleomorphic, have been identified.9-11 The blastoid and pleomorphic variants compose 10% to 20% of all MCLs and are morphologically characterized, respectively, by immature cells resembling lymphoblasts and by large pleomorphic cells with prominent nucleoli, irregular nuclear contours, and mitotic figures.12,13 The clinical behavior of these variants is distinct, likely influenced by a different genetic and mutational profile such as TP53 mutations, complex karyotype, and abnormalities in chromosomes 3, 8, 13, 17, and 18.14-16
The optimal treatment of patients with MCL remains controversial, with no standard of care induction strategy. Historically, blastoid/pleomorphic variants have largely been treated similarly to nonblastoid/pleomorphic MCL. Patients can be offered a wide range of therapies, from observation to bendamustine-based therapy and intensive chemoimmunotherapy using high-dose cytarabine.17 Many young patients are offered consolidative autologous hematopoietic cell transplantation (auto-HCT) in first remission18 based on a single randomized trial of younger patients with MCL that demonstrated improved progression-free survival (PFS) with HCT consolidation over maintenance interferon alpha.19 Limited retrospective series suggest that allogeneic HCT (allo-HCT) may be associated with long-term disease control in patients with blastoid/pleomorphic variants of MCL.13 Herein, we sought to describe characteristics, treatment pattern, and outcomes of patients with blastoid/pleomorphic variant MCL treated in the modern era in a large retrospective cohort.
Methods
Patients
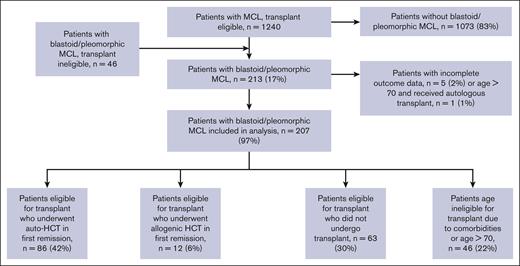
Twenty-five North American centers retrospectively identified patients with newly diagnosed blastoid/pleomorphic variant MCL who were treated between 2000 and 2015. A subset of the cohort was previously reported as part of a larger cohort of patients aged ≤65 years, newly diagnosed with MCL, and deemed transplant eligible at diagnosis by the institutional investigator, as previously described.20 From that original cohort, we limited the present analysis to patients with the diagnosis of blastoid/pleomorphic MCL as determined by a hematopathologist at each institution as per routine clinical practice. We subsequently added a cohort of patients from the same institutions with blastoid/pleomorphic morphology who had been excluded from the original analysis because they were aged >65 years or ineligible for transplantation based on age or comorbidities. Patients who underwent HCT within 6 months after induction were classified as having received HCT as consolidation. The protocol was approved by the institutional review board of each participating center.
Data collection
Data collected for each patient included the following baseline characteristics: age, sex, stage, peripheral blood or bone marrow involvement, number of extranodal sites, Eastern Cooperative Oncology Group performance status, presence of B symptoms, serum lactate dehydrogenase at diagnosis, histologic subtype (ie, blastoid/pleomorphic variant), cytogenetics, cyclin D1 protein expression, and Ki67 percentage. MCL International Prognostic Index (MIPI) scores were calculated for each patient with sufficient data as per Hoster et al.21 If a range was provided for Ki67, the highest value was used; values were rounded to the nearest 10%. Treatment data collected included induction regimen (defined as cyclophosphamide, doxorubicin, vincristine, and prednisone–like [CHOP-like]; intensive [eg, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyperCVAD)]; maxi-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone); dexamethasone, high-dose cytarabine, and cisplatin [DHAP] based; bendamustine based; or novel [lenalidomide, ibrutinib, or bortezomib containing]), receipt of an anti–CD-20 antibody with induction, response to induction, and receipt of maintenance therapy. Response to induction therapy was determined by the local investigators based on respective institutional practices. In addition, data regarding HCT were collected, including receipt of consolidative HCT, time from initiation of treatment to transplantation, and reason for no transplantation if not received (investigator preference, patient preference, other reason, or unknown). Finally, data on date of last follow-up, occurrence and date of progression, cause of death (if deceased), and development of treatment-related myelodysplastic syndrome or acute myeloid leukemia were collected.
Statistical analysis
In order to account for different practice patterns, we divided the cohort into 4 separate groups mainly based on age and transplant eligibility: patients deemed by the local investigator to be eligible for transplantation who received auto-HCT in first remission, eligible for transplantation who received allo-HCT in first remission, eligible for transplantation who did not receive transplantation, and ineligible for transplantation because of comorbidities or age >70 years. We sought to characterize survival outcomes for patients with blastoid/pleomorphic MCL for the entire group and in each group separately, using descriptive statistics. We then compared patient, tumor, and treatment factors between the different groups using χ2, Fisher exact (with P values computed by simulation), and Wilcoxon rank-sum tests, as appropriate. Survival outcomes were estimated using the Kaplan-Meier method, which used a landmark survival analysis that was restricted to patients surviving >6 months. Cox proportional hazard models were used to analyze the association of HCT consolidation with survival after adjusting for confounders (MIPI, induction regimen, response to induction [complete response vs partial response], and Eastern Cooperative Oncology Group performance status) in the subset of eligible patients. Because of the differential time points of receiving HCT, this was included in the Cox model as a time-varying covariate.22
Results
Patients and disease characteristics
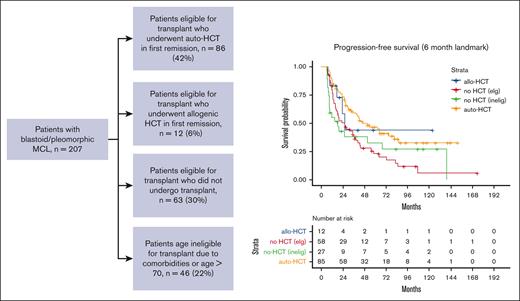
In total, 207 patients with blastoid or pleomorphic variant MCL were included in this analysis (Figure 1). Baseline characteristics are reported in Table 1. Median age at diagnosis was 58 years (range, 33-88 years). Most patients, 163 (79%), were men. Ki67 was reported in 113 patients (54%), with a median of 70% (20%-100%). MIPI score was reported for 158 patients and was low for 68 patients (43%), medium for 38 (24%), and high for 52 (33%) patients. Overall, 174 patients (84%) had stage IV disease at diagnosis. TP53 abnormalities were detected in 13 patients (8%) patients, and data were missing for 41 patients (20%). Induction therapy was CHOP for 75 patients (36%), intensive (hyperCVAD, DHAP-based, or Nordic regimen) for 113 (55%), and bendamustine for 16 (8%); cytarabine was included in induction for 110 patients (53%), and rituximab for 184 patients (89%). Best response to induction was complete response in 140 patients (69%), partial response in 38 (19%), and stable disease or progression in 9 (4%). At a median follow-up of 82 months (range, 0.1-174 months), the median PFS was 38 months (95% confidence interval [95% CI], 28-66), and overall survival (OS) was 68 months (95% CI, 45-96). Out of 207 patients, 76 died; 5 because of treatment-related mortality and 19 of other causes. In total, 131 patients were alive at last follow-up.
Comparison between groups
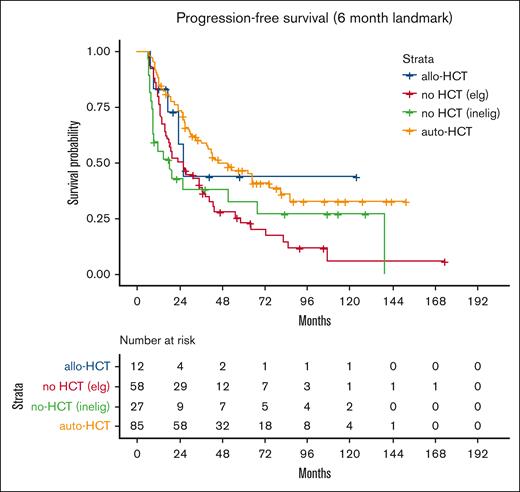
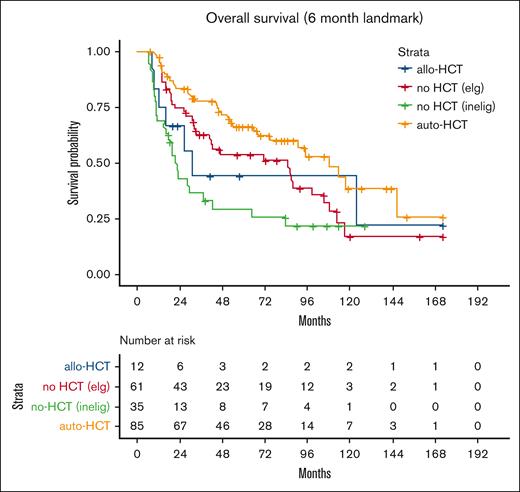
Patients were then divided into 4 groups; 42% (86 patients) were deemed by the local investigator to be eligible for transplantation and received auto-HCT in first remission; 6% (12 patients) were transplant eligible and received allo-HCT in first remission, 63 patients (30%) were transplant eligible but did not receive transplant, and 46 patients (22%) were transplant ineligible because of comorbidities or age >70 years. Patient baseline characteristics for each group are included in Table 1; differences between groups were statistically significant on univariate analysis for age at diagnosis, MIPI, induction regimen, cytarabine-containing induction, and best response to induction. On unadjusted landmark analysis for patients surviving at least 6 months after start of induction therapy, the median PFS for these 4 groups was 46, 26, 24, and 18 months, respectively (P < .008; Table 2; Figure 2). Median OS for the groups was 108, 31, 85, and 21 months, respectively (P < .001; Table 2; Figure 3). When analyzing the entire cohort, on multivariate analysis, the 2 factors associated with improved PFS were receipt of HCT (hazard ratio [HR], 0.52; 95% CI, 0.31-0.80; P < .01) and complete response to induction therapy (HR, 0.29; 95% CI, 0.17-0.51; P < .01), whereas MIPI score intermediate (HR, 2.3; 95% CI, 1.2-4.3; P < .02), and MIPI score high (HR, 3.8; 95% CI, 2.0-7.4; P < .01) had worse PFS compared with MIPI score low. Factors associated with OS were MIPI intermediate score (HR, 5.7; 95% CI, 2.5-13.2; P < .01), MIPI high score (HR, 10.8; 95% CI, 4.7-24.9; P < .01), MIPI unknown (HR, 5.2; 95% CI, 2.4-11.6; P < .01), and complete response to induction (HR, 0.44; 95% CI, 0.23-0.86; P < .02). To evaluate for any difference in the receipt of rituximab with induction, a sensitivity analysis was performed, excluding the ∼10% of patients who did not receive rituximab (R), with nearly identical results (supplemental Table 1). Induction chemotherapy, Ki-67, and R-maintenance were not associated with PFS and OS. Because of the very small number of patients with information on del17p and/or TP53 mutation, we did not perform a statistical analysis to assess the impact of these well-established, poor risk factors on outcomes.
PFS for each group. auto-HCT, autologous hematopoietic cell transplant. elg, eligible; inelig, ineligible.
PFS for each group. auto-HCT, autologous hematopoietic cell transplant. elg, eligible; inelig, ineligible.
Discussion
We report patient characteristics and outcomes of 207 patients with blastoid and pleomorphic variant MCL. To our knowledge, this is the largest reported cohort of patients with these variants of MCL treated in North America in the rituximab era. Survival for these patients at high risk was, as previously shown,20 lower than for patients with nonblastoid/pleomorphic MCL, with a median PFS of only 38 months and median OS of 68 months. Patients in our cohort who were not eligible for transplant because of comorbidities had the worst outcomes.
Smaller retrospective cohort studies have previously suggested inferior outcome for patients with blastoid/pleomorphic variant MCL. Our study, to our knowledge, provides the largest series, to date, of outcome for these morphologic variants and is in line with the existing data. We similarly showed an inferior outcome for patients with these high-risk variants of MCL.
There is a high variability in the clinical outcomes of MCL23; however, as demonstrated by small case series, blastoid and pleomorphic variants generally are associated with an aggressive clinical course and a particularly poor prognosis.24-26 These variants are associated with high Ki-67 expression and higher frequencies of TP53 mutations and CDKN2A/p16 deletions.16 In our series, a high percentage of missing data regarding TP53 mutational status limits determination of the impact of this alteration on outcome. Nevertheless, of the patients with available data, less than 10% had chromosome 17p deletions.
The majority (88%) of patients in this cohort received maintenance rituximab. Interestingly, in contrast to the extensive literature demonstrating a survival advantage when using maintenance rituximab in MCL,27-29 this factor was not associated with outcome in this cohort.
The major strength of this report is the large size of the cohort for this uncommon subset of a rare disease, with >200 patients having blastoid/pleomorphic variants. Although rituximab was approved in 1997, not all patients (9%) received an anti-CD20 antibody with induction. Nevertheless, a sensitivity analysis excluding patients who did not receive rituximab did not show significant differences in our results, confirming that this population was treated with therapies considered appropriate in the modern therapy era. There are numerous limitations inherent to an observational retrospective study design. First, the lack of central pathology review and high interrater variability in the diagnosis of these uncommon cancers limit confidence that these cases were accurately classified. Similarly, other true cases of blastoid or pleomorphic variant MCL might have been missed. During data collection, we grouped blastoid and pleomorphic variants together, with no difference in outcome between the 2. Second, the retrospective nature of the study allowed for inherent selection bias that might have informed the treatment decisions, including the decision for undergoing HCT. Although auto-HCT after induction for patients with blastoid/pleomorphic variants of MCL was associated with improved outcomes on multivariant analysis, the analysis was unadjusted and not propensity weighted. On multivariable analysis, we described similarly improved PFS and OS for patients with MCL who were eligible for transplantation, with only a PFS benefit remaining after propensity-score weighted analysis. Third, there was a high rate of missing data, most importantly TP53 mutation status, making it difficult to determine whether the inferior outcome was driven by this factor rather than pathologic morphology alone. Fourth, very few patients underwent allo-HCT, which limits any interpretation of outcome after this therapy. Lastly, although we categorized the reason for determining transplant ineligibility (supplemental Table 2), an investigator’s decision is inherently biased and cannot be fully accounted for.
In summary, we describe a large cohort of patients with blastoid/pleomorphic variant MCL treated in the rituximab era and establish their suboptimal outcomes. For transplant-eligible patients, multivariable analysis suggests that the use of consolidative autologous HCT after induction was associated with improved PFS, although this might have been confounded by a selection bias. In our analysis, very few patients received upfront treatment that included biological agents. Increasing data suggest improved outcomes in high-risk MCL by the addition of Bruton tyrosine kinase (BTK) inhibitors, such as ibrutinib,30 acalabrutinib,31 and zanubrutinib,32,33 in both the frontline (most notably the recently reported SHINE trial34) and the relapsed setting, which may be particularly relevant for patients with TP53 aberrations and blastoid/pleomorphic variants of MCL. The combination of a BTK inhibitor with the B-cell lymphoma 2 protein inhibitor venetoclax with or without an anti CD20 monoclonal antibody35,36 has also shown significant promise, even for patients with TP53 aberrations. Two very novel therapies that were not available to the patients included in our study outside of clinical trials are pirtobrutinib, a highly selected, reversible BTK inhibitor,37 and CD19-directed chimeric antigen receptor T-cell therapy (specifically brexucabtagene autoleucel). Although only a small number of patients with blastoid MCL (31%) were included in the pivotal trial of brexucabtagene autoleucel, subgroup analysis suggests its activity in blastoid and pleomorphic variant MCL.38 Cellular and noncytotoxic therapies, such as targeted biological agents and bispecific antibodies, may prove to be agnostic to histologic variant and TP53 mutational status and may become a preferred treatment modality, as evidenced by the recently presented TRIANGLE study data.39 Although the treatment approaches used in our patient population will, most likely, become outdated soon, our results provide a benchmark that can be used to contrast outcomes with noncytotoxic and more targeted therapies.
Authorship
Contribution: J.N.G., E.H., D.V., A.S.G., P.C., S.L., L.J.M., M.W., J.B.C., M.C., B.T.H., Y.S., F.J.H.-I., S.K., J.M.V., M.B., T.S.F., S.N., K.J.M., D.B., V.B., B.K., J.C., B.S., F.L., T.B., A.M.D., N.W.-J., M.M., A.M., J.K.A., N.R., A.E.K., D.J.L., M.G., D.J.I., K.R., R.K., J.B.K., P.F.C., S.R., A.E., A.K., E.U., B.P., J.E.A., J.K.L., C.D., R.I.F., and S.K.B. designed the research, performed research, and performed statistical analysis; and J.N.G., E.H., and S.K.B. analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.N.G. declares research funding from Loxo and consulting for Genetech. E.H. declares research funding from Pfizer (to institution). D.V. declares honoraria from Roche Canada, Janssen, Lundbeck Canada, Seattle Genetics, Gilead Sciences, Acerta Pharma/AstraZeneca, Celgene, and Merck; consulting for, or advisory role with Roche Canada, Janssen, Lundbeck Canada, Seattle Genetics, Gilead Sciences, Accerta Pharma/AstraZeneca, and Celgene; research funding from Roche (to institution); and travel, accommodation, and expenses funds from Roche Canada, Janssen, Lundbeck Canada, and Accerta Pharma. A.S.G. declare honoraria from Janssen; consulting for, or advisory role with Janssen, AbbVie, and Seattle Genetics; and research funding from AbbVie (to institution), Accerta (to institution), Roche Canada (to institution), and Lundbeck Canada (to institution). P.C. declares patents, royalties, and other intellectual property (intellectual property of recombinant oncoloytic viruses: the invention relates to recombinant oncolytic viruses; more specifically, the present invention relates to recombinant oncolytic viruses expressing a heterologous B-cell–attractant polypeptide or a T-cell–attractant polypeptide). M.W. declares honoraria from Janssen Research & Development, AstraZeneca, the National Cancer Institute, Medscape, and PeerView; consulting for or advisory role with AstraZeneca, Janssen Research & Development, Celgene, and MoreHealth; research funding from AstraZeneca, Janssen Research & Development, Pharmacyclics, Kite Pharma, Juno Therapeutics, BeiGene, Novartis, and Accerta Pharma; and travel, accommodation, and expense funds from Janssen Research & Development and AstraZeneca. J.B.C. declares consulting for, or advisory role with Pharmacyclics, Celgene, Millennium Pharmaceuticals, Seattle Genetics, Novartis, Infinity Pharmaceuticals, and AbbVie; and research funding from Bristol Myers Squibb, Janssen, Novartis, and Takeda Pharmaceuticals. B.T.H. declares honoraria from Pharmacyclics, Gilead Sciences, Genentech, AbbVie, Seattle Genetics, Bayer HealthCare Pharmaceuticals; consulting for, or advisory role with Seattle Genetics, Novartis, and AbbVie/Genentech; and research funding from AbbVie (to institution), Karyopharm Therapeutics (to institution), Celgene (to institution), Takeda Pharmaceuticals (to institution), and Amgen (to institution). F.J.H.-I. declares consulting for or advisory role with Celgene, Amgen, Seattle Genetics, Pharmacyclics, Takeda Pharmaceuticals, Novartis, and GlaxoSmithKline. S.K. declares stock and other ownership interests in Portola Pharmaceuticals. J.M.V. declares honoraria from Novartis, AbbVie, Epizyme, Roche, Legend Biotech, Karyopharm Therapeutics, Sandoz, Vaniam Group, Janssen Scientific Affairs, Kite Pharma/Gilead Sciences, Accerta Pharma/AstraZeneca, and Nordic Nanovector; consulting for or advisory role with Bio Connections; and research funding from Celgene (to institution), Genentech (to institution), Incyte (to institution), Accerta Pharma (to institution), Kite Pharma (to institution), Seattle Genetics (to institution), Novartis (to institution), Bristol Myers Squibb (to institution), Merck Sharp & Dohme (to institution), AstraZeneca. T.S.F. declares stock and other ownership interests in Merck; honoraria from Sanofi, AstraZeneca, Celgene, Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, and Genentech; consulting for or advisory role with Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, and Genentech; served on the speakers’ bureau for Sanofi, AstraZeneca, Seattle Genetics, and Celgene; and received travel, accommodation, and expenses funds from Sanofi, AstraZeneca, Celgene, Adaptive Biotechnologies, Janssen Oncology, Seattle Genetics, and Genentech. K.J.M. declares honoraria from Pharmacyclics, Bayer HealthCare Pharmaceuticals, Novartis, and Teva Pharmaceuticals Industries; and research funding from Pharmacyclics, Merck, Bristol Myers Squibb. V.B. declares consulting for or advisory role with Seattle Genetics, and Kite Pharma; research funding from Gamida Cell, Unum Therapeutics (to institution), Novartis (to institution); and received travel, accommodation, and expenses funds from Amgen. B.K. declares stock and other ownership interests in Amgen. J.C. declares consulting for, or advisory role with Kite Pharma/Gilead Sciences, Novartis, Genentech, and Bayer HealthCare Pharmaceuticals and is a member of the speaker’s bureau of Kite Pharma/Gilead Sciences, Novartis, Genentech, Janssen, and Merck. B.S. declares honoraria from Pharmacyclics/Janssen; consulting for or advisory role with Adaptive Biotechnologies; and research funding from Incyte, Jazz Pharmaceuticals (to institution). F.L. declares consulting for, or advisory role with Spectrum Pharmaceuticals, Celgene, and Seattle Genetics; and reports research funding from Spectrum Pharmaceuticals (to institution). N.W.-J. declares consulting for, or advisory role with Juno Therapeutics, ADC Therapeutics, Janssen Oncology, and Gilead Sciences; and reports research funding from Merck, Novartis/Pfizer, Genentech, and Astex Pharmaceuticals. A.M. declares stock and other ownership interests in Witty Health; consulting for, or advisory role with Spectrum Pharmaceuticals, Aileron Therapeutics, Bristol Myers Squibb, Seattle Genetics, Kite Pharma, and Carevive; is a member of the speaker’s bureau of Spectrum Pharmaceuticals, AstraZeneca, Kite Pharma, and Gilead Sciences; and reports research funding from Incyte (to institution), Roche/Genentech (to institution), Merck (to institution), Bristol-Myers Squibb (to institution), Juno Therapeutics (to institution), Gilead Sciences (to institution), Forty Seven (to institution), Takeda Pharmaceuticals (to institution), Astex Pharmaceuticals (to institution), Pharmacyclics/Janssen (to institution), Epizyme (to institution), Aileron Therapeutics (to institution), and Carevive (to institution). N.R. declares consulting for or advisory role with Celgene, AbbVie, Bristol Myers Squibb, Adaptive Biotechnologies; is a member of the speaker’s bureau of Gilead Sciences; and reports research funding from Bristol Myers Squibb (to institution). A.E.K. declares stock and other ownership interests in Lixte Biotechnology. D.J.L. declares consulting or advisory role with Celgene and Curis; and research funding from Takeda Pharmaceuticals (to institution), Triphase Accelerator (to institution), and Curis, Curis (to institution). M.G. declares employment with ExactSciences-; and research funding from Genentech. R.K. declares consulting for, or advisory role with Kite Pharma/Gilead Sciences, and Juno Therapeutics; is a member of the speaker’s bureau of AstraZeneca and Kite Pharma/Gilead Sciences; and received research funding from Bristol Myers Squibb (to institution) and Takeda Pharmaceuticals (to institution). J.B.K. declares consulting for, or advisory role with, Seattle Genetics; research funding from Janssen (to institution) and Seattle Genetics (to institution); and travel, accommodation, and expenses funds from Curis. P.F.C. declares consulting for, or advisory role with, Genentech/Roche and Kite Pharma; is a member of the speaker’s bureau of Spectrum Pharmaceuticals; and hold patents, receives royalties, and has other intellectual property with XaTEC as patent holder (I). A.E. declares honoraria from Seattle Genetics, Celgene, Spectrum Pharmaceuticals, Pharmacyclics, Affimed Therapeutics, Merck, Accerta Pharma, AbbVie, Janssen Biotech, Novartis, Bayer HealthCare Pharmaceuticals, Verastem, Kite Pharma/Gilead Sciences, and Research to Practice; consulting for, or advisory role with Celgene, Spectrum Pharmaceuticals, Seattle Genetics, Affimed Therapeutics, Merck, Kite Pharma, Janssen Oncology, Bayer HealthCare Pharmaceuticals, and AbbVie/Genentech; received research funding from Tesaro, Seattle Genetics, and Merck; and received travel, accommodation, and expenses funds from Seattle Genetics, Research to Practice, Bayer HealthCare Pharmaceuticals, Affimed Therapeutics, Pharmacyclics, Janssen Biotech, Novartis, Merck, Verastem, AbbVie/Genentech, Spectrum Pharmaceuticals, and Celgene. A.K. declares honoraria from Takeda Pharmaceuticals consulting for, or advisory role with Shire; and received travel, accommodation, and expenses funds from Takeda Pharmaceuticals. J.E.A. declares honoraria from Janssen; and research funding from Appia Pharmaceuticals. C.D. declares stock and other ownership interests in Gilead Sciences; consulting for or advisory role with Seattle Genetics, Bayer HealthCare Pharmaceuticals, Bristol Myers Squibb, Genentech/Roche, and Merck; and research funding from Seattle Genetics (to institution), Genentech (to institution), Incyte (to institution), LAM Therapeutics (to institution), Merck (to institution), Bristol-Myers Squibb (to institution), Millennium Pharmaceuticals (to institution), MEI Pharma (to institution). R.I.F. declares consulting for, or advisory role with Pharmacyclics/Janssen, Roche, Kite Pharma, Seattle Genetics, Sandoz, Celgene, Genentech, Bayer HealthCare Pharmaceuticals, AstraZeneca, Adaptive Biotechnologies, and Ion Solutions; and provided expert testimony for Roche. The remaining authors declare no competing financial interests.
Correspondence: Stefan K. Barta, Hematology and Oncology, University of Pennsylvania Abramson Cancer Center, 3400 Civic Center Blvd, M South 12-177, Philadelphia, PA 19104; email: stefan.barta@pennmedicine.upenn.edu.
References
Author notes
Data are available on request from the corresponding author, James N. Gerson (james.gerson@gmail.com).
The full-text version of this article contains a data supplement.