Key Points
Dynamin-2 haplodeficient female mice develop neutropenia with aging.
Neutropenia is because of defective production from dysmyelopoiesis and migration defects.
Abstract
The dynamins are a family of ubiquitously expressed GTPase proteins, best known for their role in membrane remodeling. Their contribution to hematopoiesis is incompletely recognized. Individuals with Charcot-Marie-Tooth disease with dynamin-2 (DNM2) mutations often develop neutropenia. We previously reported that dynamin (DNM) inhibition impairs SDF1a-mediated migration in megakaryocytes. Here, we report on conditionally Dnm2 deleted mice in hematopoietic tissues using the Vav-Cre murine strain. Homozygous Dnm2 deletion in blood tissues is embryonic lethal. Dnm2het male mice only developed a slightly decreased hemoglobin level. Dnm2het female mice developed leukopenia by 40 weeks of age and neutropenia by 65 weeks of age. Flow cytometry revealed decreased lineage-negative cells and granulocyte-monocyte progenitors in Dnm2het female mice. Immunohistochemical staining of bone marrow (BM) for mature neutrophils with Ly6G was decreased and myelodysplastic features were present in the BM of Dnm2het female mice. A linear distribution of Ly6G+ BM cells along blood vessels was observed in fewer Dnm2het mice than in controls, suggesting that the migration pattern in the marrow is altered. Marrow neutrophils treated with dynamin inhibitor, dynasore, showed increased cell surface CXCR4, suggesting that abnormal migration results in marrow neutrophil retention. Dnm2het female mice also developed splenomegaly secondary to germinal center hyperplasia at younger ages, suggesting perturbed immunity. In summary, female mice with BM Dnm2 haploinsufficiency developed neutropenia as they aged with decreased granulocyte progenitor production and migration defects. Our studies indicate a potential mechanism for the development of chronic idiopathic neutropenia, a disease that predominantly presents in middle-aged women.
Introduction
Dynamins (DNM) are membrane-bound large GTPases best known for enabling the intracellular release of endocytic vesicles. Three different forms of DNM have been identified; those can be specifically expressed in different organs and systems. However, dynamin-2 (DNM2) is the only 1 with ubiquitous expression.1,2 The physiological relevance of DNM2 has emerged, because genetic mutations were identified in patients with Charcot-Marie-Tooth (CMT) disease, a congenital syndrome that is characterized by peripheral neuropathy and muscle atrophy. Specifically within the blood lineage, individuals with CMT who carry mutations in DNM2 are characterized by the development of cytopenia, particularly neutropenia.3-6 Patients with CMT disease may suffer from impaired wound healing,7,8 a process partially dependent on intact neutrophil migration. More recently, patients with clonal hematopoiesis have presented with hotspot protein–altering mutations in DNM2.9 This overall supports a critical role of DNM2 in hematopoietic lineage survival and development, however, its exact role within various blood lineages is incompletely understood.10,11
Circulation neutrophil numbers depend on neutrophil egress from the bone marrow (BM). The dynamic regulation of endocytic signal termination and the recycling of chemotaxis receptors will direct neutrophil BM trafficking. Consequently, the decreased expression of CXCR4 within specific granulocyte populations is known to permit migration from the BM compartment into the blood circulation, and those with increased amounts of CXCR4 will remain in the BM. Interestingly, as neutrophils age, the expression of CXCR4 increases.12 We have previously reported that loss-of-function of DNM2 leads to an increase in surface expression of CXCR4 in megakaryopoiesis, confirming that DNM2 plays a role in endocytosis and CXCR4 recycling.10 We therefore hypothesize a potential role for DNM2 in regulating the migration of granulocytes from the BM compartment.10,13
Circulating neutrophil numbers also vary with age. We know that during the normal lifespan of individuals, the risk of development of myelodysplastic syndrome (MDS) increases over the years. An impaired native immune system and unregulated inflammation are evoked along with driver genetic mutations in MDS, and the exact nature of the relationship between MDS and inflammation/immunity has been scrutinized.14 Interestingly, mutations in proteins directly involved in intracellular trafficking such as SAMD9/SAMD9L have recently been described for MDS.15,16 In addition, a recent mouse transcriptome analysis found an enrichment with membrane-associated products in aging clonal hematopoiesis.17 Because of the emerging function of DNM2 in vesicular trafficking during hematopoietic maturation, the specific role of DNM2 in aberrant hematopoiesis and the development of MDS remains to be explored and could involve autophagy among other mechanisms. In this study, we assessed the specific role of DNM2 during hematopoietic maturation in mice with lineage-specific knock–out of Dnm2 in hematopoietic elements.
Materials and methods
Animals
Genetically engineered mouse strains Dnm2flox (B6.129S1 (Cg)-Dnm2tm1.1Pdc/J stock number: 013542) and Vav1-Cre (B6.Cg-Commd10Tg(Vav1-icre) A2Kio/J, stock number: 008610) were obtained either from the Jackson Laboratory (Bar Harbor, ME) or from the Crispino Lab for some of the Vav1-Cre mice. Littermates with the genotype Dnm2flox/−/Vav-Cre−/− or Dnm2flox/flox/Vav-cre−/− were used as control (CTRL) mice. The ethical use of the mice was approved by both the Northwestern University and the University of Illinois Animal Care and Use Committees. All euthanasia methods met the specifications of the American Veterinary Medical Association Panel on Euthanasia.
Cell cultures
Cultures were kept in humidified incubators at 37 °C with 5% CO2. Cells were cultured in Iscove modified Dulbecco medium (Gibco; Thermo Fisher Scientific, Waltham, MA) with 10% fetal bovine serum (Hyclone; GE Healthcare, Chicago, IL), supplemented with penicillin/streptomycin and L-glutamine (Gibco).
Antibodies
The following primary antibodies were used for western blot and immunofluorescence imaging: DNM2 Ab, Santa Cruz (#sc-6400, #C-18; Dallas, TX); and CXCR4 Ab, Santa Cruz (#sc-9046, H-118). The following secondary antibodies were used: horseradish peroxidase–conjugated secondary antibodies were either from Rockland (Limerick, PA) or from Santa Cruz; Alexa Fluor secondary antibodies were from Thermo Fisher Scientific. The following antibodies were used for immunohistochemistry: Ly6G Ab, Cell Signaling (#87048; Danvers, MA), BCL-6 Ab, Abcam (#ab243150; Waltham, MA), and LC3B Ab, Cell Signaling (#83506). The following antibodies were used for flow cytometry experiments: CXCR4-FITC, BD Biosciences (#561735; Franklin Lake, NJ), and Ly6G-APC, BD Biosciences (#560599), c-kit APC, BD Pharmingen (Franklin Lake, NJ, #553356), IL7R BV421 (#135023; Biolegend), CD34-PE (#551387; BD Pharmingen), CD16/32-PE-Cy7 (#25-0161-82; ThermoFisher), Sca-1-FITC (#122505; Biolegend). In Cyto-ID autophagy experiments, Sca-1 APC (#108112; Biolegend) was used.
Immunologic analysis
Western blotting and flow cytometry were performed as described elsewhere.10 4’,6-diamidino-2-phenylindole was purchased from Invitrogen (#D1306). Data were acquired on an LSR/Fortessa 6-Laser Analyzer (BD, Franklin Lake, NJ), a Cytomics FC 500 (Beckman Coulter, Brea, CA), or a CytoFLEX S (Beckman Coulter) and analyzed with FlowJo (Ashland, OR).
PCR
Reactions were performed on a BioRad iCycler as previously prescribed.10 Real-time polymerase chain reaction (PCR) was performed on a 7500 Real-Time PCR System (Applied Biosystems, Waltham, MA). The following primers used were used for mouse genotyping: Dnm2 (forward: 5’ CCC TGC TAG TGA CCT TTC TTG A 3’, reverse: 5’ GCA GGA AGA CAC ACA ACT GAA C 3’), Vav1-Cre (forward: 5’ AGA TGC CAG GAC ATC AGG AAC CTG 3’. reverse: 5’ ATC AGC CAC ACC AGA CAC AGA GAT C 3’). For Dnm2 quantitative PCR, the forward primer was CAC AGA GCA GAG GAA TGT CTA C, and the reverse primer was CTT GTG CTC CAT CCT CAT TCT. For Actin quantitative PCR, the primers were: forward, 5’ ACC CCA GCT GAG AGG GTA AT 3’); and reverse, 5’ AGG AAG AGG ATG TGG CAG TG 3’.
Migration assays
Chemotaxis assays were performed as previously described.10 BM mouse neutrophils were isolated with Percoll gradient as described.18-21 Their migration was studied in Transwell migration assays using 3 μm pore inserts (#CLS3398; Sigma, Saint-Louis, MO) with the chemoattractant, n-formylmethionyl-leucyl-phenylalanine, purchased from Millipore Sigma (#F3506; Burlington, MA). 600 ul RPMI with 10 uM n-formylmethionyl-leucyl-phenylalanine was loaded into the lower compartment. SDF1a gradient (#250-20A; PeproTech, Cranburg, NJ), 100 ng/mL in the lower compartment) for 3 hours at room temperature.10
Stress-associated neutrophilia induction
Mice received an intraperitoneal injection with a 500 microliters sterile mixture of aluminum and magnesium hydroxide (or alum) mixed with ovalbumin mix (mixed 20-1 with ovalbumin) as previously described22 to induce stress-associated neutrophilia, or sterile saline for CTRL mice. Complete blood counts (CBCs) were obtained from the tail vein before injection of this alum mix and on the following day after injection.
Time course of CXCR4 surface expression in the presence of SDF1a
CBCs and histologic staining
Blood was collected in a Microvette (#20.1278.100; Sarstedt, Newton, NC) with EDTA. Blood was immediately run on a HemaVet (Drew Scientific, Miami Lakes, FL) or a Heska Element HT5 (#5200; Loveland, CO) for total blood counts. The femurs and sterna were decalcified, fixed, sectioned, and stained with hematoxylin and eosin by standard methods. BM sections were stained with hematoxylin and eosin. Images were captured with an Aperio Image Analysis (Sausalito, CA) camera or Olympus BH2 microscope with an Olympus (Central Valley, PA) C35-AD-2 microscopic camera. Immunohistochemistry staining quantification was performed at the core pathology facility at the University of Illinois Chicago, using Halo software from Indica Labs (Albuquerque, NM).
BM cell collection for flow cytometry
BM cell suspensions were obtained from femurs flushed with RPMI or Iscove modified Dulbecco medium and 2% fetal bovine serum then filtered through a 70-μm nylon strainer to remove bone debris. A sample was set aside for erythroid lineage flow cytometry analysis. Red blood cells were removed with ACK lysis buffer in the reminder BM cell solution. A sample was set aside for RNA collection and western blotting experiments. At the same time, another sample underwent lineage depletion using Miltenyi Direct Lineage Depletion Kit (#130-110-470; Gaithersburg, MD) and Miltenyi columns for flow cytometry analysis of hematopoietic progenitors. Lineage negative, Sca-1 positive, and c-Kit positive (LSK)cells were defined as lineage negative, R-IL7 negative, Sca-1 positive, and c-Kit positive cells. Granulocyte-monocyte progenitor (GMP) cells were defined as lineage negative, R-IL7 negative, c-Kit positive, Sca-1 negative, CD34 positive, and CD16/32 high.
Flow cytometry quantification of autophagy
Autophagy was detected in lineage-negative BM cells, using Cyto-ID Autophagy Kit (#ENZ-51031-0050; Enzo, Farmingdale, NY). Because Cyto-ID uses the fluorescein isothiocyanate filter, Sca-1 was detected by allophycocyanin staining, whereas c-Kit cells were positively selected with c-Kit selection beads (#130-091-224; Miltenyi).
Statistical analysis
Statistical analysis was performed with the GraphPad software (La Jolla, CA), using a t-test to compare the mean ± standard error of the mean, or Fisher exact test, with a P-value < .05 considered as significant.
Results
Dnm2 null mice in the hematopoietic system are incompatible with life
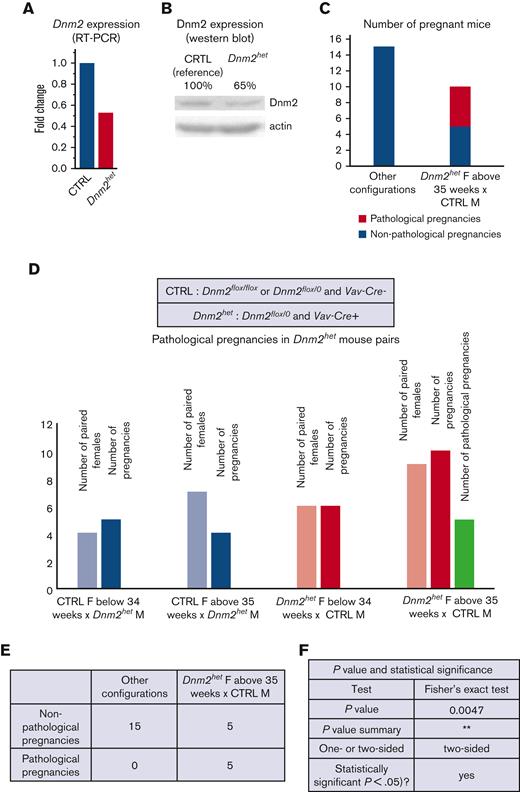
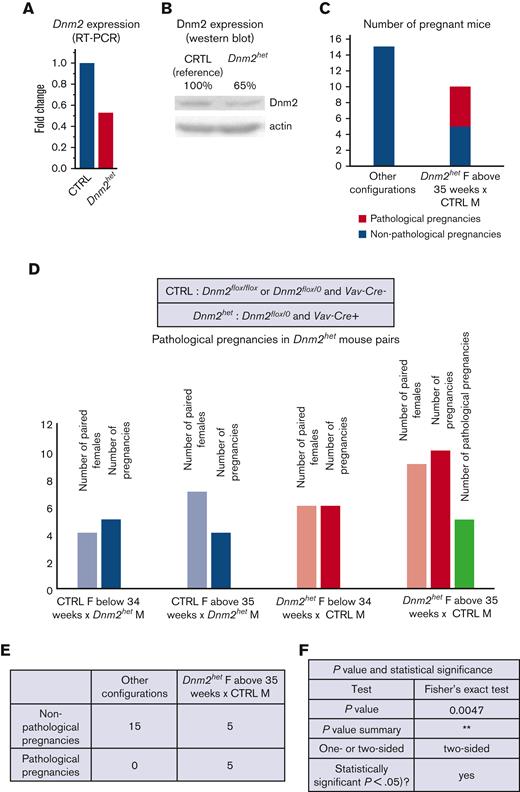
The role of Dnm2 in hematopoietic lineage differentiation and migration was tested by breeding Dnm2flox mice with Vav-Cre mice. From a total of 70 pups born, approximately one-third of these were born with the Vav-Cre+/Dnm2flox+/− (Dnm2het). Interestingly, no viable pups with the Vav-Cre+/Dnm2flox+/+ genotype were obtained, indicating that a complete knockout of Dnm2 in the entire hematopoietic system is lethal in utero. Further analysis validated partial knock-down of Dnm2, as Dnm2het mice had expression of DNM2 mRNA and protein levels reduced on average by half compared with CTRL mice (Figure 1A-B; supplemental Figure 1). We further noticed while breeding mouse colonies that some Dnm2het females (positive for Vav-Cre and Dnm-2flox) became distressed when pregnant at middle age (between 35 weeks and 45 weeks of age). We named this distressed state as pathologic pregnancies, for the purpose of designating this group in the present study (Figure 1C-F).
DNM2 expression in the offspring of Vav-Cre x Dnm2flox (targeting the hematopoietic system) mice.Dnm2 expression, normalized to actin, in Dnm2het mice and in CTRL mice by RT-PCR (A) and western blot (B) in BM cells. Different breeding combinations based on genotypes were tested to determine aspects of Dnm2het phenotype. The number of pregnancies was documented. Among breeding mouse colonies, some Dnm2het females (positive for Vav-Cre and Dnm-2flox) became distressed when pregnant, at middle age (between 35 weeks and 45 weeks of age). We named this distressed state as pathologic pregnancies for the purpose of designating this group in this study. This phenotype was not observed in pregnant mice younger than 34 weeks of age or in any of the mice that harbored other sets of genotypical combinations. The comparison of pathologic pregnancies in different mouse group is indicated and illustrated (C,D,E,F).
DNM2 expression in the offspring of Vav-Cre x Dnm2flox (targeting the hematopoietic system) mice.Dnm2 expression, normalized to actin, in Dnm2het mice and in CTRL mice by RT-PCR (A) and western blot (B) in BM cells. Different breeding combinations based on genotypes were tested to determine aspects of Dnm2het phenotype. The number of pregnancies was documented. Among breeding mouse colonies, some Dnm2het females (positive for Vav-Cre and Dnm-2flox) became distressed when pregnant, at middle age (between 35 weeks and 45 weeks of age). We named this distressed state as pathologic pregnancies for the purpose of designating this group in this study. This phenotype was not observed in pregnant mice younger than 34 weeks of age or in any of the mice that harbored other sets of genotypical combinations. The comparison of pathologic pregnancies in different mouse group is indicated and illustrated (C,D,E,F).
Female Dnm2het mice develop neutropenia
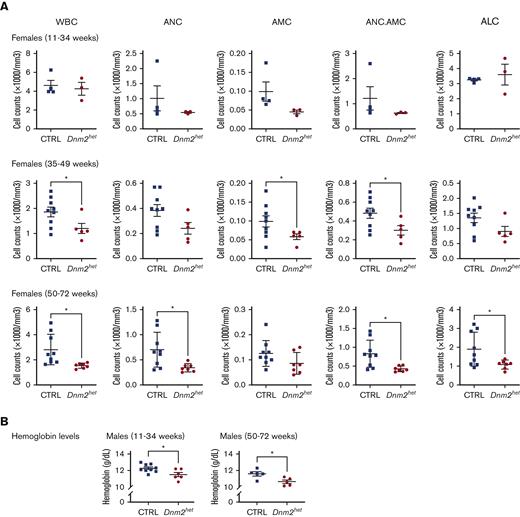
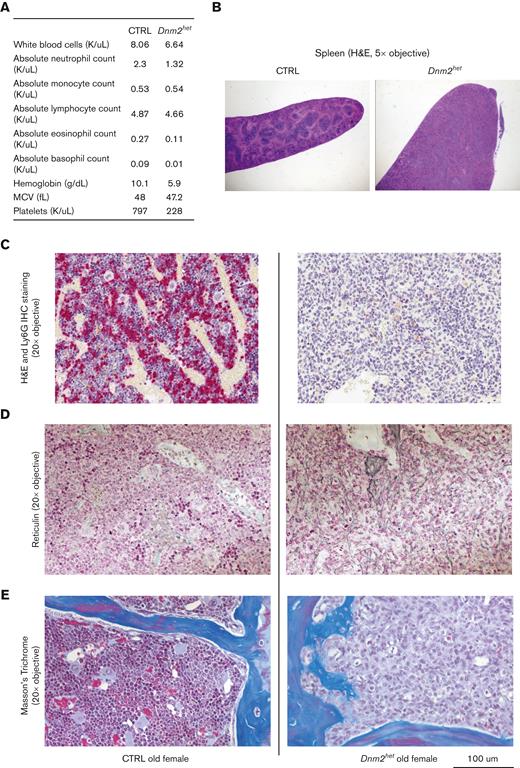
To evaluate the differentiation and production of hematopoietic elements, CBCs were measured in CTRL and Dnm2het mice at different ages (Figure 2; supplemental Figure 2). The analysis showed the development of absolute leukopenia in Dnm2het mice from 40 weeks of age (average granulocyte-monocyte count: CTRL 532/mm3 vs Dnm2het 483/mm3; P = .028). Neutropenia was persistent when assessed at 65 weeks of age (average neutrophil counts, CTRL 700/mm3 vs Dnm2het 343/mm3; P = .016). This was not observed in male mice, although a trend toward lower counts became apparent in the group age above 50 weeks old. (supplemental Figure 2). Mild abnormalities in the red blood cell lineage (slight decrease in hemoglobin concentration) were observed in Dnm2het males but not in Dnm2het females. Dnm2het mice showed a trend for higher platelet counts than CTRLs, but this was statistically nonsignificant.
Complete blood count (CBC) abnormalities in Dnm2het when compared with CTRL mice. (A) Comparison of WBC counts in female Dnm2het and in CTRL mice aged 11 weeks and above (11-34 weeks: N = 4, 35-49 weeks: N = 5 and above, 50-72 weeks: N = 7 and above). Older female Dnm2het mice have significantly decreased WBC compared with CTRL mice, affecting mainly neutrophils, starting at middle-age (∼40 weeks; age range, 11-34 weeks). This persists at older ages (age range, 50-72 weeks). (B) Comparison of hemoglobin levels in male Dnm2het and CTRL mice aged 11 weeks and above (11-34 weeks: N = 6 and above, 50-72 weeks: N = 5 and above). Mildly decreased hemoglobin was observed in male Dnm2het only. Error bars indicate SEM. Only marked, group-wise comparisons are significant. ∗P < .05, t-test. ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; ANC.AMC, combined absolute neutrophil and monocyte count; SEM, standard error of the mean; WBC, white blood cells.
Complete blood count (CBC) abnormalities in Dnm2het when compared with CTRL mice. (A) Comparison of WBC counts in female Dnm2het and in CTRL mice aged 11 weeks and above (11-34 weeks: N = 4, 35-49 weeks: N = 5 and above, 50-72 weeks: N = 7 and above). Older female Dnm2het mice have significantly decreased WBC compared with CTRL mice, affecting mainly neutrophils, starting at middle-age (∼40 weeks; age range, 11-34 weeks). This persists at older ages (age range, 50-72 weeks). (B) Comparison of hemoglobin levels in male Dnm2het and CTRL mice aged 11 weeks and above (11-34 weeks: N = 6 and above, 50-72 weeks: N = 5 and above). Mildly decreased hemoglobin was observed in male Dnm2het only. Error bars indicate SEM. Only marked, group-wise comparisons are significant. ∗P < .05, t-test. ALC, absolute lymphocyte count; AMC, absolute monocyte count; ANC, absolute neutrophil count; ANC.AMC, combined absolute neutrophil and monocyte count; SEM, standard error of the mean; WBC, white blood cells.
Female Dnm2het mice develop splenomegaly secondary to germinal center hyperplasia
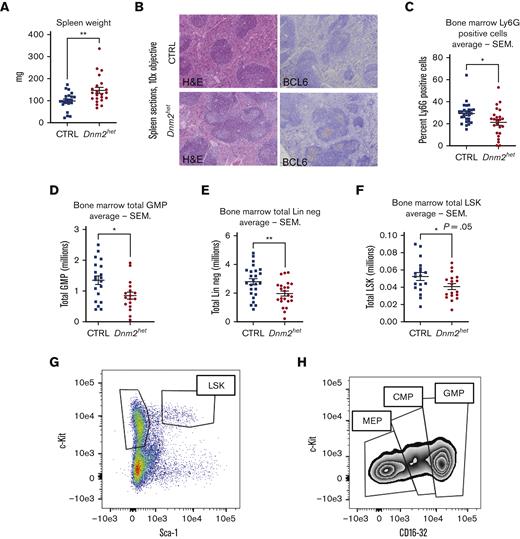
The hematopoietic differentiation downstream of Dnm2 was further evaluated by macroscopic and microscopic assessments of the spleens in Dnm2het mice (Figure 3A-B). On comparison of groups involving all ages, the analysis showed that Dnm2het female mice developed splenomegaly (average spleen weight: Dnm2het 146 mg vs CTRL 99 mg; P = .006). This started during young adulthood, as the histological analysis from the spleen sections of mice aged 12 to 25 weeks revealed that Dnm2het mice featured an expansion of the white pulp compartment. Further characterization of the white pulp expansion showed that this was secondary to an increased number of reactive, secondary follicles that were composed of BCL-6 positive germinal center lymphocytes and a mixture of mature CD3 positive T and CD20 positive B-cell lymphocytes. To rule out any infiltration by precursor myeloid precursors, CD34 immunostaining was also performed, and it was negative.
Spleen histology, BM neutrophil content and hematopoietic progenitors in female Dnm2het mice. (A) Comparison of spleen weights in female Dnm2het and CTRL mice. Overall, when comparing groups involving all ages (N = 18 and above), female Dnm2het mice developed splenomegaly. (B) Spleen histology in 12- to 25-week-old female Dnm2het and CTRL mice, showing florid reactive germinal center hyperplasia with the expansion of BCL-6 positive lymphocytes within secondary reactive follicles. CTRL mice: sections demonstrate a normal proportion and white and red pulp distribution. BCL-6 immunostain shows the absence of secondary reactive follicles. Dnm2het mice: the white pulp was mildly expanded by reactive, secondary follicles. BCL-6 highlights germinal center lymphocytes. Objective: 10×. (C) Average percentage of Ly6G positive BM cells in female Dnm2het and CTRL mice when comparing groups involving all ages (N = 24 and above). Scoring by immunohistochemistry demonstrated decreased BM Ly6G positive neutrophil. Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise. Quantification was performed by Halo Image analysis. (D) Average total GMP (lineage negative, R-IL7 negative, c-Kit positive, Sca-1 negative, CD34 positive, CD16/32 high) in the BM of female Dnm2het and CTRL mice when comparing groups involving all ages (N = 17 and above). (E) Average total BM lineage negative (Lin neg) cells in the BM of female Dnm2het and CTRL mice (N = 23 and above). (F) Average total BM lineage negative, R-IL7 negative, Sca-1 positive, c-Kit positive (LSK) cells in the BM of female Dnm2het and CTRL mice when comparing groups involving all ages (N = 18 and above). Dnm2het female mice displayed a decrease in BM Lin neg cells counted after immunomagnetic lineage depletion, decreased LSK and GMP measured by flow cytometry when comparing groups involving all ages. (G) Representative plot of LSK BM cells flow cytometry analysis. (H) Representative plot of megakaryocyte-erythrocyte progenitor (MEP), common myeloid progenitor (CMP), and GMP flow cytometry analysis. Data are shown as mean +/− SEM. ∗P < .05, ∗∗P < .01, t-test.
Spleen histology, BM neutrophil content and hematopoietic progenitors in female Dnm2het mice. (A) Comparison of spleen weights in female Dnm2het and CTRL mice. Overall, when comparing groups involving all ages (N = 18 and above), female Dnm2het mice developed splenomegaly. (B) Spleen histology in 12- to 25-week-old female Dnm2het and CTRL mice, showing florid reactive germinal center hyperplasia with the expansion of BCL-6 positive lymphocytes within secondary reactive follicles. CTRL mice: sections demonstrate a normal proportion and white and red pulp distribution. BCL-6 immunostain shows the absence of secondary reactive follicles. Dnm2het mice: the white pulp was mildly expanded by reactive, secondary follicles. BCL-6 highlights germinal center lymphocytes. Objective: 10×. (C) Average percentage of Ly6G positive BM cells in female Dnm2het and CTRL mice when comparing groups involving all ages (N = 24 and above). Scoring by immunohistochemistry demonstrated decreased BM Ly6G positive neutrophil. Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise. Quantification was performed by Halo Image analysis. (D) Average total GMP (lineage negative, R-IL7 negative, c-Kit positive, Sca-1 negative, CD34 positive, CD16/32 high) in the BM of female Dnm2het and CTRL mice when comparing groups involving all ages (N = 17 and above). (E) Average total BM lineage negative (Lin neg) cells in the BM of female Dnm2het and CTRL mice (N = 23 and above). (F) Average total BM lineage negative, R-IL7 negative, Sca-1 positive, c-Kit positive (LSK) cells in the BM of female Dnm2het and CTRL mice when comparing groups involving all ages (N = 18 and above). Dnm2het female mice displayed a decrease in BM Lin neg cells counted after immunomagnetic lineage depletion, decreased LSK and GMP measured by flow cytometry when comparing groups involving all ages. (G) Representative plot of LSK BM cells flow cytometry analysis. (H) Representative plot of megakaryocyte-erythrocyte progenitor (MEP), common myeloid progenitor (CMP), and GMP flow cytometry analysis. Data are shown as mean +/− SEM. ∗P < .05, ∗∗P < .01, t-test.
Loss of Dnm2 is associated with decreased granulocyte maturation
The role of Dnm2 during hematopoietic differentiation was further evaluated with histological analysis from BM sections. The findings showed that the myeloid to erythroid ratio was not significantly different between the Dnm2het and CTRL animals (data not shown). Analysis of granulocyte maturation was performed with Ly6G immunostaining (Figure 3C), and the analysis involving all ages showed that the proportion of mature granulocytes was higher in CTRL mice (46% n = 31) than in Dnm2het mice (34%, n = 23, P < .01). Further analysis of hematopoietic lineage maturation by flow cytometry (Figure 3D,E,H) indicated that lineage-negative cells and GMPs were decreased in Dnm2het mice (average BM lineage-negative cells: CTRL 2.8×106 vs Dnm2het 1.97 × 106; P = .0056; average granulocyte-monocyte progenitors: CTRL 1.35×106 vs Dnm2het 0.85 × 106; P = .01), on comparison of groups involving all ages. Moreover, in groups involving all ages, absolute LSK cell numbers were decreased with a borderline statistical significance: CTRL 0.05237 × 106 vs Dnm2het 0.04072 × 106; P = .05 (Figures 3F-G).
Loss of Dnm2 leads to the development of morphologic myelodysplastic changes
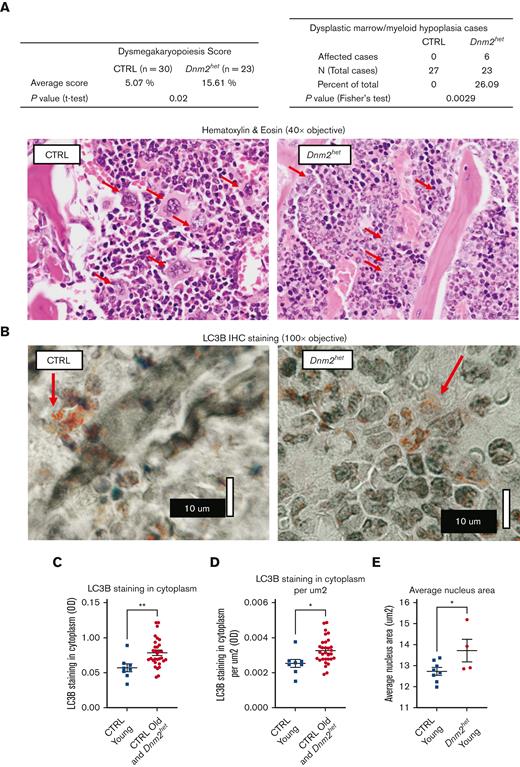
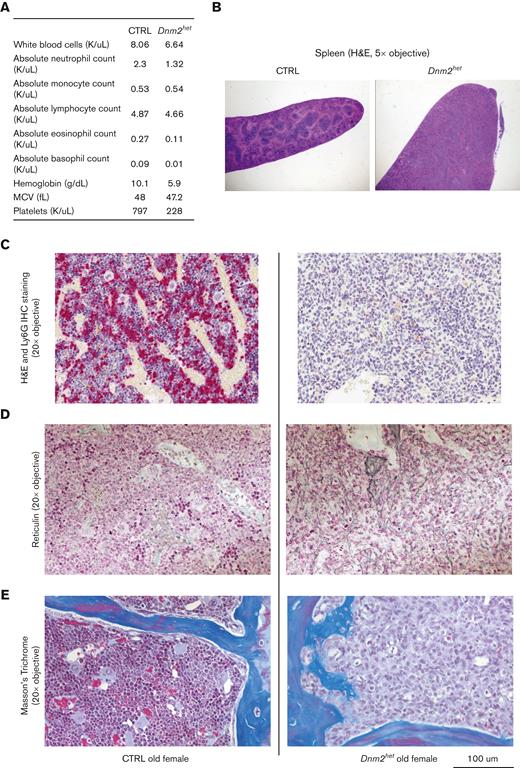
Two older Dnm2het mice (above 67 weeks) developed signs of distress (reduced activity, poor grooming, ruffled furs, and hunched posture) warranting euthanasia. These mice were nonpaired with a male and nonpregnant at the time of visible sickness but had delivered litters at younger ages. One of these mice (Figure 4) showed a marked cytopenia (Figure 4A), splenomegaly (Figure 4B) and marked decrease in Ly6G+ neutrophils, which constituted only 0.3% of the total BM cells. The BM was replaced by immature myeloid progenitors, consistent with the development of acute leukemia (Figure 4C,F). Reticulin staining and Masson’s trichrome staining showed evidence for early fibrotic changes in this mouse (Figure 4D-E). The second mouse also showed a similar marrow anomaly at an earlier stage (see Figure 6E). The development of dysplastic maturation was examined in the BM compartment of other mice. After 50 weeks of age, 26% of Dnm2het mice (out of N = 23), but no CTRL mice, displayed marrow dysplasia (Figure 5A).
Hematological findings in an old distressed Dnm2het mouse. CBC findings in a distressed old female Dnm2het mouse. One old distressed female Dnm2het mouse had severe cytopenia (A) and a marked splenomegaly (B). Its BM was entirely effaced by immature myeloid precursors (C). Special stains were performed. Ly6G staining (C) was dramatically reduced. Reticulin staining (D) and Masson’s trichome staining (E) showed early fibrotic changes in the BM of this distress old Dnm2het mice. Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise.
Hematological findings in an old distressed Dnm2het mouse. CBC findings in a distressed old female Dnm2het mouse. One old distressed female Dnm2het mouse had severe cytopenia (A) and a marked splenomegaly (B). Its BM was entirely effaced by immature myeloid precursors (C). Special stains were performed. Ly6G staining (C) was dramatically reduced. Reticulin staining (D) and Masson’s trichome staining (E) showed early fibrotic changes in the BM of this distress old Dnm2het mice. Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise.
BM dysplastic feature analysis and autophagy marker LC3B staining pattern in older female Dnm2het mice. (A) BM dysplastic morphology analysis in female and Dnm2het CTRL mice. After 50 weeks of age, 26% of Dnm2het mice (out of N = 23), but no CTRL mice, displayed marrow dysplasia. On hematoxylin & eosin stain, with red arrows indicating megakaryocytes, Dnm2het mice show frequent dysmorphic megakaryocytes with small and hyposegmented nuclei with a scant amount of cytoplasm, whereas CTRL mice showed normal segmented megakaryocytes with variable amounts of cytoplasm. Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise. (B) Autophagy marker LC3B pattern in older female Dnm2het mice (>34 weeks of age). For imaging of LC3B cluster dots, we used an Olympus BH2 microscope with a 100X objective, NA 1.3, and Olympus C-35AD-2 camera. Additional nonlinear adjustments such as changes to gamma settings using the Photoshop software were applied to improve discernibility on the digital images. Autophagy marker LC3B stained dotted clusters were more conspicuous and more readily observed in the cytoplasm in the BM of young CTRL (<34 weeks of age) than in the BM in the other groups (old CTRL above 34 weeks combined with Dnm2het all ages). (C,D) Halo Image analysis of the average cytoplasm LC3B staining in different mouse groups. The average cytoplasm LC3B staining was lower in the young CTRL (<34 weeks) than in other groups taken together (old CTRL above 34 weeks plus Dnm2het all ages), N = 8 and above. (E) Halo Image analysis of the average nuclear size in young Dnm2het females (<34 weeks) than in young CTRL females (<34 weeks). The average nuclear size was greater in young Dnm2het females than in young CTRL females, N = 4 and above. Data are shown as mean +/− SEM. ∗P < .05, ∗∗P < .01, t-test.
BM dysplastic feature analysis and autophagy marker LC3B staining pattern in older female Dnm2het mice. (A) BM dysplastic morphology analysis in female and Dnm2het CTRL mice. After 50 weeks of age, 26% of Dnm2het mice (out of N = 23), but no CTRL mice, displayed marrow dysplasia. On hematoxylin & eosin stain, with red arrows indicating megakaryocytes, Dnm2het mice show frequent dysmorphic megakaryocytes with small and hyposegmented nuclei with a scant amount of cytoplasm, whereas CTRL mice showed normal segmented megakaryocytes with variable amounts of cytoplasm. Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise. (B) Autophagy marker LC3B pattern in older female Dnm2het mice (>34 weeks of age). For imaging of LC3B cluster dots, we used an Olympus BH2 microscope with a 100X objective, NA 1.3, and Olympus C-35AD-2 camera. Additional nonlinear adjustments such as changes to gamma settings using the Photoshop software were applied to improve discernibility on the digital images. Autophagy marker LC3B stained dotted clusters were more conspicuous and more readily observed in the cytoplasm in the BM of young CTRL (<34 weeks of age) than in the BM in the other groups (old CTRL above 34 weeks combined with Dnm2het all ages). (C,D) Halo Image analysis of the average cytoplasm LC3B staining in different mouse groups. The average cytoplasm LC3B staining was lower in the young CTRL (<34 weeks) than in other groups taken together (old CTRL above 34 weeks plus Dnm2het all ages), N = 8 and above. (E) Halo Image analysis of the average nuclear size in young Dnm2het females (<34 weeks) than in young CTRL females (<34 weeks). The average nuclear size was greater in young Dnm2het females than in young CTRL females, N = 4 and above. Data are shown as mean +/− SEM. ∗P < .05, ∗∗P < .01, t-test.
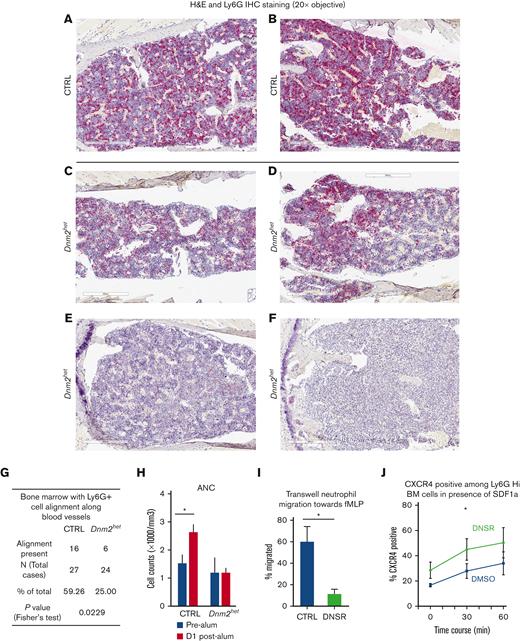
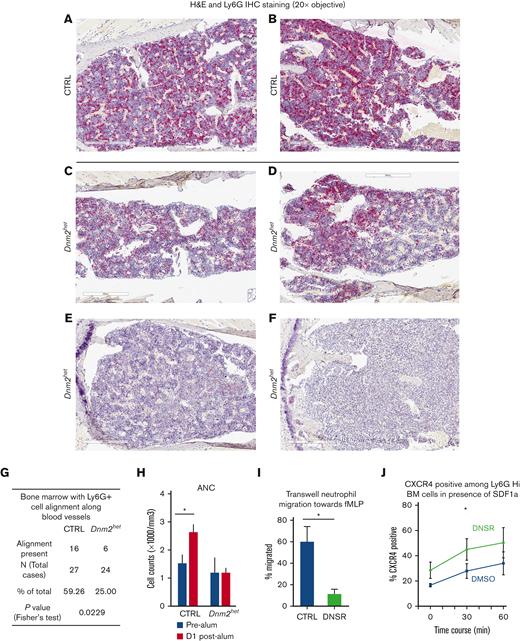
Granulocyte BM distribution, migratory response and CXCR4 expression in Dnm2-defective mouse granulocytes. (A-F) Immunohistochemical staining for mature granulocytes with Ly6G in the BM of representative CTRL (panels A-B) and Dnm2het female mice (panels C-F). Fewer Ly6G positive cells (granulocytes) were present in the BM of Dnm2het mice when comparing groups involving all ages (N = 30 and above). Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise. (G) A linear pattern of distribution of Ly6G positive cells (granulocytes) was observed in fewer mice among the Dnm2het group than in the CTRL group, indicating that the migration pattern within the BM is altered in the Dnm2het group (N = 30 and above). (Panel F) The BM from 1 Dnm2het mouse was completely effaced by immature myeloid precursors. (H) ANC in the peripheral blood 1 day after intraperitoneal alum injection in old female Dnm2het and CTRL mice. Older Dnm2het female mice (>52 weeks, N = 3) failed to show a rise of ANC, indicating a loss of rapid egress of neutrophils from the BM reserve. (I) Transwell migration assay of BM neutrophils from wild-type young female adults (aged 12-24 weeks, N = 3) treated with the DNM inhibitor DNSR, compared with CTRL neutrophils. Wild-type mouse neutrophils separated from the BM and treated with DNM-GTPase inhibitor, DNSR have reduced migration toward n-formylmethionyl-leucyl-phenylalanine, a chemoattractant for neutrophils, indicating that DNM function is necessary for this chemotactic response. (J) CXCR4 surface expression analyzed by flow cytometry in DNSR-treated and in CTRL BM Ly6G-high granulocytes from wild-type young female adults (aged 12-24 weeks, N = 4), when exposed for varying time periods to chemoattractant SDF1a (100 ng/mL). After 30 minutes of SDF1a exposure, surface CXCR4 was expressed to a greater extent among DNSR-treated neutrophils than in CTRL neutrophils. (Panels H-J) Each graph summarizes at minimum 3 to 4 independent experiments. Error bars indicate SEM. Only marked, group-wise comparisons are significant. ∗P < .05, t-test.
Granulocyte BM distribution, migratory response and CXCR4 expression in Dnm2-defective mouse granulocytes. (A-F) Immunohistochemical staining for mature granulocytes with Ly6G in the BM of representative CTRL (panels A-B) and Dnm2het female mice (panels C-F). Fewer Ly6G positive cells (granulocytes) were present in the BM of Dnm2het mice when comparing groups involving all ages (N = 30 and above). Histology slide images were captured using the Aperio AT2 slide scanner at 20X or the CRI Nuance Spectral Imaging microscope, unless specified otherwise. (G) A linear pattern of distribution of Ly6G positive cells (granulocytes) was observed in fewer mice among the Dnm2het group than in the CTRL group, indicating that the migration pattern within the BM is altered in the Dnm2het group (N = 30 and above). (Panel F) The BM from 1 Dnm2het mouse was completely effaced by immature myeloid precursors. (H) ANC in the peripheral blood 1 day after intraperitoneal alum injection in old female Dnm2het and CTRL mice. Older Dnm2het female mice (>52 weeks, N = 3) failed to show a rise of ANC, indicating a loss of rapid egress of neutrophils from the BM reserve. (I) Transwell migration assay of BM neutrophils from wild-type young female adults (aged 12-24 weeks, N = 3) treated with the DNM inhibitor DNSR, compared with CTRL neutrophils. Wild-type mouse neutrophils separated from the BM and treated with DNM-GTPase inhibitor, DNSR have reduced migration toward n-formylmethionyl-leucyl-phenylalanine, a chemoattractant for neutrophils, indicating that DNM function is necessary for this chemotactic response. (J) CXCR4 surface expression analyzed by flow cytometry in DNSR-treated and in CTRL BM Ly6G-high granulocytes from wild-type young female adults (aged 12-24 weeks, N = 4), when exposed for varying time periods to chemoattractant SDF1a (100 ng/mL). After 30 minutes of SDF1a exposure, surface CXCR4 was expressed to a greater extent among DNSR-treated neutrophils than in CTRL neutrophils. (Panels H-J) Each graph summarizes at minimum 3 to 4 independent experiments. Error bars indicate SEM. Only marked, group-wise comparisons are significant. ∗P < .05, t-test.
Dnm2 depletion is associated with decreased autophagy
Because Dnm2 can participate in autophagy,23 any potential dysregulation in autophagy was evaluated in the BM compartment. Younger CTRL female and male mice showed well-defined cytoplasmic multiple punctate structures. However, in older Dnm2het females, visible punctate patterns were less conspicuous and more often surrounded with less densely punctate, and dimly stained areas (Figure 5B). LC3B background average staining intensity was quantified by Halo Image software analysis and found to be lower in the young CTRL group (<34 weeks) when compared with all other mice as a group (per cell: young CTRL 0.05699 Intensity Units vs CTRL aged above 34 weeks/Dnm2het all ages 0.07817 Intensity Units, P = .0071; and per μm2: Young CTRL (<34 weeks) 0.002510 Intensity Units vs CTRL aged above 34 weeks /Dnm2het all ages 0.003235 Intensity Units, P = .0167; Figure 5C-D). A possible interpretation is that cytoplasm depletion in the young CTRL group (aged <34 weeks) may be because of LC3B molecules being sequestered or clustered into small, dotted structures with active autophagy. Meanwhile, in other mouse groups, greater amounts of LC3B molecules remain in solution in the cytoplasm where autophagy is less active. This suggests that the autophagy function is optimal in young mice with intact Dnm2 function. On detection of autophagy by flow cytometry using Cyto-ID, we found that the geometric mean fluorescence intensity was reduced in the LSK population in female Dnm2het (aged 16-35 weeks) compared with CTRL mice (supplemental Figure 3). Interestingly, while measuring morphologic parameters of BM cells, we noted that the average size of nuclei is greater in young Dnm2het mice (<34 weeks) than in young CTRL mice (<34 weeks) (CTRL 12.72 μm2 vs Dnm2het 13.71, P = .0491; Figure 5E).
Loss of Dnm2 function impairs chemotactic neutrophil migration
We hypothesized a potential role for dysregulated granulocyte migration as a contributor to neutropenia and aberrant myeloid maturation. We observed the following in support of our hypothesis. First, a linear distribution pattern of Ly6G positive BM cells along blood vessels was seen in fewer mice in the Dnm2het group than in the CTRL group (25% vs 59%, P = .02) (Figure 6A-G), in groups involving all ages. Second, neutrophil response to stress was tested by measuring the peripheral blood neutrophil counts in response to intraperitoneal injection with an ovalbumin/alum mixture vs saline CTRL.22 Older Dnm2het (above 52 weeks) female mice failed to show a rise of neutrophil counts on the day after being stressed with alum intraperitoneal injection when, compared with age-paired CTRL female mice (Figure 6H). Third, using Transwell migration assays, mouse neutrophils separated from the BM of wild-type young female adults and treated with dynamin-GTPase inhibitor DNSR had reduced migration toward the chemoattractant n-formylmethionyl-leucyl-phenylalanine (Figure 6I), supporting the fact that dynamin-GTPase activity is involved in neutrophil chemotactic migration.
Dynamin activity CTRLs CXCR4 surface expression in mature BM neutrophils
Because marrow neutrophils trafficking within the BM is regulated by CXCR4,13 and we had previously found that DNM2 regulate CXCR4 endocytosis in megakaryocytes,10 we measured the surface expression of CXCR4 on mature, Ly6G-high BM neutrophils from wild-type young female adults in the presence of SDF1a at a concentration of 100 ng/mL (Figure 6J), with or without DNM2 activity inhibitor (DNSR). We found that during a time course, the expression of surface CXCR4 was increased at 30 minutes in DNSR-treated neutrophils compared with CTRL. This reflects delayed endocytosis of the receptor, thus prolonging the exposure of the neutrophils to CXCR4-SDF1a axis effects.
Discussion
In this study, we explored the role of Dnm2 knockout in hematopoiesis in transgenic Vav-Cre mice. We found that only mice with heterozygous Dnm2 knockout were born, suggesting that homozygosity for the Dnm2 knockout gene causes embryonic lethality. Furthermore, in our study, the monocytic/granulocytic lineage appears to be more sensitive to partial Dnm2 depletion than other hematopoietic lineages. This is possibly because neutrophil turnover is higher (neutrophil half-life can be a few hours) than that of other lineages, therefore more susceptible to the biological strain caused by reduced Dnm2 levels.
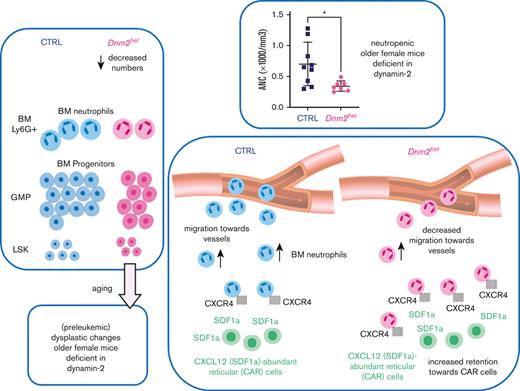
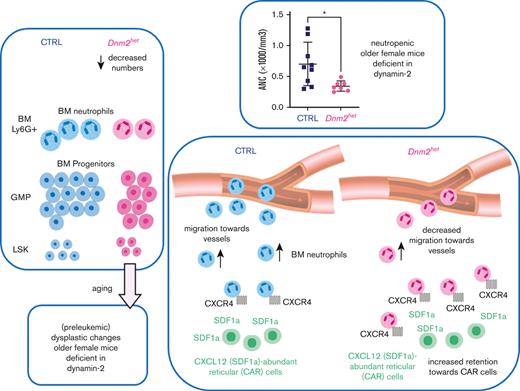
Searching for mechanisms, we established that both decreased precursor numbers and impaired migration within the BM contribute to neutropenia in mice with DNM2 deficient BM (refer to the summary in supplemental Figure 4). The decreased numbers of GMPs might be because of reduced differentiation of hematopoietic stem cells into the monocyte-granulocyte lineage, or because of excess cell death. One mechanism could be decreased autophagy.24 Autophagy is also involved in the activity of the innate immune system.25,26 Because DNM2 reportedly participates in autophagy,23 we tested autophagy by staining the BM with autophagy marker LC3B by immunohistochemistry. With BM staining of LC3B, we found autophagy to be impaired in older Dnm2 haplodeficient females. Also, the average size of nuclei was greater in Dnm2het mice than in CTRL mice, possibly because of perturbed cytokinesis in Dnm2het mice, as DNM was previously found to participate in centrosome cohesion and cytokinesis.27,28
Thus, the dysplastic features developing in the BM cells in older female Dnm2het mice may be because of perturbed autophagy and perturbed cell division in case of defective dynamin activity. Furthermore, the circulating neutrophils in older Dnm2het female mice likely show a poor egress into the peripheral blood owing to being retained within the BM secondary to prolonged SDF1a acting on excess CXCR4 surface receptors, as in myelokathexis.29-31 Interestingly, as neutrophils age, the expression of CXCR4 increases.12 This would suggest that aging neutrophils remain in the BM and do not migrate into circulation. Thus, neutrophils in Vav-Cre/Dnm2flox (also known as Dnm2het) female mice, at least when they age, may show a poor increase in the peripheral blood owing to being retained within the BM, secondary to prolonged SDF1a acting on CXCR4 receptors, whose endocytosis is delayed compared with CTRL cells. Consequently, it appears that a decrease in both the production and the migration of neutrophils are responsible for the observed neutropenia in older Dnm2het female mice.
There also appears to be additional inflammatory or immune dysregulation, as we observed prominent splenic germinal centers in younger Dnm2 haplodeficient mice. Immune dysregulation may be because of reduced endocytosis of receptors on immune cells.32 Interestingly, lymphopenia in T-cell specific Dnm2 knockout is reportedly because of defective egress rather than T-cell maturation defect.33 Migration is likely affected in other hematopoietic lineages such as already reported in lymphocytes; this could be further characterized in future studies.
We observed sex differences in the hematopoietic lineage, in which female mice showed significant neutropenia, unlike male mice. In contrast, Dnm2het male mice had lower hemoglobin levels than CTRL males, which wasn’t observed in female mice. This comes in parallel with recent reports in a human population cohort in the Netherlands, in which older women show a tendency toward lowering their neutrophil numbers, whereas older men showed decreasing hemoglobin levels.34 The mechanism for the male/female difference is unclear. It might implicate differences in inflammation profiles.35 Estrogens and androgens also influence hematopoiesis differentially.36-39 Among humans and mice models, certain types of myelodysplasia/leukemia preferentially develop in older females, such as the 5q- syndrome, and in mouse models with haploinsufficiency of cytoskeletal effector mDia1, that recapitulates many features of 5q- syndrome.40,41 Although clonal hematopoiesis emerges as a still incompletely understood contributor to myelodysplasia in older humans,42 a recent study has reported human cases of clonal hematopoiesis harboring hotspots for protein-altering mutations in DNM2.9 Interestingly, in recent reports, DNM2 (in particular, with loss of function mutation) is associated in a molecular signature with RUNX/RUNXT1 leukemia,43 and DNM2 was associated with MDS at risk for transformation in the Oncomine database. Furthermore, RUNX1 is a potential transcription factor controlling the expression of DNMs according to the TRANSFAC Predicted Transcription Factor Targets Dataset44 and to other literature,45 as are STAT3 and Ikaros. 32,46
Potential applications of the new knowledge in human disease are multiple. Because there are several molecule modulators of DNM function,47,48 DNM could serve as a new druggable target in a wide array of diseases that depend on the immune and inflammatory response. Notably, the Bis-T-23 compound promotes DNM oligomerization and was used in mouse models with success.49
Acknowledgments
The authors thank William Vainchenker, Najet Debili for critical review of the manuscript; Constadina Arvanitis, and Wensheng (Wilson) Liu for help with imaging; Suchitra Swaminathan for help with flow cytometry analysis; John Crispino for providing the Vav-Cre mice, Maria Sverdlov and Ryan Deaton for advanced pathology analysis, Robin McWherter and Jeanette Purcell for advice on animal care and experiments, Donald Lavelle, Vinzon Ibanez, Susan Quaggin, Hrishikesh Mehta, Usua Oyarbide, Robert Molokie, Damiano Rondelli, VK Gadi for additional support and helpful suggestions, Courtney Colvin for graphics. This work was financially supported by grants from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute grants K08 HL114871 and UIC Start-Up Funds (Y.C.); flow cytometry cell sorting was performed on a BD FACSAria SORP system, purchased through the support of NIH Office of the Director Award 1S10OD011996-01.
Authorship
Contribution: A.J.W., S.J.C., C.M.-Z., S.S.K., E.A.E., and Y.C. designed experiments, performed research and/or analyzed data; E.A.E. provided critical materials and reagents; and A.J.W., S.J.C., C.M.-Z., K.K., J.Q., E.A.E., and Y.C. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yolande Chen, Department of Medicine, Hematology/Oncology, University of Illinois at Chicago, 840 S Wood St, 820-E CSB, MC 713, Chicago, IL 60612; e-mail: ychenmd@uic.edu.
References
Author notes
Data are available on request from the corresponding author, Yolande Chen (ychenmd@uic.edu).
The full-text version of this article contains a data supplement.