TO THE EDITOR:
Hemophilia A (HA) is an X-linked hemorrhagic disorder caused by coagulation factor VIII (FVIII) protein deficiency. Standard-of-care treatment for severe HA (FVIII ≤1 international unit (IU)/dL) is prophylaxis with FVIII concentrate or emicizumab to prevent bleeding.1 Frequent infusions and consistent access to FVIII affect the quality of life for people with HA. Gene therapy offers a potential alternative in which 1 treatment provides endogenous FVIII production and prolonged bleeding protection.2-4
Valoctocogene roxaparvovec is a replication-incompetent adeno-associated virus (AAV) serotype 5 vector carrying a B-domain–deleted FVIII coding sequence controlled by a hepatocyte-selective promotor.5 One valoctocogene roxaparvovec infusion in participants with severe HA generated endogenous FVIII production that reduced bleeding and FVIII use in phase 1/2 and 3 trials.6-8 The most common adverse event (AE; 119/134 participants [88.8%]) was alanine aminotransferase (ALT) elevation, which was effectively managed with immunosuppression.6,9
Some older individuals with hemophilia have transfusion-associated HIV infection.10 They could benefit from valoctocogene roxaparvovec, but data are lacking on AAV-mediated gene transfer in this population. We report safety and efficacy of valoctocogene roxaparvovec in 3 clinical trial participants with HIV infection and severe HA.
GENEr8-1 (301; NCT03370913) and GENEr8-2 (302; NCT03392974) were open-label, single-group, phase 3 trials evaluating safety and efficacy of valoctocogene roxaparvovec at 6 ×1013 and 4 ×1013 vector genome per kg (vg/kg), respectively.6 Participants were men aged ≥18 years with FVIII ≤1 IU/dL receiving prophylactic FVIII concentrate before enrollment.6 Study design of 301 was published previously; 2 participants with HIV infection enrolled before a protocol amendment made HIV infection an exclusion criterion because of events in 302 as described in the subsequent section.6 After review of interim 301 results, enrollment into 302 was stopped. Only 1 participant enrolled in 302 who also had HIV infection.11 Both studies were approved by the Institutional Review Boards or Independent Ethics Committees of participating sites. Participants provided written informed consent.
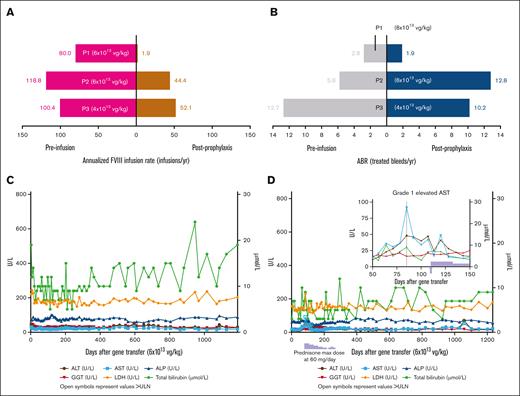
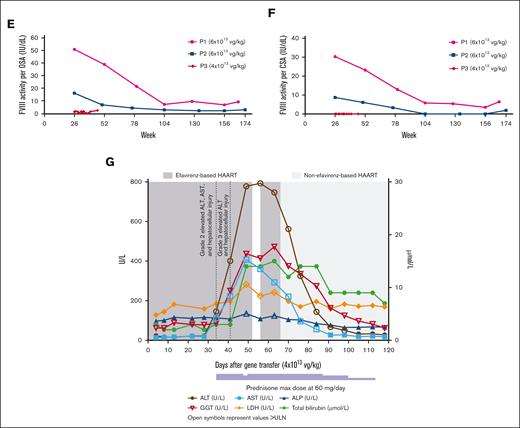
Participants 1 (P1) and 2 (P2) enrolled in 301 and received 6 ×1013 vg/kg valoctocogene roxaparvovec (supplemental Table 1).6 They were included in previously reported safety results as part of the intent-to-treat population, but not in efficacy results.6 At baseline, P1 and P2 had history of cleared hepatitis C and hepatitis B infections and had normal liver function tests. P1 (body mass index, 26.3 kg/m2) was receiving emtricitabine, rilpivirine, and tenofovir disoproxil fumarate as highly active antiretroviral therapy (HAART), and P2 (body mass index, 21.7 kg/m2) was receiving darunavir, dolutegravir, and ritonavir. Valoctocogene roxaparvovec provided endogenous FVIII production and decreased annualized FVIII infusion rate for both participants (Figure 1A). The annualized treated bleed rate (ABR) of P1 decreased from baseline (Figure 1B), but P2 experienced bleeding episodes requiring treatment beyond week 105 as FVIII activity declined. Neither has resumed FVIII prophylaxis. P1 experienced no elevations in liver enzymes, and P2 developed grade 1 elevated aspartate aminotransferase that resolved with prednisone (Figures 1C-D).
Participant efficacy data and liver function tests. (A) Annualized FVIII infusion rate (infusions/yr). (B) ABR (treated bleeds/yr). (C) Liver function test results for P1. (D) Liver function test results for P2. (E) FVIII measured by 1-stage assay (IU/dL). (F) FVIII measured by chromogenic assay (IU/dL). (G) Liver function test results for P3. ∗The postprophylaxis period began either at the start of week 5 or 3 days after the end of FVIII prophylaxis (whichever was later) and ended at the data cutoff date. P1 and P2 were enrolled in 301 and received 6×1013 vg/kg valoctocogene roxaparvovec. P3 was enrolled in 302 and received 4×1012 vg/kg valoctocogene roxaparvovec. In Figure 1C, 1D, and 1G, open symbols denote values above the ULN, and all depicted measurements were from a central laboratory. In Figure 1D, the light blue stepwise box below the X-axis denotes prednisone use in P2, and the light blue arrow located in the insert denotes when prednisone was started in P2. In Figure 1G, local laboratory measurements of liver function tests were also performed to support participant monitoring; these demonstrated ALT levels worsened again after resuming efavirenz by day 63. Bleeds and any FVIII treatment that was administered were self-reported by participants. Treated bleeds were defined as bleeds followed by use of standard half-life, extended half-life, or plasma-derived FVIII products within 72 hours. Bleeds associated with surgery or procedures were excluded from the analysis. ABR was defined as number of bleeding episodes/total number of days x 365.25. Annualized FVIII utilization rate was defined as number of FVIII infusions/total number of days x 365.25. The black arrow in Figures 1E and 1F denotes when P3 resumed FVIII prophylaxis and measurements of FVIII activity beyond this time were excluded. Values for FVIII activity detected below the lower limit of quantification (1 IU/dL for OSA, 3 IU/dL for CSA) were reported as 0 IU/dL and any measurements of FVIII activity were excluded if obtained within 72 hours of an infusion of exogenous FVIII. ABR, annualized bleeding rate; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CSA, chromogenic assay; FVIII, factor VIII; GGT, gamma-glutamyl transferase; HAART, highly active antiretroviral therapy; LDH, lactate dehydrogenase; OSA, one-stage assay; P, participant; ULN, upper limit of normal; vg, vector genomes; yr, year.
Participant efficacy data and liver function tests. (A) Annualized FVIII infusion rate (infusions/yr). (B) ABR (treated bleeds/yr). (C) Liver function test results for P1. (D) Liver function test results for P2. (E) FVIII measured by 1-stage assay (IU/dL). (F) FVIII measured by chromogenic assay (IU/dL). (G) Liver function test results for P3. ∗The postprophylaxis period began either at the start of week 5 or 3 days after the end of FVIII prophylaxis (whichever was later) and ended at the data cutoff date. P1 and P2 were enrolled in 301 and received 6×1013 vg/kg valoctocogene roxaparvovec. P3 was enrolled in 302 and received 4×1012 vg/kg valoctocogene roxaparvovec. In Figure 1C, 1D, and 1G, open symbols denote values above the ULN, and all depicted measurements were from a central laboratory. In Figure 1D, the light blue stepwise box below the X-axis denotes prednisone use in P2, and the light blue arrow located in the insert denotes when prednisone was started in P2. In Figure 1G, local laboratory measurements of liver function tests were also performed to support participant monitoring; these demonstrated ALT levels worsened again after resuming efavirenz by day 63. Bleeds and any FVIII treatment that was administered were self-reported by participants. Treated bleeds were defined as bleeds followed by use of standard half-life, extended half-life, or plasma-derived FVIII products within 72 hours. Bleeds associated with surgery or procedures were excluded from the analysis. ABR was defined as number of bleeding episodes/total number of days x 365.25. Annualized FVIII utilization rate was defined as number of FVIII infusions/total number of days x 365.25. The black arrow in Figures 1E and 1F denotes when P3 resumed FVIII prophylaxis and measurements of FVIII activity beyond this time were excluded. Values for FVIII activity detected below the lower limit of quantification (1 IU/dL for OSA, 3 IU/dL for CSA) were reported as 0 IU/dL and any measurements of FVIII activity were excluded if obtained within 72 hours of an infusion of exogenous FVIII. ABR, annualized bleeding rate; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CSA, chromogenic assay; FVIII, factor VIII; GGT, gamma-glutamyl transferase; HAART, highly active antiretroviral therapy; LDH, lactate dehydrogenase; OSA, one-stage assay; P, participant; ULN, upper limit of normal; vg, vector genomes; yr, year.
P3 (body mass index, 28.7 kg/m2) enrolled in 302 and was not reported previously. P3 was taking efavirenz, lamivudine, and tenofovir disoproxil fumarate for HAART at baseline (supplemental Table 1). P3 had a history of cleared hepatitis C that resolved spontaneously and was hepatitis C RNA negative; his liver function tests were normal at baseline, except gamma-glutamyl transferase (1.1x upper limit of normal). P3 received 4 ×1013 vg/kg valoctocogene roxaparvovec. He stopped FVIII prophylaxis during week 4 after infusion, as his FVIII activity was at detectable levels; however, beyond week 5, most assessments were below the limit of quantitation of the chromogenic assay (<3 IU/dL) (Figures 1E-F). P3 then experienced 21 bleeding events, 16 requiring treatment by week 46, and resumed FVIII prophylaxis at week 49.
The poor efficacy outcomes of P3 could be related to the low valoctocogene roxaparvovec dose13 or serious liver enzyme elevations experienced after infusion. On day 34, asymptomatic grade 2 AEs of elevated ALT, elevated aspartate aminotransferase, and hepatocellular injury were reported, and prednisone treatment (60 mg/day) was initiated (Figure 1G). On day 41, ALT levels and hepatocellular injury worsened to grade 3 serious AEs. A hepatic workup found a normal liver ultrasound and no evidence of autoimmune hepatitis or recent viral infection. On day 52, the efavirenz component of P3’s HAART was halted per recommendation of the investigator and a hepatologist. Efavirenz is a nonnucleoside reverse transcriptase inhibitor (NNRTI) associated with hepatic injury,12,14,15 and the pause was associated with plateaued ALT levels. P3 requested efavirenz, which had effectively managed his HIV since 2004, be reinstated. The ALT levels of P3 worsened again by day 63 according to the measurements performed at a local laboratory. After a hepatologist and the investigator consulted with P3, his HAART regimen was replaced with raltegravir, emtricitabine, and tenofovir alafenamide, a tenofovir prodrug with improved safety compared with tenofovir disoproxil fumarate.16 Four days later, his liver enzyme tests dropped ∼25% and continued to improve until day 105, when the AEs of liver enzyme elevation and hepatocellular injury were considered resolved. Throughout these AEs, he remained asymptomatic. Whether prednisone mitigated the hepatotoxicity P3 experienced is unknown. In an abundance of caution, further enrollment of HIV-positive participants into 301 was suspended.
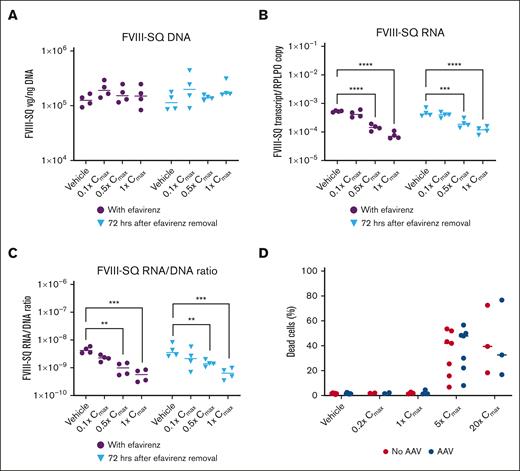
To explore possible drug interactions between efavirenz and valoctocogene roxaparvovec leading to hepatocellular toxicity, primary hepatocytes were transduced with valoctocogene roxaparvovec at a multiplicity of infection of 1×106 and cultured with efavirenz up to 20x the reported mean ± standard deviation maximum plasma concentration (Cmax) of 12.9 ± 3.7 μM.17,18 Cell culturing and FVIII-SQ RNA and DNA quantification were performed as previously described.19 Combined, AAV and efavirenz dose-dependently decreased the ratio of transgene RNA to DNA by 87%, which persisted after efavirenz withdrawal, suggesting transcription was affected (Figure 2A-C), but cytotoxicity was not increased compared with vehicle-treated control (Figure 2D). This may explain low circulating FVIII in P3, but it is not known why efavirenz interferes with transcription of episomal AAV. From a clinical standpoint, efavirenz-related liver damage has been associated with alcohol use, obesity, and active viral hepatitis,20 none of which were present in P3. From a biochemical standpoint, efavirenz is an NNRTI that binds reverse transcriptase at a site different from the nucleoside binding component, resulting in allosteric inhibition. Whether efavirenz inhibits RNA polymerases, and thereby inhibits AAV transcription, is unknown. In mice, decline in transgene RNA expression over time is mediated by epigenetic regulation of AAV episomes, with reduced interaction of the AAV genome with active histone marks and reduced RNA production.21 Efavirenz is known to modify cardiac cells through the same histone marks.22 This could explain inhibition of RNA production from the AAV episome by efavirenz in the absence of in vitro hepatotoxicity.
In vitro evaluation of efavirenz. Effect of efavirenz on transgene (A) DNA expression, (B) RNA expression, and (C) the ratio of transgene RNA/DNA. (D) Effect of efavirenz and AAV on cytotoxicity. ∗P <.05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 using a 2-way ANOVA followed by a Dunnett’s multiple comparisons test. In panels A–C, all samples were treated with valoctocogene roxaparvovec. In panel D, samples treated with AAV received valoctocogene roxaparvovec, and samples not treated with AAV received a vehicle control. AAV, adeno-associated virus; ANOVA, analysis of variance; Cmax, maximum plasma concentration; FVIII-SQ, factor VIII SQ-variant; hrs, hours; RPLPO, housekeeping gene for normalizing transcript expression.
In vitro evaluation of efavirenz. Effect of efavirenz on transgene (A) DNA expression, (B) RNA expression, and (C) the ratio of transgene RNA/DNA. (D) Effect of efavirenz and AAV on cytotoxicity. ∗P <.05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001 using a 2-way ANOVA followed by a Dunnett’s multiple comparisons test. In panels A–C, all samples were treated with valoctocogene roxaparvovec. In panel D, samples treated with AAV received valoctocogene roxaparvovec, and samples not treated with AAV received a vehicle control. AAV, adeno-associated virus; ANOVA, analysis of variance; Cmax, maximum plasma concentration; FVIII-SQ, factor VIII SQ-variant; hrs, hours; RPLPO, housekeeping gene for normalizing transcript expression.
Our model failed to demonstrate synergistic effects on cytotoxicity explaining the hepatocellular injury. The in vitro hepatocyte model is not well-suited for modeling protein production, limiting assessment of FVIII protein and efavirenz interactions.19 Efavirenz negatively affects the endoplasmic reticulum,23,24 and FVIII protein is difficult for the endoplasmic reticulum to fold and secrete.25,26 The combination of efavirenz and valoctocogene roxaparvovec perhaps triggered endoplasmic reticulum stress leading to hepatocellular injury. Active viral hepatitis also increases the risk of efavirenz-associated hepatic injury27; whether AAV-mediated gene transfer carries similar risk when combined with efavirenz requires additional study. In a separate trial evaluating an AAV vector for hepatocyte-mediated FVIII expression in HA, 3 participants with HIV infection had responses similar to those of P1 and P2 reported here,28 supporting that the AEs of P3 were not caused by HIV infection alone. Data are also required regarding whether the effect is unique to efavirenz or a drug class effect for all NNRTIs. Although efavirenz carries a relatively high risk, other NNRTIs are also associated with hepatic injury, including rilpivirine, which P1 took without eliciting similar AEs. Hepatotoxic drugs may require caution in liver-directed gene therapy. In the 301 trial, the highest ALT elevation after treatment was attributed to hepatotoxic effects of carbamazepine and/or amphotericin B in an HIV-negative participant.6 Generally, drug hepatotoxicity is more frequent in individuals with hemophilia than those without, owing to chronic hepatitis.29
In summary, 2 participants with severe HA and HIV infection experienced endogenous FVIII production, reduced FVIII infusion rates, and no or only grade 1 elevations in liver enzymes. P1 experienced a slight decrease in ABR, but ABR of P2 increased because of bleeding after week 105. A third participant with severe HA and HIV infection had limited FVIII production and multiple liver toxicity-related AEs, likely because of negative interactions between valoctocogene roxaparvovec and efavirenz, which is a known hepatotoxic agent. Many participants in 301 and in other gene therapy trials for hemophilia experienced mild ALT elevations which were controlled by immune suppression.6,8,13,28,30,31 Although the timing of elevated ALT in P3 was consistent with previous reports, the magnitude and duration of elevated ALT, and lack of improvement with immunosuppression, warranted concern. Careful history for hepatotoxic medications should be taken before liver-directed gene therapy initiation, and they should be used with caution or avoided, if possible, as with efavirenz.
Acknowledgments: The authors thank the trial participants, their families, and study site personnel. Medical writing support was provided by Tony Sallese of AlphaBioCom and funded by BioMarin Pharmaceutical Inc.
Contributions: M.V.R. and E.M. are investigators who conducted the clinical studies; M.V.R. conceptualized the research question and proposed this publication; D.B.R. and T.M.R. oversaw the clinical studies; H.Y. performed the statistical analyses; S.F., B.Y., S.S., and L.R. designed and performed the in vitro analyses; and all authors contributed to the interpretation of the data and critically reviewed the manuscript.
Conflict-of-interest disclosure: M.V.R. received research grants to her institution from BioMarin Pharmaceutical Inc, Sanofi, SPARK, and Takeda; and consulting fees from BeBio, BioMarin Pharmaceutical Inc, HEMAB, Sanofi, SPARK, Takeda; and speaker fees from Takeda and Institute for Clinical Economic Review (ICER).
E.M. received consulting payments from BioMarin Pharmaceutical Inc for serving on the advisory board.
S.F., B.Y., S.S., L.R., H.Y., D.B.R., and T.M.R. are employees and stockholders of BioMarin Pharmaceutical Inc.
Correspondence: Margaret V. Ragni, University of Pittsburgh Medical Center and Hemophilia Center of Western Pennsylvania, 3636 Boulevard of the Allies, Pittsburgh, PA 15213-4306; e-mail: ragni@pitt.edu.
References
Author notes
The deidentified individual participant data that underlie the results reported in this article (including text, tables, figures, and appendices) will be made available together with the research protocol and data dictionaries for noncommercial, academic purposes. Additional supporting documents may be available upon request. Investigators will be able to request access to these data and supporting documents via a data sharing portal beginning 6 months and ending 2 years after publication. Data associated with any ongoing development program will be made available within 6 months after approval of relevant product. Requests must include a research proposal clarifying how the data will be used, including proposed analysis methodology. Research proposals will be evaluated relative to publicly available criteria available at www.BioMarin.com/patients/publication-data-request/ to determine if access will be given, contingent upon execution of a data access agreement with BioMarin Pharmaceutical Inc.
The full-text version of this article contains a data supplement.