TO THE EDITOR:
Patients with relapsed/refractory (R/R) aggressive B-cell non-Hodgkin lymphoma (NHL) after anti-CD19 chimeric antigen receptor T-cell therapy (CART19) have poor prognosis with a median overall survival (OS) of ∼6 months only.1,2 Thus, this population needs access to novel therapies through clinical trials; however, in a single-center experience, Chow et al reported that only 12% of patients with aggressive B-cell NHL were treated in clinical trials at the time of disease progression after CART19.1 Identifying the potential barriers to clinical trial enrollment is needed to further guide the design of future inclusive clinical trials for this population. To address this, we evaluated the potential clinical trial eligibility and its barriers in patients with aggressive B-cell NHL at the time of disease progression/relapse after CART19.
We included adult patients who received CART19 for R/R aggressive B-cell NHL at 3 Mayo Clinic sites (Rochester, Arizona, and Florida) and had disease progression/relapse after CART19. The potential eligibility to the following key clinical trials for R/R aggressive B-cell NHL was retrospectively evaluated at the time of disease progression/relapse post-CART19.
Landmark clinical trials that resulted in recent Food and Drug Administration approvals: (a) polatuzumab + bendamustine + rituximab (Pola-BR),3 (b) tafasitamab + lenalidomide (Tafa-Len),4 (c) selinexor,5 and (d) loncastuximab.6
Anti-CD20 bispecific antibodies (bsAb) clinical trials: (a) glofitamab,7 (b) odronextamab,8 (c) epcoritamab,9 and (d) plamotamab.10
The potential eligibility for the above clinical trials was based on hemoglobin (Hb) level, absolute neutrophils count (ANC), platelets count (Plt), renal11 and liver function tests, Eastern Cooperative Oncology Group (ECOG) performance status, and central nervous system (CNS) disease involvement at the time of disease progression/relapse post-CART19. The study was approved by the institutional review board at Mayo Clinic and was conducted according to the Declaration of Helsinki.
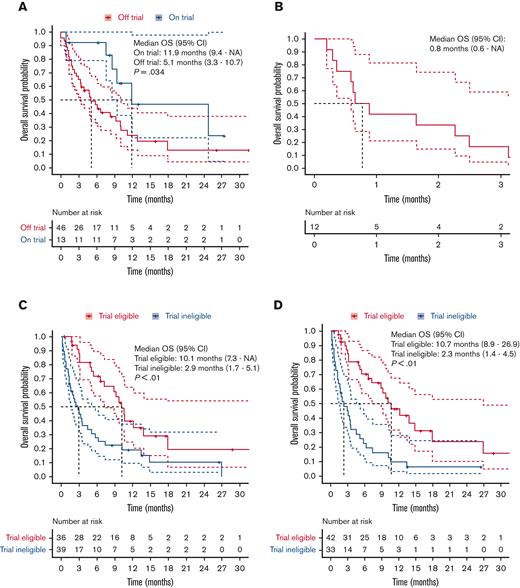
A total of 75 patients with aggressive B-cell NHL had disease progression after axicabtagene ciloleucel (N = 73, 97%) or tisagenlecleucel (N = 2, 3%) and were included in this study, and their characteristics are summarized in supplemental Table 1. Only 13 patients (18%) with aggressive B-cell NHL were treated in trials at the time of disease progression post-CART19. Among the 59 patients (78%) who actually received salvage therapy for disease progression post-CART19, the median OS of patients treated on (n = 13) or off (n = 46) trials was 11.9 months (95% confidence interval [CI], 9.4-not available) vs 5.1 months (95% CI, 3.3-10.7), respectively (Figure 1A). Patients who did not receive salvage therapy at the time of disease progression post-CART19 had a dismal survival, and the median OS was only 0.8 months (95% CI, 0.6-not available) (Figure 1B).
OS after disease progression to CART19. (A-D) OS after disease progression to CART19 estimated by Kaplan-Meier and compared by long-rank test; 95% CI. (A) Patients treated on or off clinical trials; here, OS is defined for salvage therapy for disease progression post-CART19. (B) Patients who did not receive salvage therapy. (C) Patients eligible vs ineligible for landmark trials. (D) Patients eligible vs ineligible for anti-CD20 bispecific antibodies trials.
OS after disease progression to CART19. (A-D) OS after disease progression to CART19 estimated by Kaplan-Meier and compared by long-rank test; 95% CI. (A) Patients treated on or off clinical trials; here, OS is defined for salvage therapy for disease progression post-CART19. (B) Patients who did not receive salvage therapy. (C) Patients eligible vs ineligible for landmark trials. (D) Patients eligible vs ineligible for anti-CD20 bispecific antibodies trials.
The exclusion criteria of the landmark trials are outlined in Table 1, and based on these, 39 patients (52%) with aggressive B-cell NHL did not meet the eligibility criteria for any of the 4 recent landmark trials for R/R aggressive B-cell NHL at the time of progression post-CART19.3-6 Hematologic exclusion criteria were the most common barrier to inclusion of these patients undergoing these landmark trials, with 36 patients (48%) having at least 1 hematologic exclusion criteria that precluded them from all the landmark trials.
Next, we examined the eligibility to anti-CD20 bsAb trial because these drugs are not associated with significant myelotoxicity or renal/liver clearance/toxicity.7-10,12 The exclusion criteria for the trials are outlined in Table 2, and based on these, 33 patients (44%) with aggressive B-cell NHL still did not meet the eligibility for any of the 4 recent anti-CD20 bsAb trials for R/R aggressive B-cell NHL at the time of disease progression post-CART19.7-10 Again, the hematologic exclusion criteria were the most common barrier to inclusion of these patients on these anti-CD20 bsAb trials, with 28 (37%) having at least 1 hematologic exclusion criteria from all these anti-CD20 bsAb trials. In both landmark and anti-CD20 bsAb trials’ exclusion criteria, renal or liver dysfunction and CNS involvement were less common reasons that led to exclusion from trials (Tables 1 and 2).
These patients ineligible to undergo landmark or anti-CD20 bsAb clinical trials had significantly poor prognosis with a median OS of only 2.9 months (95% CI, 1.7-5.1) and 2.3 months (95% CI, 1.4-4.5), respectively, whereas among patients eligible for these trials, the median OS was 10.1 months (95% CI, 7.3-not available) and 10.7 months (95% CI, 8.9-26.9), respectively (Figure 1C-D).
Finally, we determined the potential trial ineligibility using hypothetical ANC and Plt cutoffs, assuming no Hb exclusion criteria (supplemental Table 2). For example, ANC < 0.75 × 109/L and Plt < 30 × 109/L or ANC < 0.5 × 109/L and Plt < 20 × 109/L would exclude 22 (29%) or 15 (20%) patients, respectively, compared with 36 (48%) patients in the commonly used exclusion criteria ANC < 1.0 × 109/L or Plt < 75 × 109/L.
In our study, we confirmed the low enrollment of patients with aggressive B-cell NHL in clinical trials at the time of disease progression/relapse after CART19 and reported the possible barriers to inclusion of these patients in clinical trials. We showed that ∼50% of this population would not meet the eligibility criteria of recent key trials for R/R aggressive B-cell NHL. We also demonstrated that patients who were not eligible for clinical trials had a very poor prognosis with a median OS of 2 to 3 months. Thus, the patient population with the most critical need for novel therapies is being deprived of the opportunity to have access to these therapies through clinical trials.
Moreover, we showed that the current hematologic exclusion criteria would be a major barrier to inclusion of these patients, excluding between 37% and 48% of these patients from clinical trials. Our study also showed high prevalence of incomplete hematologic recovery, specifically at the time of disease progression post-CART19, and it was rarely related to bone marrow (BM) involvement (supplemental Table 1). This is critical information for any future design of inclusive clinical trials for this population. Our findings are in alignment with the current literature of delayed hematologic recovery post-CART, but the data from previous reports were not specific to disease progression as it is in our study.13-15
Next, we inquired whether the current trial hematologic exclusion criteria were appropriate for the myelotoxicity risk of these study drugs. For example, the myelotoxicity of anti-CD20 bsAb is lower than that of cytotoxic drugs; however, the hematologic exclusion criteria of their trials were very similar to trials including cytotoxic drugs.3-10,16 Then, the anti-CD20 bsAb trials’ eligibility criteria only led to a potential inclusion net gain of 8% compared with that of landmark trials, and this gain was only due to the flexibility toward the platelet count of the plamotamab trial, which tolerated lower Plt (50 × 109/L compared with 75 × 109/L in most of other trials).10 Taking into consideration that the hematologic exclusion criteria are frequently not supported with the myelotoxicity risk of the study drug, as we discussed above, our data are very important for designing future trials for patients with aggressive B-cell NHL at the time of disease progression post-CART19, when incomplete hematologic recovery is still prevalent as we showed in this study. One caveat is that despite anti-CD20 bsAb not being primarily myelotoxic, these novel agents have immune effects, infection risks, and potential overlaps with still undetermined mechanisms of post-CART cytopenia, therefore warranting caution in patients with significant cytopenia.
In summary, ∼50% of the patients with aggressive B-cell NHL progressing after CART19 are excluded from clinical trials. The current hematologic exclusion criteria represent a major barrier to trial enrollment. Therefore, the clinical trials’ hematologic exclusion criteria should be adapted to be more inclusive of this population with very poor prognosis in such unmet need, especially when the study drugs are not associated with significant myelotoxicity.
Contribution: E.D.B., M.I., J.M., A.K., Y.W., M.J.M., T.E.W., G.S.N., and Y.L. contributed in the concept and design; E.D.B., M.I., J.M., R.B., M.A.H., and Y.L. contributed in the collection and assembly of the data; E.D.B., M.I., J.M., M.J.M., G.S.N., and Y.L. analyzed and interpreted the data; and all authors contributed to the manuscript writing, gave final approval of manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Lin, Division of Hematology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: lin.Yi@mayo.edu.
References
Author notes
∗E.D.B., M.I., and J.M. are joint first authors.
†G.S.N. and Y.L. are joint senior authors.
Presented in abstract form at the 63rd American Society of Hematology Annual Meeting in December 2021.
Original data are available on request from the corresponding author, Yi Lin (lin.yi@mayo.edu).
The full-text version of this article contains a data supplement.