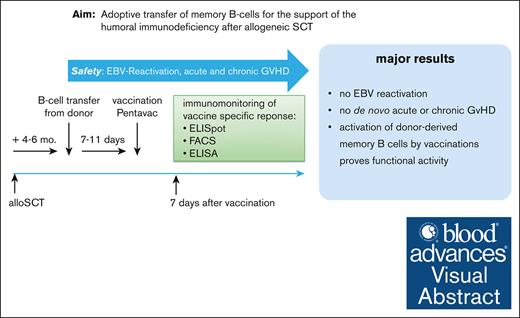
No clinically significant EBV reactivation or acute or chronic GVHD occurred after adoptive donor B-cell transfer into allo-SCT recipients.
Transferred donor B cells enhance the memory antibody response to vaccine antigens.
Visual Abstract
Immune reconstitution after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is slow and patients carry a high and prolonged risk of opportunistic infections. We hypothesized that the adoptive transfer of donor B cells can foster after HSCT immuno-reconstitution. Here, we report, to our knowledge, the results of a first-in-human phase 1/2a study aimed to evaluate the feasibility and safety of adoptively transferred donor B cells and to test their activity upon recall vaccination. Good manufactoring practice (GMP) B-cell products were generated from donor apheresis products using 2-step magnetic cell separation. Fifteen patients who had undergone allo-HSCT were enrolled and treated after taper of immunosuppression (median, day +148; range, 130-160). Patients received 4 different doses of B cells (0.5 × 106 to 4.0 × 106 B cells per kg body weight). To test the activity of infused donor memory B cells in vivo, patients were vaccinated with a pentavalent vaccine 7 days after B-cell transfer. We observed the mobilization of plasmablasts and an increase in serum titers against vaccine antigens, with a stronger response in patients receiving higher B-cell numbers. Analysis of immunoglobulin VH-sequences by next-generation sequencing revealed that plasmablasts responding to vaccination originated from memory B-cell clones from the donor. Donor B-cell transfer was safe, as no Epstein-Barr virus (EBV) reactivation was observed, and only low-grade graft-versus-host disease (GVHD) occurred in 4 out of 15 patients. This pilot trial may pave the way for further studies exploring the adoptive transfer of memory B cells to reduce the frequency of infections after allo-HSCT. This trial was registered at ClinicalTrial.gov as #NCT02007811.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for high-risk hematologic diseases. However, even in graft-versus-host disease (GVHD)–free and relapse-free allo-HSCT survivors, full reconstitution of the adaptive immune system and functional protection from opportunistic infections can take months to years. Delayed reconstitution of B cells increases the risk of infection, especially by opportunistic pathogens.1-3 To reduce the risk of vaccine-preventable infectious diseases, patients undergoing HSCT are repeatedly vaccinated according to international guidelines.4,5 Vaccine response studies in HSCT recipients have shown that antibody response rates depend on age, type of vaccine, immune reconstitution, and GVHD.6 Nevertheless, HSCT recipients have a much higher susceptibility to infection than the general population even after vaccination.7 In previous studies, pre–HSCT vaccination of donors was explored to improve vaccine-induced immunity, but only limited success against individual pathogens was observed.8-10 For a recent review, see Harris et al.11 The clinical relevance of transferred immunity is evident, particularly for cytomegalovirus (CMV), because seropositive patients are at high risk of CMV reactivation if transplanted from seronegative donors. Therefore, CMV–seronegative donors are usually omitted from CMV–seropositive recipients.12
We previously demonstrated that memory B cells from murine CMV–infected donor mice adoptively transferred into immunodeficient mice were highly effective in protecting against lethal murine CMV infections.13 These preclinical data on the efficacy of adoptively transferred virus-specific memory B cells were the rationale to examine a cell-based strategy to support humoral immune recovery in the clinical setting. We, thus, developed good manufacturing practice (GMP)–approved methods for the adoptive transfer of purified donor B cells in patients with allo-HSCT to improve both vaccine-induced and infection-triggered immunity.14 Because no adoptive B-cell transfer has thus far been performed in the allo-HSCT setting, to our knowledge, we initiated a first-in-human phase 1/2a study to test the safety and tolerability of adoptively transferred donor B cells. The most important safety and tolerability aspects were the transmission of Epstein-Barr virus (EBV) and the development of GVHD by cell products. EBV persists in B cells and can be reactivated in immunocompromised hosts, such as allo-HSCT recipients, and can cause posttransplantation lymphoproliferative disease.15 Furthermore, B cells also contribute to the development of chronic GVHD (cGVHD) and a number of B-cell–targeting drugs, such as ibrutinib14 and the B-cell–depleting antibody rituximab, have been shown to be effective in cGVHD.16
For the preparation of GMP–approved B-cell products, we applied our previously established protocol and used leukapheresis cells from the original stem cell donors to generate pure B-cell products containing only marginal amounts of contaminating T cells to minimize the risk of GVHD induction.17 Here, to our knowledge, we present the first report on the feasibility, safety, and preliminary efficacy data from this phase 1 to 2 dose-escalation clinical trial on adoptively transferred donor B cells in a post–allo-HSCT setting. For preliminary evaluation of efficacy, we examined the response to pentavalent diphtheria- tetanus- pertussis- polio-myelitis (inactivated) and Haemophilus-type b–conjugate-vaccine (DTaP-IPV-Hib) after B-cell administration based on antibody levels and memory B-cell responses.
Methods
Patients
We conducted a multicenter (University Hospital Erlangen, Essen, and Regensburg), open-label, phase 1/2a study of a donor–derived B-cell product administered once 140 ± 20 days after unmanipulated allo-HSCT in adult patients with a HLA-identical sibling donor or a 9 of 10 or 10 of 10 HLA–matched unrelated donor (Table 1). All patients had a GVHD-prophylaxis with rabbit-derived anti-thymocyte globuline (ATG, Thymoglobuline, Sanofi S.A.) of 7.5 mg/kg per b.w. in matched unrelated donor (MUD) and of 2.5 mg/kg per b.w in sibling allogeneic HSCT.
The clinical trial (EudraCT 2012-003033-42) was approved by the German higher competent authority Paul Ehrlich Institute (Federal Institute for Vaccines and Biomedicines; No.1824/01) and ethics committee of Friedrich-Alexander University Erlangen-Nürnberg (184_13 Az). It was conducted in accordance with the ethical principles of the Declaration of Helsinki, GCP, and applicable regulations. The clinical trial was registered at ClinicalTrials.gov (Identifier: NCT02007811) before enrollment. All participants and donors provided written informed consent.
Adult patients with complete remission of their malignancy and donor chimerism of >98% were eligible. Patients with an EBV reactivation of >10 000 copies per mL and acute GVHD grade 3/4 in their history were excluded. Because the GVHD prophylaxis or immunosuppressive therapy had to be reduced to a dose of cyclosporine A <50 ng/mL and of steroids <0.2 mg/kg body weight (b.w.), and sufficient T cell number >100/μL were required for a vaccine response a second evaluation (V2) was carried out before the start of the manufacturing of the cell product. Additionally, patients with cGVHD of moderate or severe risk (National Institutes of Health [NIH] criteria, 201518) were excluded from this study.
Study design and assessments
The B-cell product was administered IV at 4 different dose levels (dose group 1: 0.5 × 106; 2: 1.0 × 106; 3: 2 × 106; and 4: 3 × 106 to 4 × 106 B cells per kg b.w.). The dose-finding followed a standard 3 + 3 design to evaluate the safety.
The primary end point was safety and tolerability. The main secondary end points were differences in the mean change in the frequency of antibody–producing cells between dose groups 7 days after a single vaccination and differences in the mean change in antigen–specific antibody concentration in serum between dose groups before and up to 120 days after vaccination.
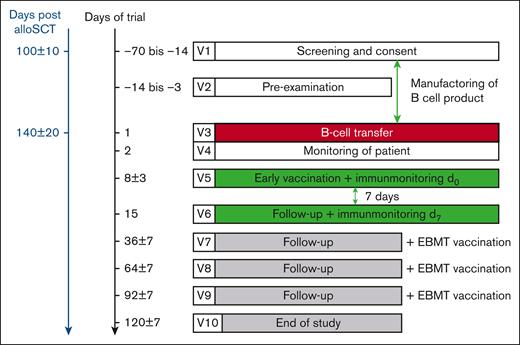
Adverse events (AEs) were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and dose-limiting toxicities were predefined as (1) any treatment-emergent nonhematologic CTCAE grade 3 toxicity; (2) a new manifestation of an acute GVHD grade 3 or 4 within ≤14 days after application of the B-cell product; (3) worsening of acute GVHD from grade 1 or 2 to grade 3 or 4; and (4) an EBV reactivation requiring treatment with rituximab within ≤14 days after application of the B-cell product. Dose progression beyond dose group 1 was based on a review of available safety and tolerability data from the data safety monitoring board. A safety interval of at least 21 days between the first and second subjects of each dose group and between the 2 dose groups was observed. The mean duration of participation of patients was 167 days (186-191), which included a screening period of 14 to 70 days (screening, donor leukapheresis, investigational medicinal product [IMP] manufacture), B-cell product transfusion, preponed single vaccination, and a 4-month safety follow-up period during which the immunization was completed with 3 vaccinations according to European Bone Marrow Transplantation Society (EBMT) guidelines (Figure 1). Clinical chemistry and hematology assessments, including measuring of EBV-DNA and CD3–positive cell numbers in the peripheral blood, were performed at screening, at the time of donor B-cell transfusion, followed by weekly assessments through day 56, and every 28 days until day 120 after B-cell transfer. Acute GVHD was graded by the investigators using the EBMT-CIBMTR-NIH criteria.19 Patients were assessed for signs and symptoms of cGVHD per the NIH guidelines 2015.18
Flowchart diagram describing clinical trial. For the time axis, the day of B-cell transfer is denoted as d1. V1 to V10, visits.
Flowchart diagram describing clinical trial. For the time axis, the day of B-cell transfer is denoted as d1. V1 to V10, visits.
IMP
IMP consisted of enriched B lymphocytes derived from the original stem cell donor by leukapheresis. The manufacturing of the B-cell product was performed using CD3 depletion followed by CD19 magnetic enrichment, as described previously.20 After 2-step separation, the median proportion of B cells was 96.1% (range, 84.9%-99.1%), and the median proportion of contaminating CD3-positive T cells was 0.08% (range, 0.01%-0.82%). The absolute number of T lymphocytes in all IMPs was below the threshold of <4 × 104 CD3+ cells per kg b.w. (mean, 0.06 × 104 kg per b.w.; range, 0.01 × 104 to 0.13 × 104). After separation, the B-cell numbers required for each dose level were separated and cryopreserved for later administration according to the specifications of the clinical study. The B-cell product contained a mean of 27.0% CD27+ memory B cells (range, 17.1%-31.3%; n = 8). CD38hi plasmablasts (PBs) and CD10+ immature B cells were below 1% in all the measured preparations.
Vaccination after B-cell transfer
The patients received the first vaccination 7 to 11 days after B-cell transfer. The immunization was completed with 3 vaccinations at an interval of at least 4 weeks, starting 28 days after the first vaccination. A pentavalent combination vaccine PENTAVAC (Sanofi Pasteur MSD GmbH) and pneumococcal conjugate vaccine Prevenar 13 (Wyeth Lederle Vaccines S.A.) were administered by intramuscular injection.
For comparison of the vaccine response, a group of volunteer healthy donors (n = 10) was vaccinated once with PENTAVAC. This is considered a booster vaccination, because all individuals had previous vaccinations with antigens in the PENTAVAC vaccine.
Flow cytometry
Flow cytometry was performed using a FACSCalibur instrument (Becton Dickinson, Heidelberg, Germany). The antibody clones used are listed in supplemental Table 1.
Measurement of EBV-DNA load and EBV–specific T-cell responses
The monitoring of EBV-DNA load value was measured at least once a week in the plasma samples of the study patients using a validated polymerase chain reaction (PCR) assay. The lower detection level of EBV-DNA was 250 copies per ml. A cut off of 50 000 EBV copies per mL in the plasma of study patients was defined to initiate a pre–emptive therapy with rituximab according to the European Conference on Infections in Leukemia (ECIL) guideline 2016.21
To monitor the frequency of EBV–specific CD4 and CD8 T cells in patients after B-cell transfer, an intracytoplasmatic IFNγ fluorescence-activated cell sorting (FACS) assay after stimulation with 15-mer peptides derived from EBV proteins EBNA1+BMLF1+BZLF1+LMP1+BRLF1 was performed according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany).
Measurement of serum antibody titers
Immunoglobulin G (IgG) serum antibody titers were measured using enzyme-linked immunosorbent assay (ELISA) for tetanus toxoid (TT) and diphtheria toxoid (DT) obtained from the Statens Serum Institute, Copenhagen, Denmark, according to the manufacturer’s instructions. ELISA 96 well plates (Greiner Bio-One GmbH) were coated with 5 μg/mL antigen. For the assessment of antibodies against the poliovirus, a commercial ELISA was used according to the manufacturer’s instructions (Demeditec Diagnostics GmbH, Kiel, Germany). The following World Health Organization standards were used for calibration: TE-3 for TT, and 10 of 262 for DT. Protective antibody concentrations were defined as: ≥0.1 IU/mL for TT and DT; ≥24 IU/mL for pertussis; and ≥1 μg/mL for Hib. A positive vaccination response was defined as ≥4 times and ≥100% increase in the antibody titer compared with the prevaccination (d + 0) and the postvaccination samples.
Isolation of PBMC and purification of B cells
Peripheral blood mononuclear cells (PBMCs) from patients and healthy donors were isolated from 80 mL of whole blood using Ficoll density gradient centrifugation (Lymphoflot; Bio-Rad, Munich, Germany). After Ficoll separation, the PBMCs were washed and untouched B cells were purified using a B Cell Isolation Kit II, human (Miltenyi Biotec). The purity of the B-cell preparations was determined by FACS analysis with CD19 antibodies for the calculation of input numbers in the enzyme-linked immunospot (ELISPOT) assay.
Quantification of ASC by ELISPOT assay
For the quantification of total and vaccine–specific IgG antibody-secreting cells (ASC), ELISPOT multiscreen plates (Millipore, Billerica, MA) were directly coated with goat antihuman IgG, Fc specific (2.5 μg/mL, DIANOVA, Hamburg, Germany), TT (2.5 μg/mL), DT (2.5 μg/mL), pertussis (1:2.000, a kind gift from SanofiPasteur, Marcy l'Etoile, France) and Hib (1 μg/mL Hib oligosaccharide-conjugated to human serum albumin; NIBSC, South Mimms, United Kingdom) in phosphate-buffered saline overnight at 4°C. Multiscreen plates were precoated with goat antipoliovirus antibody, followed by incubation with an inactivated polio vaccine preparation (types 1, 2, and 3) kindly provided by Sanofi Pasteur. After washing, the plates were blocked with 200 μL RPMI/10% fetal calf serum at 37°C. Purified B lymphocytes at different cell densities were incubated in 200 μL RPMI/10% fetal calf serum for 5 hours at 37°C. Plates were washed and incubated with HRPoat antibody against human IgG (1:1.000; DIANOVA, Hamburg, Germany) overnight at 4°C. ELISPOTs were detected using 3,3',5,5'-tetramethylbenzidine (TMB) as substrate (KPL/Seracare, Milford, MA) and analyzed using an ELISPOT reader and AID EliSpot v5.0 (AID Diagnostics, Strassberg, Germany).
Cell sorting and RNA–based BCR-repertoire analysis
Memory B cells (CD19+ CD27+) were sorted from cryopreserved donor B-cell products and PBs (CD19+ CD27hi CD38hi) from 4 recipients 7 days after experimental vaccinations on an Astrios EQ cell sorter (Beckman Coulter). RNA was purified using the RNeasy plus micro kit (Qiagen). The human B-cell receptor (BCR) Heavy Chain V-C gene kit (iRepertoire Inc) was used to perform multiplex PCR to generate full-length VH libraries for sequencing following the manufacturer’s instructions. Sequencing was performed using Illumina MiSeq (Next generation sequencing (NGS) Core Facility of FAU). From the raw sequencing data, we used the CDR3 algebra tool in the iRweb tools (iRepertoire Inc) to find shared CDR3 clones between matching and nonmatching donor recipient pairs. CDR3 sequences were normalized to 10 million reads for each sample and exported. The top 100 clones for the PB repertoire were analyzed using Microsoft Excel for shared identical CDR3 clones in the matching and nonmatching memory BCR libraries.
Statistical analysis
Comparison of means was performed using the Wilcoxon-Mann-Whitney test using the GraphPad Prism 9 software.
The clinical trial (EudraCT 2012-003033-42) was approved by the German higher competent authority Paul Ehrlich Institute (Federal institute for Vaccines and Biomedicines; no.1824/01) and Ethics committee of Friedrich-Alexander University Erlangen-Nuremberg (184_13 Az).
Results
Patient cohort
Fifteen patients who underwent allo-HSCT were included in this first-in-human phase 1/2a clinical trial. The baseline characteristics of study patients are listed in Table 1. After the informed consent form between day +90 and day +110 after allo-HSCT, the original stem cell donor was asked to undergo unstimulated leukapheresis (Figure 1). Simultaneously, GVHD prophylaxis/immunosuppressive therapy was tapered off under the ongoing screening of the inclusion/exclusion criteria. One week before the scheduled B-cell transfer, that is, at visit V2 (Figure 1), a second screening of exclusion criteria was performed. At the time of B-cell transfer, only 2 of 15 patients with a history of acute GVHD grade 2 had a residual dose of prednisolone <0.2 mg/kg BW, whereas cyclosporine was tapered off in all patients.
Safety assessment
By applying the previously described isolation technology, for all 15 patients, GMP-quality B-cell products were manufactured from donor apheresis and cryopreserved. Manufactured cryopreserved B-cell products were transfused without complications after thawing after the administration of H1 and H2 receptor antagonists.
For safety evaluation, AE for low (1 + 2) and high (3 + 4) B-cell dose levels are listed in supplemental Table 2. As expected, all patients with allo-HSCT reported at least 1 AE between B-cell transfer (V3) and completion of the observation period (V10, ie, 120 days after B-cell transfer). Most of the events were CTCAE intensity grade 1 or 2. Three patients in the high-dose group developed 1 grade 3 AE. Three AEs associated with hospitalization (3 SAEs) occurred in patients who received the B-cell product, and 1 patient with dose level 1 developed a fever 108 days after B-cell transfer. One patient with dose level 4 was admitted for CMV colitis 34 days after B-cell transfer, and another patient with dose level 4 was admitted for septic shock 62 days after B-cell transfer. Two of 3 patients with SAE recovered, whereas the patient with septic shock died. The data safety monitoring board identified no causative relationship between SAEs and B-cell transfer. Thus, no suspected unexpected serious adverse reaction (SUSAR) was reported during the study and dose modification of the B-cell product was not required.
GVHD after B-cell transfer
The onset or worsening of existing GVHD after B-cell transfer (containing low amounts of T cells) was considered an AE of special interest. Patients without acute or cGVHD before B-cell transfer (n = 3) did not develop GVHD after B-cell transfusion. Two of the 5 patients with resolved prior acute GVHD developed a maculopapular rash qualifying as stage 1 and stage 2 according to the Glucksberg criteria after B-cell transfer. The recurrence of acute GVHD resolved with topical steroids in patient BT10 and low-dose steroid therapy in patient BT20 (Table 2). In 2 of 7 patients with mild cGVHD before B-cell transfer, worsening of cGVHD was observed after B-cell transfer (Table 2). Both patients developed an increase in liver enzymes on days 83 (BT01) and 89 (BT12) after B-cell transfer, with NIH score 2. In addition, patient BT01 had new-onset mild oral (NIH score 1) and cutaneous cGVHD (NIH score 1), and patient BT12 developed keratoconjunctivitis sicca (NIH score 2). Under immunosuppression with budesonide, liver enzyme levels declined in both patients. Maculopapular erythema in patient BT01 was treated with topical steroids and keratoconjunctivitis sicca in patient BT12 was treated with steroid eye drops. At the end of the trial, cGVHD in both patients had resolved.
EBV reactivation after adoptive B-cell transfer
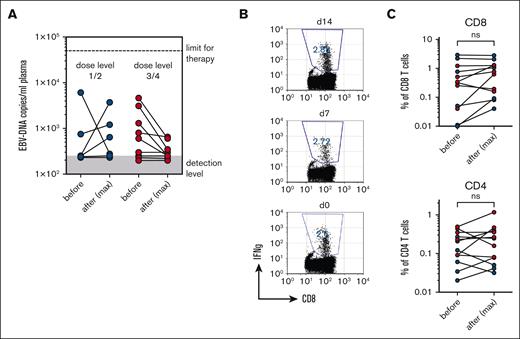
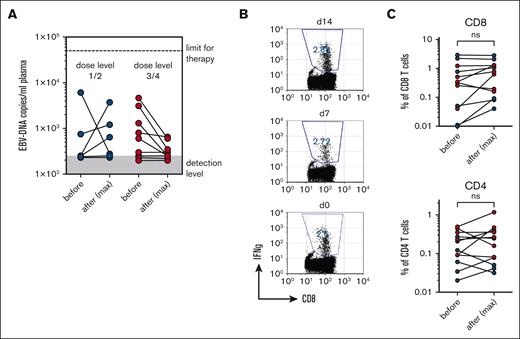
Among the 15 study patients, 13 had a positive EBV serostatus and received a graft from an EBV-seropositive donor (R1D1). Two of 15 patients had a positive EBV serostatus but received a graft from a seronegative donor (R1D0) without the risk of EBV transmission by the B-cell product. The copy numbers of EBV-DNA in plasma before B-cell transfer on V3 and the maximum copy numbers of EBV-DNA after transfer from V4 to V10 are indicated in Figure 2. Despite some fluctuations in copy numbers during the observation period, no clear rise was detectable in the low- or high-dose groups of patients (Figure 2A). Importantly, the copy number of EBV-DNA was not higher in the high-dose group, suggesting the absence of EBV reactivation even after the transfer of considerable B-cell numbers. EBV copy numbers remained below the level for therapeutic intervention (<50 000 copies per mL, Figure 2A) in all patients. To gain more insight into potential EBV reactivation, we also measured EBV–specific CD4– and CD8– T cells before and after B-cell transfer, with the reasoning that EBV reactivation might be undetectable by PCR in the plasma because of efficient T-cell responses in the patient. Peptide pools for a panel of 5 EBV proteins were used to determine the frequency of EBV–reactive CD4- and CD8- T cells using intracellular FACS analysis for specific IFNγ secretion (Figure 2B). EBV–specific CD8– and CD4– T cells were readily detectable in patients but did not significantly rise after adoptive transfer of B cells (Figure 2B). We conclude that B-cell transfer, even at the highest dose level, does not appear to increase the EBV reactivation risk in GVHD–free allo-HSCT recipients.
Reactivation of EBV after B-cell transfer. (A) EBV copy numbers before (V2) and after B-cell transfer. Maximal values of EBV copy numbers are depicted for observation periods V3 to V10. (B) Representative FACS analysis of IFNγ production in CD8+ T cells after stimulation with 5 PepTivator peptide mixes derived from EBV proteins (EBNA1, BMLF1, BZLF1, LMP1, and BRLF1). (C) Frequency of IFNγ-producing CD8 and CD4 cells after stimulation with EBV PepTivator peptide mixes. The respective maximal values of 2 time points analyzed after B-cell transfer (days 7 and day 14) are shown.
Reactivation of EBV after B-cell transfer. (A) EBV copy numbers before (V2) and after B-cell transfer. Maximal values of EBV copy numbers are depicted for observation periods V3 to V10. (B) Representative FACS analysis of IFNγ production in CD8+ T cells after stimulation with 5 PepTivator peptide mixes derived from EBV proteins (EBNA1, BMLF1, BZLF1, LMP1, and BRLF1). (C) Frequency of IFNγ-producing CD8 and CD4 cells after stimulation with EBV PepTivator peptide mixes. The respective maximal values of 2 time points analyzed after B-cell transfer (days 7 and day 14) are shown.
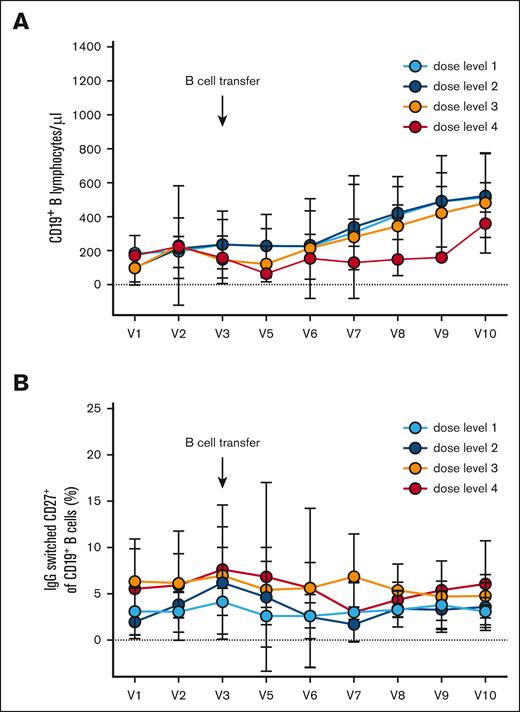
Reconstitution of B cells after adoptive B-cell transfer
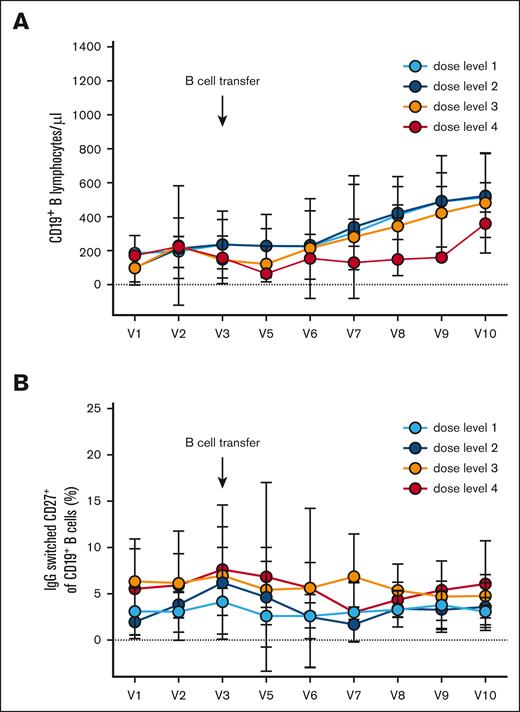
At different time points after adoptive B-cell transfer, we measured the number of total CD19+ B cells and IgG–switched CD27+ memory B cells at 4 different dose levels (Figure 3). The circulating B-cell number did not peak immediately after the transfer (from 184 ± 146 B cells per μL at V3 to 152 ± 124 B cells per μL at V5 after 7 days). B-cell counts increased from V3 to V10, however (267 ± 250 B cells per μL at V7 and 497 ± 220 B cells per μL at V10 4 months after B-cell transfer; Figure 3A). There were no significant differences in the B-cell numbers in the peripheral blood between the individual dose levels. The frequency of IgG–switched CD27+ memory B cells among all B cells did not increase significantly during the observation period (Figure 3B).
Longitudinal analysis of B-cell numbers and frequencies of IgG memory B cells. (A) Absolute number of CD19+ B cells over time. (B) Frequency of IgG–switched CD19+ CD27+ memory B cells over time. Mean ± standard deviation are shown for the 4 dose groups. V1 to V10 represent time points as shown in Figure 1.
Longitudinal analysis of B-cell numbers and frequencies of IgG memory B cells. (A) Absolute number of CD19+ B cells over time. (B) Frequency of IgG–switched CD19+ CD27+ memory B cells over time. Mean ± standard deviation are shown for the 4 dose groups. V1 to V10 represent time points as shown in Figure 1.
Effect of B-cell transfer on the mobilization of CD38high/CD27high PBs after vaccination
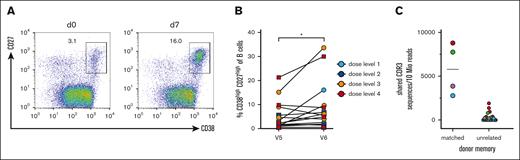
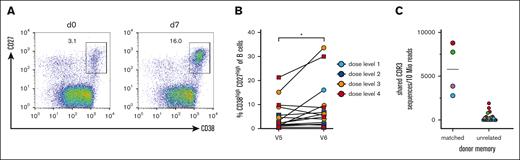
To obtain insight into the potential benefit of the transferred memory B cells, we conducted vaccination studies with a pentavalent DTaP-IPV-Hib vaccine and measured cellular responses by flow cytometry, as well as serological responses by ELISA. Seven to 10 days after the transfer of donor B cells, the patients were vaccinated. The increase in total and antigen–specific CD38high/CD27high PB on day 7 after vaccination is considered a response derived from memory B cells.22 Therefore, we analyzed the PB frequency before and on day 7 after vaccination (Figure 4A-B). Although the mobilization of circulating PBs was variable in the patients, we observed a significant increase in PBs after vaccination (mean percentage for day 0 5.6% ± 6.2% and 9.4 ± 10.3 for day 7, P < .05; Figure 4B).
PB responses after experimental vaccination. (A) Representative FACS analysis of gated CD19+ B cells for CD27 and CD38 on days 0 and 7 after vaccination. CD27high CD38high PBs are gated and the percentages of all CD19+ B cells are shown. (B) Summary of PB expansion in all patients. Dose levels of individual patients are depicted in different colors; ∗P < .05. (C) CDR3 clonotype sharing between PBs after experimental vaccination and memory cells from donor–derived B-cell products (left column) and unrelated memory preparations (right column). The color code corresponds to the PB samples. The CDR3 sequences are normalized to 10 million reads per sample. A mean of 1.6 × 105 CDR3 reads was obtained for sorted memory cells (range, 1.1 × 104 to 3.8 × 105), and a mean of 6.8 × 105 CDR3 reads was obtained from sorted PBs from 4 patients after experimental vaccination (range, 3.5 × 105 to 9.7 × 105).
PB responses after experimental vaccination. (A) Representative FACS analysis of gated CD19+ B cells for CD27 and CD38 on days 0 and 7 after vaccination. CD27high CD38high PBs are gated and the percentages of all CD19+ B cells are shown. (B) Summary of PB expansion in all patients. Dose levels of individual patients are depicted in different colors; ∗P < .05. (C) CDR3 clonotype sharing between PBs after experimental vaccination and memory cells from donor–derived B-cell products (left column) and unrelated memory preparations (right column). The color code corresponds to the PB samples. The CDR3 sequences are normalized to 10 million reads per sample. A mean of 1.6 × 105 CDR3 reads was obtained for sorted memory cells (range, 1.1 × 104 to 3.8 × 105), and a mean of 6.8 × 105 CDR3 reads was obtained from sorted PBs from 4 patients after experimental vaccination (range, 3.5 × 105 to 9.7 × 105).
To gain direct molecular evidence that memory B-cell clones from the donor were able to respond to the vaccine, we sorted PBs from patients on day 7 after vaccination. The immunoglobulin heavy chain repertoire was bulk-sequenced using immunorepertoire sequencing (iRepertoire). We compared the CDR3 sequences from reisolated and FACS-sorted CD27hi CD38hi PBs from allo-HSCT recipients after vaccination with the CRD3 sequences of donor CD27+ memory B cells in the preinfusion B cell product. As a control for recurrent CDR3 sequences in the human population, we compared CD27hiCD38hi PB CDR3 sequences with memory B-cell preparations from other unrelated donors. For both comparisons, the clonal overlap was calculated. The results for 4 patients evaluable for PB-sorting on day 7 after vaccination are shown in Figure 4C. For all 4 patients, identical CDR3 sequences were found among memory cells from the donor and corresponding PBs isolated from the patient. The frequency was much higher for matched pairs than for several unmatched memory B cells that were sequenced. These results demonstrate that in all available samples memory B-cell clones from the donor responded to the patient after vaccination.
Vaccine-specific ASC on day 7 after vaccination
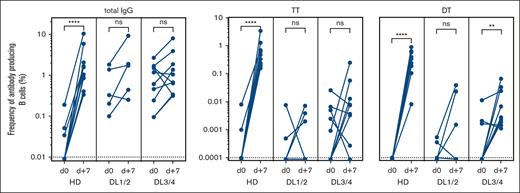
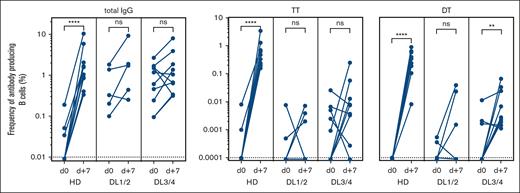
In addition to the general PB response after vaccination, we measured antigen-specific ASCs in patients using ELISPOT and compared the results with those of a small cohort of healthy donors after booster vaccination. Examples of the resulting spots for HD and DL1 patients before and 7 days after vaccination with TT- and DT-containing vaccines are shown in supplemental Figure 1. A threshold of detection of 1 of 100 000 B cells was set as the lower level of detection.
For TT and DT, specific PBs were undetectable before vaccination in the HDs and control patients. As expected, a significant and variable increase was observed in HDs after booster vaccination, whereas the response was expectedly lower for patients with allo-HSCT than for healthy donors (Figure 5).
Antigen-specific ASC after experimental vaccination. Frequencies of total IgG and TT and DT–specific antibody–producing cells were enumerated by ELISPOT on day 0 and day +7 after vaccination. The responses of 10 healthy donors (HD), 5 patients receiving low doses of B cells (DL1/2), and 9 patients receiving a high-dose (DL3/4) of B cells are depicted. The frequency was calculated as the percentage of B cells seeded in ELISPOT wells. ns, not significant; ∗∗P < .01; ∗∗∗∗P < .0001. Dotted lines indicate the lower detection limits.
Antigen-specific ASC after experimental vaccination. Frequencies of total IgG and TT and DT–specific antibody–producing cells were enumerated by ELISPOT on day 0 and day +7 after vaccination. The responses of 10 healthy donors (HD), 5 patients receiving low doses of B cells (DL1/2), and 9 patients receiving a high-dose (DL3/4) of B cells are depicted. The frequency was calculated as the percentage of B cells seeded in ELISPOT wells. ns, not significant; ∗∗P < .01; ∗∗∗∗P < .0001. Dotted lines indicate the lower detection limits.
A higher frequency of patients in the DL3/4 group responded with elevated ASCs against TT and DT compared with the DL1/2 group (78% vs 40% for TT; 78% vs 60% for DT) (Figure 5). In summary, the higher frequencies of vaccine-specific ASCs and the higher number of vaccine-responding patients in the DL3/4 study group indicated a dose-dependent effect on cellular response on day 7 after vaccination.
Serum antibody response after vaccination in patients after B-cell transfer
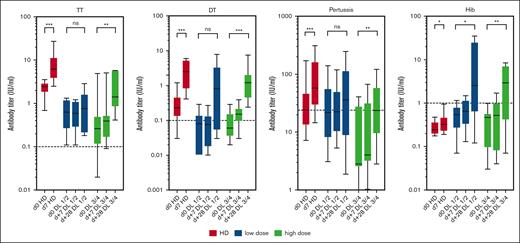
As additional information on vaccine responses after adoptive B-cell transfer, serum antibodies against TT, DT, PT, and Hib were measured serially over the entire vaccination schedule. For poliovirus, a quantitative ELISA was not available. Although booster vaccinations in healthy donors resulted in measurable antibody responses already on day 7 after vaccination, this early antibody response was absent in most of the patients (Figure 6). However, 4 weeks after booster vaccination, specific antibody titers increased significantly for the high-dose groups of our patients for all 4 antigens tested (Figure 6). Altogether, the finding that patients with a higher B-cell dose showed a stronger response to all vaccines tested suggests that memory B cells from the donor responded to recall antigens. As expected, further immunizations at weeks 4, 8, 12, and 16 after the first immunization further increased the specific antibody titers (supplemental Figure 2).
Serum responses after experimental vaccination. The serum response on days 0, 7, and 28 after a single vaccination was measured using an ELISA with calibrated standard antisera. Data are shown for HD (red bars), dose groups DL1/2 (blue bars), and DL3/4 (green bars) as box plots. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Dotted lines indicate the protective titer of individual vaccine antigens.
Serum responses after experimental vaccination. The serum response on days 0, 7, and 28 after a single vaccination was measured using an ELISA with calibrated standard antisera. Data are shown for HD (red bars), dose groups DL1/2 (blue bars), and DL3/4 (green bars) as box plots. ns, not significant; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Dotted lines indicate the protective titer of individual vaccine antigens.
Discussion
Our phase 1/2a first-in-human study on adoptively transferred donor B cells was designed to evaluate the safety (ie, EBV reactivation and GVHD) and efficacy aiming to improve the humoral immunity of patients after allo-HSCT.
To be able to identify the side effects caused by the B-cell product, only patients with resolved acute GVHD or mild cGVHD and patients with EBV-DNA <10 000 copies per mL were included in the study. The B-cell products were well tolerated without acute toxicities and only in 3 out of 15 patients higher-grade AEs were observed.
Regarding the transmission of EBV by the B-cell product from a seropositive donor, no significant increase in EBV copy numbers occurred; thus, no rituximab therapy was required. In addition, no significant T-cell reactivity against EBV–specific CD4 or CD8 epitopes was detected. These findings are important because they show that, in the absence of inflammation, the EBV lytic program appears to be controlled,23 and the risk for EBV–related posttransplantation lymphoproliferative disease is low even when high numbers of B cells are adoptively transferred.
Induction of GVHD after B-cell transfer was only observed in patients with previous acute or cGVHD. In the 3 patients without GVHD before B-cell transfer, no subsequent GVHD was observed. Only a minority of patients with a history of GVHD before B-cell transfer (4/12 patients) developed GVHD after B-cell transfer. GVHD after B-cell transfer was not severe and all patients responded to first-line therapy. In 3 of 4 cases, GVHD resolved after topical treatment (cutaneous steroids, eye drops, and budesonide for skin, eyes, and GI/liver, respectively) and only in 1 of 4 patients with GVHD reactivation after B-cell transfer required systemic low-dose steroids.
One reason for the low rate of acute GVHD could be the very low content of T cells in our B-cell products, with a maximum acceptable contamination of 3 × 104 CD3+ T cells per kg b.w. of the recipient, which is the critical threshold number for T cells in haploidentical HSCT.24 This low level of T cell contamination was achieved using a 2-stage cell separation strategy consisting of a T cell-depletion step followed by magnetic B-cell enrichment.17
Even though the study cohort was small, several patients had risk factors for cGVHD (female donor, preexisting acute GVHD) and B cells are thought to play a pathogenetic role in the development of cGVHD.16,25,26 Nevertheless, the incidence of cGVHD was low in the study cohort and it remains unclear whether B-cell transfer itself was causative for GVHD progression. B cells were applied on day 148 (mean value) after allo-HSCT when the withdrawal of calcineurin inhibitors was completed, but B lymphopenia persisted. Our observation that the transfer of significant B-cell numbers at this time point after allo-HSCT did not induce or aggravate cGVHD suggests that dysregulation of the B-cell compartment for cGVHD induction occurs at earlier time points. Our study was done in HCT recipients who received ATG as a GVHD prophylaxis. Whether a similar safety profile can be obtained with other GVHD prophylaxis regimens is unknown.
For patients receiving high numbers of donor B cells, we expected a rise in peripheral B-cell counts. However, we did not detect a measurable increase in total B cells or memory B cells in the PB. Based on the BCR-repertoire analysis, adoptively transferred B cells seem to persist, yet in B lymphopenic hosts, they seem to preferentially repopulate major lymphoid organs, such as the spleen and lymph nodes, which usually harbor 5 × 1010 to 10 × 1010 B cells in healthy individuals.27 In the human memory B-cell compartment, it has recently been shown that the spleen represents an archive of recirculating memory B cells.28 Although detailed studies on the B-cell compositions of lymphoid organs after allo-HSCT are not available, it can be expected that conditioning and the graft-versus-leukemia effect eradicate most, if not all, recipient B cells from the spleen. The long-lasting deficiency in memory B cells (despite the normalization of absolute B-cell numbers over time) supports the model that the memory archive only slowly replenishes after allo-HSCT.20,29 Adoptive transfer of memory B cells from the SCT donor might improve memory B-cell replenishment.
In addition to test the safety and feasibility of adoptive B-cell transfer, we also examined whether donor memory B cells are functionally active in the recipient. We examined the mobilization of PBs as early as day 7 after the first vaccination and analyzed the response of the memory B cells. A significant increase in total PB frequency and DT-specific ASCs in the high-dose B-cell transfer group indicated a memory response. The molecular relationship of in vivo expanded CDR3-clonotypes among responding PBs to clonotypes in the donor memory B-cell pools proved their donor origin. We cannot formally rule out that individual memory clones were already transferred with the initial stem cell graft, but the dose-dependent efficacy data strongly suggest a contribution from adoptively transferred B cells. Overall, these data for the first time provide direct molecular evidence that memory B cells from the donor get activated in the recipient. The fact that serum antibody responses measured 4 weeks after the first immunization resulted in higher antibody titers in the high B-cell transfer groups than in the low-dose groups for the 4 vaccine antigens also indicates a specific memory response from the transferred donor B cells. In summary, direct and indirect evidence indicates that donor memory B cells responded in the recipient to antigenic challenges. Similar observations on humoral immunity transfer have recently been shown against severe acute respiratory syndrome coronavirus 2in donor and recipient pairs after allo-HSCT.30
In conclusion, our data showed that the transfer of donor B cells into allo-HSCT recipients is feasible and generally safe. Furthermore, adoptive B-cell transfer results in enhanced memory B-cell responses. These results warrant further investigations on adoptive B-cell immunity against viral infections for patients who underwent allo-HSCT.
Acknowledgments
The authors thank all research and clinical staff for their work supported this trial and all patients, their families, and volunteer stem cell donors.
This study was supported by Deutsche Forschungsgemeinschaft (SFB643 project number 5486111) to J.W., A.M., M.M., and T.H.W. and (TRR221 project no. 324392634) to J.W., D.W., A.M., M.E., and T.H.W.
Authorship
Contribution: J.W., S.M., M.M., A.M., and T.H.W. conceived and designed the study; J.W., H.T., I.V., J.S., S.H., L.K., D.W., and M.E. recruited patients and collected data; J.W., H.T., A.S., S.H., B.M.S., R.R., and T.H.W analyzed the data; J.W., M.E., A.M., and T.H.W. wrote the manuscript; and all authors interpreted the data, were involved in the development and review of the manuscript, and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julia Winkler, Department of Internal Medicine 5, Hematology/Oncology, University Hospital Erlangen, Ulmenweg 18, Erlangen N/A 91054, Germany; email: julia.winkler@uk-erlangen.de.
References
Author notes
Data are available on request from the corresponding author, Julia Winkler (julia.winkler@uk-erlangen.de).
The full-text version of this article contains a data supplement.