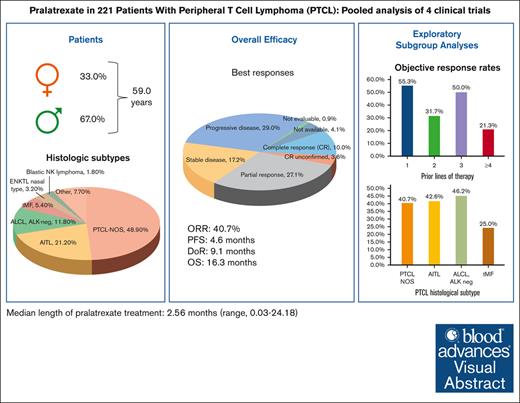
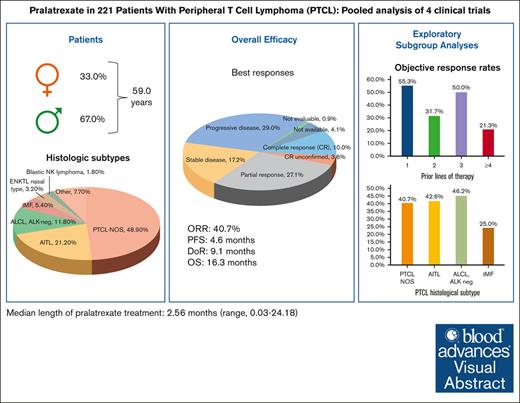
Pooled analysis confirms pralatrexate clinical activity in heavily pretreated patients with R/R PTCL.
Exploratory analyses suggest that certain populations of patients might derive more benefit from pralatrexate therapy.
Visual Abstract
Patients with relapsed or refractory (R/R) mature natural killer cell and T-cell lymphoma have limited treatment options. To evaluate pralatrexate’s performance and factors influencing its safety and efficacy in R/R peripheral T-cell lymphoma (PTCL), we performed a pooled analysis of data from 4 similarly designed, regulatory-mandated prospective clinical trials. Of 221 patients (median age, 59 years; 67.0% male) in the study population, 48.9% had PTCL not otherwise specified (PTCL-NOS), 21.3% angioimmunoblastic T-cell lymphoma, and 11.8% ALK-negative anaplastic large cell lymphoma (ALCL). Patients received pralatrexate for a median of 2.56 months (range, 0.03-24.18) and had a 40.7% objective response rate with a median duration of response of 9.1 months, progression-free survival 4.6 months, and overall survival 16.3 months. The most common treatment-related all-grade adverse events were stomatitis, thrombocytopenia, white blood cell count decrease, pyrexia, and vomiting. Subgroup exploratory analyses suggest improved efficacy with 1 prior line of chemotherapy vs 2 or ≥4 prior lines; PTCL-NOS or ALCL vs transformed mycosis fungoides; chemotherapy and transplant before pralatrexate vs chemotherapy alone or chemotherapy with other nontransplant treatments. In conclusion, these pooled analysis results further support using pralatrexate in patients with R/R PTCL. Prospective studies are needed to confirm the findings of subgroups analyses.
Introduction
Peripheral T-cell lymphomas (PTCLs), a category of rare non-Hodgkin lymphomas, also referred to as mature natural killer (NK) cell and T-cell lymphoma, include 29 subtypes.1,2 The estimated US annual incidence of the 2 most common forms of PTCL is 2500 cases of PTCL not otherwise specified (PTCL-NOS) and 1800 cases of angioimmunoblastic T-cell lymphoma (AITL).2 There is no widely accepted standard of care for patients with these diseases due to limited and inconclusive data from mostly small noncomparative studies and a few randomized prospective trials. Patients eligible for anthracycline with PTCL-NOS, AITL, and other aggressive PTCLs often receive firstline chemotherapy with cyclophosphamide, doxorubicin, vincristine, prednisone, and with or without etoposide.3 Despite inconclusive data, many physicians in Western countries recommend consolidation therapy with autologous stem cell transplantation (SCT) for patients with PTCL who achieve first remission.2 Patients with PTCL-NOS and AITL have a 32% estimated 5-year overall survival (OS).4
Patients with relapsed or refractory (R/R) PTCL marginally benefit from traditional chemotherapy.5 A registry data analysis reported a median OS of 29.1 months for relapsed disease and 12.3 months for refractory disease.6 Another analysis from this registry noted longer OS and progression-free survival (PFS) with monotherapies (ie, pralatrexate, romidepsin, belinostat, brentuximab vedotin, bendamustine, alisertib, denileukin diftitox, and lenalidomide) than with combination chemotherapy in R/R PTCL.7 A large cohort study reported median 4-month event-free survival, 9.1-month OS, and 34% 3-year OS in patients with R/R PTCL and disease progression.8
There is no consensus on a standard of care for patients with R/R PTCL, and there is a lack of comparative effectiveness data for monotherapies.2 Studies on patients with R/R PTCL are difficult to perform and interpret because these diseases are rare with nearly 30 distinct subtypes. Patients who are not eligible for SCT or unable to participate in a clinical trial are candidates for palliative chemotherapy, typically as a monotherapy to reduce untoward toxicity and attempt to control disease-related symptoms.2 Combination chemotherapy is typically associated with more toxicity, which can limit the number of cycles that can be administered, and short duration of benefit. Chemotherapy agents such as gemcitabine and, to a lesser extent, bortezomib, bendamustine, lenalidomide, and etoposide have all been used off-label in R/R PTCL, despite limited data supporting their use.2
To date, 4 drugs have been approved by the US Food and Drug Administration for patients with R/R PTCL. These include the antifolate pralatrexate, the histone deacetylase inhibitors belinostat and romidepsin (recently withdrawn secondary to a negative phase 4 clinical trial9), and the anti-CD30 monoclonal antibody brentuximab vedotin, which was only approved in the R/R setting for patients with anaplastic large T-cell lymphoma.2 The histone deacetylase inhibitor chidamide (also called tucidinostat) was approved in China for the treatment of R/R PTCL.10,11 Tucidinostat was approved in Japan for R/R PTCL12 and R/R adult T-cell leukemia-lymphoma.13,14 The defucosylated anti-CCR4 monoclonal antibody mogamulizumab has approval in Japan for previously untreated and R/R adult T-cell leukemia-lymphoma15-17 and for R/R PTCL.18 These approved monotherapies for R/R PTCL have distinct advantages and limitations. Without comparative studies, medical oncologists/hematologists must critically assess the limited available data to individualize treatment recommendations.
Pralatrexate was the first drug approved in the United States for R/R PTCL, based on data from the single-arm, phase 2, multicenter PROPEL trial including patients from Canada, Europe, and the United States.19 Subsequently, pralatrexate attained drug registration/regulatory approval in other countries based on results from PROPEL19 and the following locally mandated, single-arm regulatory studies: a confirmatory phase 3 trial (FOT12-CN-301) conducted in China20; a phase 4 trial (FOT14-TW-401) conducted in Taiwan21; and a phase 1/2 trial (PDX-JP1) conducted in Japan.22 Here, we have performed a pooled analysis of the PROPEL, FOT12-CN-301, FOT14-TW-401, and PDX-JP1 (only phase 2 part) trials to generate a more robust data set for evaluating pralatrexate’s performance and factors influencing its safety and efficacy in R/R PTCL.
Methods
Clinical trials
We analyzed pooled patient-level or summary-level data from the clinical study reports of 4 prospective clinical trials of pralatrexate monotherapy in patients with R/R PTCL (supplemental Figure 1).19-22 Of 231 patients in the safety analysis set (defined as all patients who received ≥1 pralatrexate dose), 221 were deemed evaluable for efficacy. Data were collected in accordance with the original protocol for each study and, when available, updated with longer survival follow-up. The institutional review boards or ethics committees at the participating centers approved the respective studies, which were conducted in accordance with the Declaration of Helsinki and its relevant amendments and with the International Conference on Harmonization Guidelines for Good Clinical Practice.
Patients
The 4 pooled studies had very similar inclusion and exclusion criteria (supplemental Table 1).19-22 In each study, the PTCL histologic subtypes were confirmed by central review. All participating patients provided written informed consent.
The PROPEL study19 required that patients have a diagnosis of PTCL per the 2000 Revised European Lymphoma (REAL) and World Health Organization (WHO) classification of lymphoid neoplasm criteria,23 with documented disease progression after ≥1 prior treatment. The FOT12-CN-301 study20 required patients have a PTCL WHO 2008 diagnosis,24 with disease progression after ≥1 prior systemic therapy and with an enlarged lymph node or extranodal mass 1.5 cm. The FOT14-TW-401 study21 required patients have a PTCL diagnosis per National Comprehensive Cancer Center diagnosis criteria, revised REAL, and WHO classification with documented disease progression after prior therapy. The PDX-JP1 study22 required that patients have PTCL per 2008 WHO classification24 after ≥1 prior antitumor therapy (not including systemic corticosteroid monotherapy).
Treatments and assessments
In all 4 trials, patients received pralatrexate at a dose of 30 mg/m2 IV over 3 to 5 minutes, weekly for 6 weeks every 7 weeks (1 week off), and started vitamin B12 and folic acid supplementation ≥10 days before the first dose of pralatrexate.19-22 All 4 studies assessed patients every 2 cycles. In the PROPEL19 and FOT12-CN-30120 studies, response assessments were performed within 7 days before the first dose of every even-numbered subsequent cycle. In the FOT14-TW-401 study,21 response assessments were performed at the end of cycles 1 (week 7), 3 (week 21), and 5 (week 35) and after the last dose of treatment beyond cycle 5. In the PDX-JP1 study,22 response assessments were performed at week 7 of odd-numbered cycles.
Statistical analysis
The primary efficacy end point across all trials was the objective response rate (ORR), assessed by central review using the International Working Group criteria.19-22,25,26 Secondary efficacy end points included duration of response (DoR), PFS, OS, and responses in patients who had undergone SCT after pralatrexate treatment. DoR was assessed from the first day of documented response until progressive disease (PD) or death and PFS and OS from the first treatment day until an event or censoring in the PROPEL study.19
Data from the studies were combined and reported as observed. Assessments of the heterogeneity between the studies indicated that there was some variability in the observed treatment effects in the studies.
ORR, PFS, and OS were analyzed for the following subgroups: R/R status at most recent treatment, number of lines of prior chemotherapy, type of prior chemotherapy, and histologic subtype. Relapse was defined as achieving a complete response (CR) or partial response (PR) on prior therapy lasting for ≥3 months. Refractory was defined as stable disease or PD on prior therapy or relapsed disease <3 months of achieving CR or PR. These analyses considered fixed and random effects models.
Results
Patient characteristics
The pooled efficacy population (n = 221) had a median age of 59 years (range, 21-89) and was predominantly male (67.0%) (Table 1). Most patients (89%) had an Eastern Cooperative Oncology Group performance status of <2. The 3 most common PTCL histologic subtypes were PTCL-NOS (48.9%), AITL (21.3%), and ALK-negative anaplastic large cell lymphoma (ALCL; 11.8%). Patients had received a median of 2.0 (range, 1-14) prior systemic regimens, and most patients had received 1 (34.4%) or 2 prior lines of systemic therapy (27.1%). At the most recent prior therapy, 53.9% of patients had refractory disease, and 24.4% had relapsed. Among the types of prior treatment for PTCL, all patients had received chemotherapy, 22.2% radiation therapy, and 13.1% SCT. The most commonly used types of prior chemotherapy were anthracycline (89.6%), platinum agents (40.7%), and gemcitabine (30.3%). As the best response to their most recent prior regimen, 19.9% of patients had a CR and 17.6%, a PR. Baseline patient characteristics in the individual studies are shown in supplemental Table 2.
Exposure and safety
The median pralatrexate treatment duration was 2.56 months (range, 0.03-24.18), with a median dose intensity of 25.8 mg/m2 per week (range, 11.7-31.7). Table 2 lists treatment-emergent AEs (TEAEs) reported in ≥10% of the total safety population from the 4 studies. The most frequently reported all-grade TEAEs were stomatitis (65.8%), anemia (39.8%), nausea (33.8%), thrombocytopenia (32.9%), and pyrexia (30.3%).
The safety analyses by worst grade severity (grades 3-5; shown in Table 2) and by relationship to study treatment excluded patients in the PDX-JP1 study21 due to a lack of accessible data from that study. Among the safety population of 209 patients from the PROPEL, FOT12-CN-301, and FOT14-TW-401 studies, the most common grade 3 and 4 TEAEs were thrombocytopenia (18.2% and 12.9%), stomatitis (17.7% and 1.9%), anemia (16.3% and 2.9%), and neutropenia (12.9% and 6.7%). The most frequently (ie, ≥10%) reported all-grade TEAEs related to study treatment were stomatitis (35.4%), thrombocytopenia (26.3%), white blood cell count decrease (18.2%), pyrexia (17.2%), vomiting (16.3%), anemia (11.0%), nausea (11.0%), and epistaxis, fatigue, mucosal inflammation, and rash (10.0%, each). The 4 pooled studies were conducted before the implementation of leucovorin prophylaxis to mitigate stomatitis and oral mucositis.
Serious TEAEs occurred in 110 patients (47.6%). Of the 6 patients (2.6%) who had TEAEs leading to death, 1 patient had infectious diarrhea, pneumonia, and sepsis, and 1 patient each had pneumonia, febrile neutropenia, lung infection, septic shock, or acute hepatic failure.
In the PROPEL study,19 23% of patients had a dose reduction for mucositis and withdrew from treatment due to AEs, primarily mucositis and thrombocytopenia. In FOT12-CN-301,20 46% of patients had dose reductions, primarily due to mucositis. In PDX-JP1,22 28% of patients had dose reductions, primarily due to mucositis, and 24% had AEs leading to treatment discontinuation. In FOT14-TW-401,21 22.2% of patients had dose reductions, primarily due to mucositis, neutropenia, and thrombocytopenia.
Efficacy
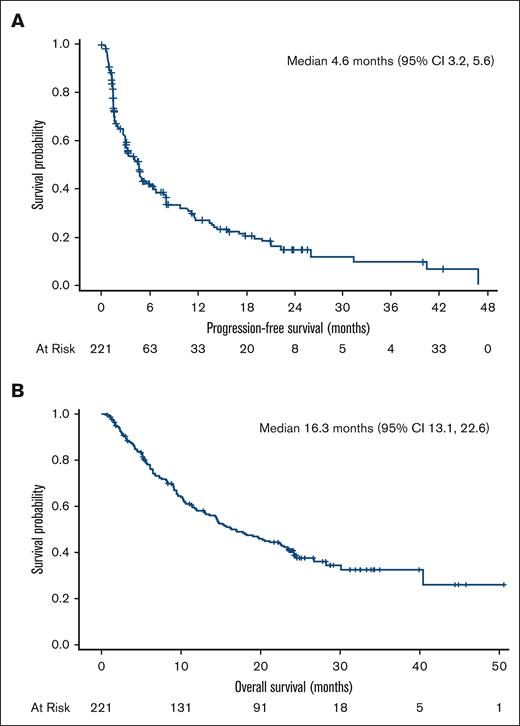
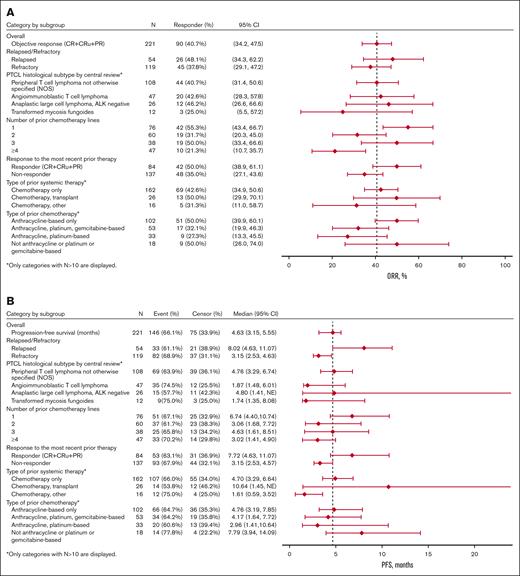
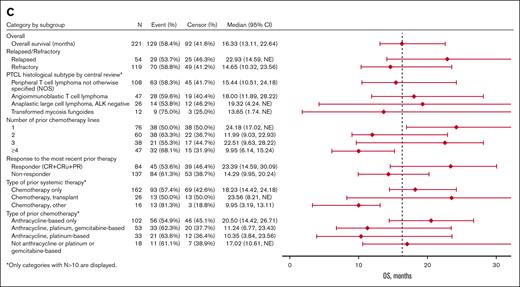
Among the pooled efficacy population of 221 patients, 10.0% had a CR, 3.6% had a CR unconfirmed (CRu), and 27.1% had a PR as their best overall response determined by central review, leading to a 40.7% ORR (95% confidence interval [CI], 34.2-47.5) (Table 3). The 90 responders with a CR, CRu, or PR had a median DoR of 9.1 months (95% CI, 7.4-10.8). The median PFS was 4.6 months (95% CI, 3.2-5.6), and the median OS was 16.3 months (95% CI, 13.1-22.6) (Figure 1A-B).
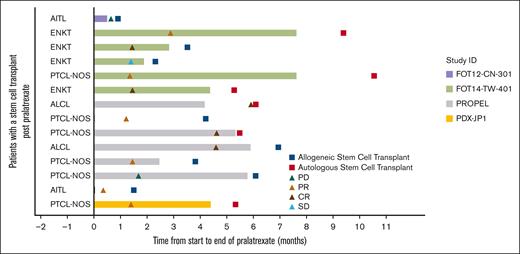
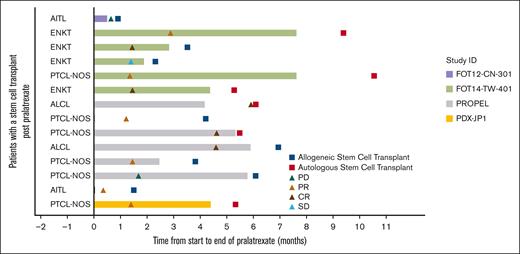
Fourteen patients (6.3%) underwent autologous (n = 6) or allogeneic SCT (n = 8) after pralatrexate treatment. The best overall responses reported in these patients were 5 CRs (35.7%), 6 PRs (42.9%), 1 stable disease (7.1%), and 2 PD (14.3%). This subgroup of patients had 78.6% ORR. The swimmer plot in Figure 2 illustrates the timeline of events for this group of patients.
Response in patients with SCT after pralatrexate treatment. AITL, angioimmunoblastic T-cell lymphoma; ENKT, extranodal NK/T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, analplastic large T-cell lymphoma.
Response in patients with SCT after pralatrexate treatment. AITL, angioimmunoblastic T-cell lymphoma; ENKT, extranodal NK/T-cell lymphoma; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; ALCL, analplastic large T-cell lymphoma.
Subgroup analyses
Exploratory subgroup analyses showed nonstatically significant trends favoring certain subgroups (Table 4; Figure 3). In the subgroup analysis by R/R status at most recent treatment, the relapse subgroup had numerically higher ORR (48% vs 38%), median PFS (8.0 vs 3.2 months), and median OS (22.9 vs 14.7 months) than the refractory subgroup, respectively. In the subgroup analysis by prior chemotherapy lines, the 1 prior line subgroup had higher ORR (55%), PFS (6.7 months), and OS (24.2 months) than the 2 prior lines (ORR, 32%; PFS, 3.1 months; OS, 12.0 months) and ≥4 prior lines (ORR, 21%; PFS, 3.0 months; OS, 10.0 months) subgroups. The efficacy outcomes of the 1 prior line subgroup were similar to the 3 prior line subgroup (ORR, 50%; PFS, 4.6 months; OS, 22.5 months). In the subgroup analysis by the type of prior chemotherapy, the anthracycline-based chemotherapy only subgroup (ORR, 50%; PFS, 4.8 months; OS, 20.5 months) and the no anthracycline or platinum or gemcitabine-based chemotherapy subgroup (ORR, 50%; PFS, 7.8 months; OS, 17.0 months) had numerically higher ORR, PFS, and OS than the other subgroups. In the histology subtype analysis, the PTCL-NOS (ORR, 41%; PFS, 4.8 months; OS, 15.4 months) and ALCL (ORR, 46%; PFS, 4.8 months; OS, 19.3 months) subgroups had numerically higher efficacy outcomes than the transformed mycosis (ORR, 25%; PFS, 1.7 months; OS, 13.6 months) subgroup. The AITL (ORR, 43%; PFS, 1.9 months; OS, 18 months) subgroup had similar ORR but shorter PFS and OS than the PTCL-NOS and ALCL subgroups.
Regarding therapies before initiation of pralatrexate (Table 4; Figure 3), the prior chemotherapy and transplant subgroup had higher ORR, median PFS, and median OS (50%, 10.6 months, and 23.6 months, respectively) than the chemotherapy alone (43%, 4.7 months, and 18.2 months, respectively) or chemotherapy and other agents (31%, 1.6 months, and 10.0 months, respectively) subgroups.
Discussion
Before the availability of novel drugs, there had been little guidance on treatment for PTCL, and clinicians had to extrapolate treatments based on their experience treating patients with B-cell lymphomas, with minimal recognition that T-cell malignancies are different from B-cell neoplasms. PTCLs are very aggressive and heterogenous lymphoid malignancies recognized for their relative resistance to traditional chemotherapy. Extrapolation of treatments from B-cell lymphoma experiences often failed to recognize the paucity of data for a treatment in patients with PTCL, which has substantially greater unmet medical need. In fact, early phase experiences with pralatrexate demonstrated sharply greater activity in patients with PTCL than those with B-cell lymphomas, hence the basis for PROPEL. Our data provide insights into the activity of pralatrexate in this heterogeneous R/R population from the global clinical trial experience and provide substantially more data for pralatrexate across not only a more diverse population of patients but also in some of the rarer PTCL subtypes.
The results of our pooled analysis confirm the marked clinical activity of pralatrexate in heavily pretreated patients with R/R PTCL, with a 40.7% ORR and a median DoR of 9.1 months, PFS 4.6 months, and OS 16.3 months. Patients who received SCT after pralatrexate had 78.6% ORR. The most frequently reported, treatment-related all-grade TEAEs were stomatitis, thrombocytopenia, white blood cell count decrease, pyrexia, and vomiting.
The 4 analyzed pralatrexate monotherapy studies reported variable ORRs of 29% (CR, 10%; CRu, 1%; PR, 18%) in PROPEL,19 52% (CR, 9%; CRu, 11%; PR, 32%) in FOT12-CN-31,20 71% (CR, 14%; OR, 57%) in FOT14-TW-401,21 and 45% (CR, 10%; PR, 35%) in PDX-JP1.22 Study sample size and differences in the number of lines of prior therapy are the most likely reasons for this wide variation in ORR. FOT14-TW-40121 and PDX-JP122 only included 20 to 21 patients compared with 109 patients in PROPEL19 and 71 patients in FOT12-CN-31.20
Results of our pooled subgroup exploratory analyses suggest that certain populations of patients with R/R T-cell lymphoma may derive the most benefit from pralatrexate therapy. Numerically higher ORR, PFS, and OS were seen among patients with only 1 prior line of chemotherapy vs 2 and ≥4 prior lines, patients with PTCL-NOS or ALCL vs transformed mycosis, and patients who had received chemotherapy and transplant before pralatrexate vs chemotherapy alone or chemotherapy with other nontransplant treatments. Interestingly, patients with AITL exhibited similar ORR to patients with PTCL-NOS and ALCL.
The subgroup of 47 patients with R/R AITL had an ORR of 43% (95% CI, 28%-58%), which is lower than the ORR of 52% (95% CI, 34-70) reported in a pooled subset analysis27 of data from 29 patients with R/R AITL treated with single-agent pralatrexate in the FOT12-CN-301 and PDX-JP1. This discrepancy is likely attributed to smaller patient numbers and may be influenced by the lower 8% ORR (95% CI, 0-36) in the 13 patients with R/R AITL in PROPEL.19 Interestingly, the ORR was 55% (95% CI, 32-77) among 20 patients with R/R AITL in FOT12-CN-301,20 71% among 5 patients with R/R AITL in FOT14-TW-401,21 and 44% (90% CI, 17-75) among 9 patients with R/R AITL in PDX-JP1.22 Given the rarity of AITL and the need to conduct subset analysis to tease out these effects, results of individual trials are not powered to adequately define the “real” ORR in AITL or any other PTCL subtype. Studies that include real-world data provide some further evidence of pralatrexates’ activity in AITL. For example, a retrospective real-world analysis that reported a 41% ORR (95% CI, 21-64) among 27 patients with R/R PTCL-NOS or AITL treated with pralatrexate noted no significant differences in ORR between the 2 small subgroups, albeit there was 1 CR among patients with AITL and 4 among patients with PTCL-NOS.28 A propensity score case-matched analysis reported a median OS of 9.8 months (95% CI, 2.2-10.2) for 12 patients with R/R AITL treated with pralatrexate in the PROPEL study and 5.5 months (95% CI, 0.4-8.2) for 12 patients with R/R AITL treated with other therapies.29 In contrast, the 47 patients with R/R AITL included in our pooled analysis had a median OS of 18 months (95% CI, 11.9-28.2).
The 4 pooled studies shared a very similar design, and patient inclusion/exclusion circumvented some common limitations of pooled analyses such as heterogeneity in study methodology and patient populations. However, there were some differences in the included histologic subtypes. For example, the PROPEL study19 allowed the inclusion of patients with extranodal NK cell/T-cell lymphoma unspecified, whereas the 3 other studies20-22 allowed the inclusion of only patients with extranodal NK cell/T-cell lymphoma nasal type. Interpretation of our analysis is limited by the fact that pralatrexate clinical trials were designed before the availability of other novel single agents for R/R PTCL. Despite this, our pooled analysis provides clinically applicable insights, considering the current lack of consistency in the use of monotherapies for patients with most types of R/R PTCL. After the clinical development and approval of pralatrexate, researchers have tried to limit their study populations to include fewer PTCL subtypes, reducing heterogeneity at the expense of limiting availability of data for underrepresented subtypes. Despite the small numbers in individual studies, our pooled analysis captures the broader R/R PTCL population and provides some insights on pralatrexate activity in this difficult-to-treat population.
Pralatrexate is being studied in combination regimens in patients with PTCL. A phase 1 trial of romidepsin plus pralatrexate reported a 71% ORR (including 4 CRs) and a median PFS of 4.4 months (95% CI, 1.2 to not achieved) in 14 patients with PTCL.30 The ongoing NCT03240211 phase 1b study is testing pembrolizumab with decitabine and/or pralatrexate in patients with PTCL or cutaneous T-cell lymphoma. The ongoing NCT03598998 phase 1/2 trial is studying the combination of pembrolizumab and pralatrexate in patients with R/R PTCL.
In conclusion, data from 3 additional regulatory-mandated clinical studies meaningfully augment the US registration PROPEL data supporting the use of pralatrexate in patients with R/R PTCL. Furthermore, results from pooled subgroup exploratory analyses suggest that certain populations of patients with R/R PTCL might derive more benefit from pralatrexate therapy. Prospective studies in these subgroups are needed to confirm these findings.
Acknowledgments
The authors thank the patients who participated in the included studies. The authors also thank Diep Gray, Pia Patron, and Monica Araujo for support in data collection and statistical analyses.
This research was funded by Mundipharma Singapore Holdings Pte Ltd. O.A.O. is an American Cancer Society research professor. Medical writing assistance was provided by Phillips Gilmore Oncology Communications, Inc, and funded by Mundipharma Singapore Holdings Pte Ltd.
Authorship
Contribution: O.A.O. and E.-M.Y. designed and planned the study; O.A.O., B.-S.K., M.-C.W., D.M., Y.S., and K.T., who were also investigators of the individual country studies, acquired and analyzed the data; O.A.O. and E.-M.Y. interpreted the data; and all authors wrote, reviewed, and/or revised the manuscript and read and approved the final manuscript.
Conflict-of-interest disclosure: O.A.O. has received research support from Merck and Astex; and is on the board of directors for Kymera, Myeloid Therapeutics, and Dren Pharmaceuticals, for which he receives an honorarium and is an equity holder. D.M. receives research funding from Astellas Pharma, Celgene, Novartis, Chugai, Ono, Takeda, Janssen, Sanofi, MSD, Otsuka, Eisai, AbbVie, Amgen, and BMS; and receives honoraria from Celgene, Chugai, Ono, Takeda, Janssen, Sanofi, MSD, Eisai, AbbVie, BMS, Mundipharma, Kyowa Kirin, Zenyaku, AstraZeneca, Nippon, and SymBio. E.-M.Y. is employed by Mundipharma Singapore Holdings Pte Ltd. N.M. is employed by Mundipharma Research Limited. K.T. receives honoraria from Celgene, Chugai, Eisai, Daiichi Sankyo, HUYA Bioscience International, Kyowa Kirin, Mundipharma, Ono Pharmaceutical, Solasia Pharma, Takeda, Yakult, and ZenyakuKogyo; and consults for Celgene, Daiichi Sankyo, HUYA Bioscience International, Mundipharma, Ono Pharmaceutical, Takeda, and Zenyaku Kogyo. The remaining authors declare no competing financial interests.
Correspondence: Owen A. O’Connor, Division of Hematology-Oncology, Translational Orphan Blood Cancer Research Center, University of Virginia Comprehensive Cancer Center, 1300 Jefferson Park Ave, MSB, Room 6002, Charlottesville, VA 22908-0716; email: owenaoconnor27@gmail.com.
References
Author notes
Data are available on request from the corresponding author, Owen A. O’Connor (owenaoconnor27@gmail.com).
The full-text version of this article contains a data supplement.