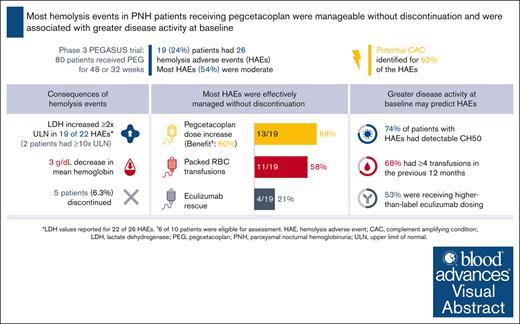
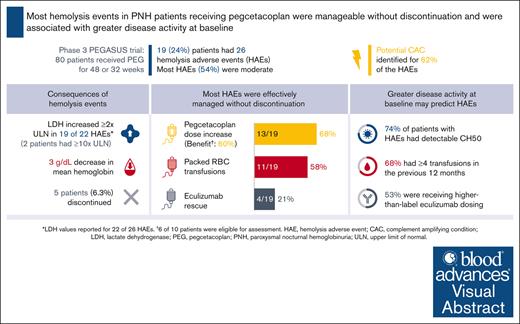
Most hemolysis events in difficult-to-treat patients with PNH on pegcetacoplan were manageable without pegcetacoplan discontinuation.
Higher disease activity at baseline may indicate patients at increased risk of hemolysis.
Visual Abstract
Patients with paroxysmal nocturnal hemoglobinuria (PNH) experience complement-mediated intravascular hemolysis leading to anemia, fatigue, and potentially life-threatening thrombotic complications. Pegcetacoplan, a C3 inhibitor, demonstrated sustained improvements in hematologic and clinical parameters in the phase 3 PEGASUS trial in patients with PNH who remained anemic despite C5 inhibitor therapy. The present post hoc analysis describes 26 hemolysis adverse events (AEs) experienced in 19 patients during pegcetacoplan therapy in PEGASUS and baseline patient characteristics potentially associated with increased hemolysis risk. Lactate dehydrogenase (LDH) ≥2× the upper limit of normal (ULN) was observed in 19 events, including 2 with LDH ≥10× ULN. All patients experienced decreased hemoglobin during hemolysis (mean decrease, 3.0 g/dL). In 16 events (62%), a potential complement-amplifying condition underlying the event could be identified. Hemolysis AEs led to study discontinuation in 5 patients. However, of 26 hemolysis AEs, 17 (65%) were manageable without pegcetacoplan discontinuation. A greater proportion of patients with hemolysis AEs (n = 19) had key characteristics of higher disease activity at baseline compared to patients without hemolysis AEs (n = 61), namely higher-than-label eculizumab dose (53% vs 23%), detectable CH50 (total complement function; 74% vs 54%), and ≥4 transfusions in the previous 12 months (68% vs 51%). These characteristics may be useful predictors of potential future hemolysis events. This trial was registered at www.ClinicalTrials.gov as #NCT03500549.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired, rare, and potentially life-threatening hematologic disease1 characterized by chronic complement–mediated intravascular hemolysis (IVH), thrombosis, and fatigue.2 Complement C5 inhibitors eculizumab and ravulizumab have improved outcomes and survival in patients with PNH by reducing IVH and thrombosis risk.3-6 Despite this, 72% to 86% of patients remain anemic and approximately one-third continue to require blood transfusions, mostly due to C3-mediated extravascular hemolysis (EVH).7-11
Pegcetacoplan is the first complement C3 inhibitor approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of PNH.12,13 In the pivotal phase 3 PEGASUS study in patients with PNH who remained anemic despite C5 inhibitor therapy, pegcetacoplan demonstrated superiority in change in hemoglobin level from baseline to 16 weeks compared with eculizumab owing to its broad control of IVH and emergent EVH under C5 inhibition.14 Further assessment of efficacy and safety of pegcetacoplan to 48 weeks demonstrated sustained improvements in hematologic outcomes and quality of life measures.15
Breakthrough hemolysis events were first described in patients treated with C5 inhibitors when IVH reoccurred despite treatment.3 Breakthrough events while on C5 inhibition can be pharmacokinetic in nature and typically occur toward the end of a dosing interval.16 Alternatively, complement-amplifying conditions (CACs) such as infection, vaccination, or surgery can drive pharmacodynamic breakthrough despite adequate complement inhibitor dosing.16-19
Adverse events (AEs) of hemolysis were reported by investigators in patients treated with pegcetacoplan in the PEGASUS study.14,15 The PEGASUS study enrolled a selection of eculizumab-treated patients with severe EVH, with most patients requiring regular transfusions. Additionally, around a third of patients were on a higher-than-label dose of eculizumab before entry.14
This analysis aims to better describe hemolysis events under C3 inhibition to identify potential predictors associated with hemolysis during pegcetacoplan treatment to improve timely and adequate management.
Methods
Study design and population
PEGASUS (NCT03500549) was a phase 3, randomized, open-label, multicenter, and active-comparator controlled trial. The study design and results have been previously described.14,15 In this analysis, data from patients who experienced hemolysis events during the 48-week study period were investigated. Hemolysis events were not defined in the study protocol but were assessed based on AE reporting, as determined by the investigator (AEs coded to system organ class and preferred term using MedDRA Version 20.0).
Characterization of hemolysis events
To further characterize hemolysis events, changes in hemoglobin and lactate dehydrogenase (LDH) levels before and during the event were described, based on laboratory values that were sampled most proximal to the event. For pegcetacoplan, central and local laboratory values were collected. For values obtained at certified local laboratories, units and normal ranges were normalized to maintain consistency with the study’s central laboratory (hemoglobin normal ranges were 12.0-16.0 g/dL for female participants and 13.6-18.0 g/dL for male participants, and LDH normal range was 113-226 U/L). Potential CACs that were proximal to the time of the event were reviewed jointly by authors and analysis sponsors; these were identified either as an AE reported by the investigator or inferred from records of concomitant medications. The severity of hemolysis, its relationship to pegcetacoplan, and study discontinuations due to hemolysis were also reviewed. Definitions that were considered when severity and relationship of AEs to study drug were evaluated by the investigators are included as a data supplement available with the online version of this article.
Evaluation of hemolysis risk
The key parameters of interest were baseline patient characteristics to assess disease activity, including higher-than-label eculizumab dose, detectable CH50 (total complement function) level while on eculizumab, and ≥4 transfusions within 12 months before study entry.
Management of hemolysis
Management strategies for hemolysis AEs were not included in the PEGASUS study protocol. Based on preliminary clinical experience, dose adjustment of pegcetacoplan, red blood cell (RBC) transfusions to improve anemia-related symptoms, or acute administration of eculizumab to control IVH could be considered.20
If a patient did not respond adequately to the twice-weekly dose of pegcetacoplan (LDH levels >2× upper limit of normal [ULN]), dosing could be increased to 1080 mg up to every 3 days according to the present prescribing information.13 Benefit of increased dosing was defined as pegcetacoplan concentration change with a minimum of 1 month at increased dosing, demonstration of LDH decrease, resolution of hemolysis (as reported by the investigator), or no new events after dose escalation, and completion of study.
The PEGASUS study was approved by the institutional review board or independent ethics committee at participating trial sites and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was obtained from all patients.
Results
Study population and baseline characteristics
Of 80 patients, 19 (24%) treated with pegcetacoplan in PEGASUS experienced a hemolysis AE. Patient characteristics on entry into PEGASUS for patients with and without hemolysis events are presented in Table 1. At baseline, patients who experienced hemolysis AEs were older with a mean age of 54.5 years and most were male (63%) compared with those without a hemolysis event (47.0 years and 31%, respectively). Patients who experienced hemolysis AEs were more likely to have a history of thrombosis at baseline than those without a hemolysis event (42% and 28%, respectively) and had a higher mean CH50 level at baseline (19.7 U/mL and 5.4 U/mL, respectively).
Incidence and characterization of hemolysis on pegcetacoplan
Up to week 16 of the randomized controlled period, 4 patients (10%) on pegcetacoplan experienced hemolysis AEs as reported by the investigators.14 During the following 32-week open-label period (OLP) (weeks, 17-48), 15 patients (19.5%) on pegcetacoplan experienced a hemolysis AE.15
The 19 patients with reported hemolysis AEs experienced 26 events (Table 2). Of 22 events with LDH values, 19 (86%) were associated with increased LDH (defined as LDH elevation ≥2× ULN21,22). Using the highest value recorded during each event, mean LDH increased from 324 U/L before hemolysis to 1128 U/L during hemolysis. Extremely high LDH levels (≥10× ULN) were an exception, occurring in only 2 patients (9%). No thrombotic events were reported in either of these patients. Using the lowest value recorded during each event, mean hemoglobin decreased from 11.3 g/dL before hemolysis to 8.3 g/dL during hemolysis. Of the 26 hemolysis events, 16 (62%) occured within 30 days of a potential CAC, such as infection or vaccination.
Of the 26 hemolysis events, 14 (54%) were considered moderate in severity. Hemolysis events led to study discontinuation in 5 patients, 3 during the randomized controlled period before study day 56 (LDH >1000 U/L for all; 2 with moderate severity; and 1 patient received eculizumab to manage the event) and 2 during the OLP (LDH <1000 U/L for both). Three additional patients discontinued the study due to AEs unrelated to hemolysis (1 pneumonitis 20 days before the hemolysis event, 1 fatal COVID-19 infection, and 1 sepsis; Table 2). Of the 26 hemolysis events, 17 (65%) were manageable without pegcetacoplan discontinuation.
Predictors of hemolysis
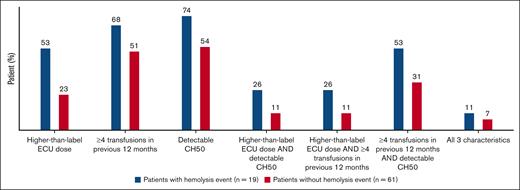
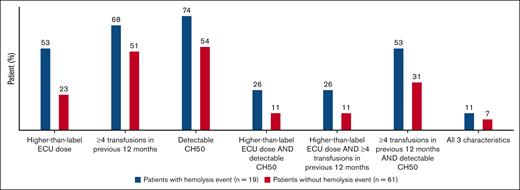
Patients with hemolysis AEs on pegcetacoplan in the PEGASUS study were associated with more key characteristics of higher disease activity at baseline than patients without hemolysis AEs (Figure 1). Over half of the patients (10 of 19) recording a hemolysis event were on a higher-than-label dose of eculizumab at study baseline. Of the 19 patients who experienced hemolysis AEs, 14 (74%) had detectable CH50 at baseline when compared with 54% of patients who did not experience hemolysis AEs. Thirteen patients (68%) in the hemolysis group and 31 (51%) in the nonhemolysis group required ≥4 transfusions in the 12 months before study entry. The 3 characteristics were evaluated in different combinations; between 26% and 53% of patients with hemolysis AEs presented with 2 of 3 characteristics (Figure 1).
Predictors of hemolysis in patients with and without hemolysis events. Assessment of key characteristics of higher disease activity at PEGASUS study baseline in patients with and without hemolysis events include higher-than-label eculizumab dose at screening (>900 mg every 2 weeks), ≥4 transfusions within 12 months before study entry, and detectable CH50 levels while on eculizumab treatment. CH50, total complement function; ECU, eculizumab.
Predictors of hemolysis in patients with and without hemolysis events. Assessment of key characteristics of higher disease activity at PEGASUS study baseline in patients with and without hemolysis events include higher-than-label eculizumab dose at screening (>900 mg every 2 weeks), ≥4 transfusions within 12 months before study entry, and detectable CH50 levels while on eculizumab treatment. CH50, total complement function; ECU, eculizumab.
Management of hemolysis
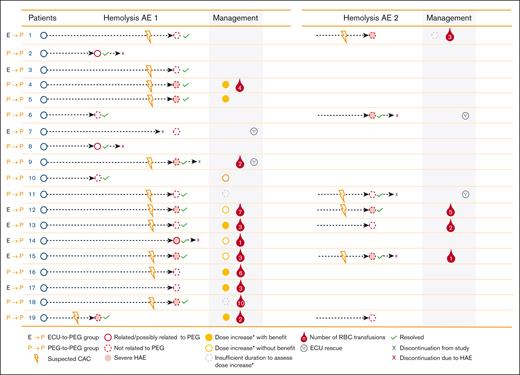
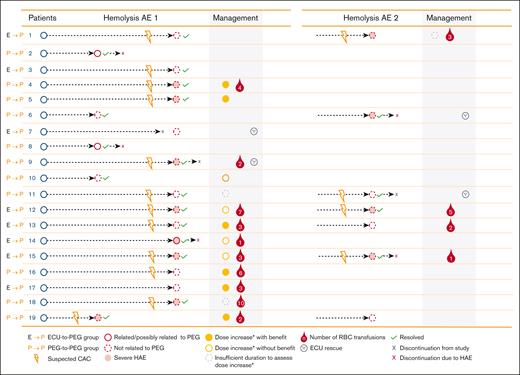
Pegcetacoplan dose was increased in 13 patients (68%) who experienced a hemolysis AE during the PEGASUS study (Figure 2). Of the 13 patients, 6 (46%) showed benefit with the increased dosing, 4 (31%) did not demonstrate benefit, and 3 (23%) had insufficient duration of increased dosing to assess the effect of escalated dosing. Eleven patients (58%) required packed RBC transfusions, and eculizumab was administered as rescue therapy in 4 patients (21%) in 4 different centers (Figure 2).
Management of hemolysis AEs in PEGASUS.∗If a patient did not respond adequately to the twice-weekly dose of pegcetacoplan (LDH levels ≥2× ULN), dosing could be increased to 1080 mg every 3 days according to the prescribing information.13 CAC, complement-amplifying condition; ECU, eculizumab; HAE, hemolysis adverse event; PEG, pegcetacoplan; RBC, red blood cell.
Management of hemolysis AEs in PEGASUS.∗If a patient did not respond adequately to the twice-weekly dose of pegcetacoplan (LDH levels ≥2× ULN), dosing could be increased to 1080 mg every 3 days according to the prescribing information.13 CAC, complement-amplifying condition; ECU, eculizumab; HAE, hemolysis adverse event; PEG, pegcetacoplan; RBC, red blood cell.
Discussion
This post hoc analysis of patients from the phase 3 PEGASUS study describes hemolysis events experienced during pegcetacoplan therapy. The identified characteristics of higher disease activity at baseline potentially associated with increased hemolysis risk may support physicians in identifying patients at risk and managing clinically relevant hemolysis events in pegcetacoplan-treated patients.
PEGASUS enrolled a study population with difficult-to-control disease. Despite receiving eculizumab treatment for a mean of 5 years, patients in the PEGASUS study had mean hemoglobin levels below the normal reference range and most required regular transfusions.14 Around a third of patients were on a higher-than-label dose of eculizumab before entry, suggesting a population prone to experiencing hemolysis. However, even in this patient population pegcetacoplan treatment was effective in managing the disease. Moreover, patients treated with pegcetacoplan did not have a higher risk for hemolysis events compared with eculizumab in the study. Up to week 16, more patients treated with eculizumab experienced hemolysis AEs (n = 9) compared with pegcetacoplan (n = 4).14 In addition, no hemolysis AE was reported during 26 weeks in the phase 3 PRINCE study evaluating pegcetacoplan in complement inhibitor–naïve patients (46 patients treated with pegcetacoplan overall).23
For nearly two-thirds of the reported hemolysis events, a potential CAC could be identified. Although hemolysis events in the context of eculizumab C5 inhibition have been identified as largely pharmacokinetic in nature,16,24 the mechanisms underlying events in pegcetacoplan-treated patients remain under investigation. A real-world study of compassionate use of pegcetacoplan in patients previously treated with eculizumab described 5 of 7 hemolysis events related to a CAC, with a mean LDH increase of 4.61× ULN for 6 of 7 hemolysis events.25
Despite the greater proportion of PNH RBCs rendered susceptible to lysis because of the high efficacy of C3 inhibition,14,15 our analysis underlines that most patients in the PEGASUS study did not experience severe acute hemolysis AEs, as discussed in recent reviews of the PEGASUS data.1,16,26,27 Most hemolysis events (54%) were considered moderate in severity and 11 patients from this difficult-to-treat patient population continued pegcetacoplan treatment. Of note, management strategies for hemolysis AEs were not included in the PEGASUS study protocol. Therefore, the discontinuations of pegcetacoplan due to hemolysis AEs need to be interpreted with caution and might not necessarily be associated with the severity of the event.
High disease activity may confer an increased risk of hemolysis in a subgroup of patients with PNH on pegcetacoplan. Three characteristics of higher disease activity at baseline were identified, namely higher-than-label eculizumab dose, detectable CH50, and ≥4 transfusions in the previous 12 months, which may be useful predictors of potential future hemolysis events. Establishing measures of disease activity associated with hemolysis events, such as detectable CH50 at the onset of pegcetacoplan treatment, may allow early identification of patients at risk for such events. However, management will vary between centers.
Not all patients on proximal inhibition are at risk for hemolysis events. However, it is advised to monitor patients switching from C5 inhibitors to pegcetacoplan for signs of hemolysis. Patients and physicians should be alert to possible CACs and in the event of pharmacodynamic influence, the identified CAC should be treated in parallel with increased pegcetacoplan dosing per approved prescribing information.20,25 If a hemolysis event occurs, pegcetacoplan dose adjustment should be performed immediately. RBC transfusion or an extra dose of eculizumab may be considered if IVH is not controlled quickly. Acute intravenous or intensive subcutaneous pegcetacoplan as a new approach for treatment of acute hemolysis events is currently being investigated as part of the long-term extension study (NCT03531255). Based on preliminary data, intensive intravenous of subcutaneous pegcetacoplan dosing is effective in managing acute hemolysis events in patients on pegcetacoplan.28
Study limitations include the timing of laboratory sample collection. Although laboratory values were collected for patients receiving pegcetacoplan to build a comprehensive picture of hemolysis events, the collection of these samples did not necessarily coincide with the hemolysis AE. As a result, this analysis reviewed available laboratory values post hoc and used values recorded most proximal to the actual event. A similar approach was used in assessing potential CACs underlying each hemolysis event, in which a post hoc review of investigator-reported AEs and concomitant medications may not have provided a comprehensive clinical picture of the patients described in this analysis. Further study limitations include the lack of additional laboratory data to better define the severity of the hemolysis AEs, as well as their relationship to CACs. Given the inclusion criteria of the PEGASUS study, the identified characteristics of high disease activity associated with a higher risk for hemolysis may only be applicable for a subset of patients with PNH. Finally, the study is descriptive in nature with no formal statistics to analyze the different patient populations.
In summary, progress has been made in the management of hemolysis events during pegcetacoplan treatment; however, risk-mitigation strategies continue to be necessary with a focus on patient and clinician education. Patients can remain on pegcetacoplan, with available prescribing information allowing for a pegcetacoplan dose increase from twice weekly to up to every 3 days in cases of LDH >2× ULN.12,13 Higher disease activity at baseline may confer an increased risk of a severe hemolysis event in a subgroup of patients with PNH on pegcetacoplan, and further investigation into predictors of hemolysis events is warranted to augment current risk-mitigation strategies.
Acknowledgments
The PEGASUS study was funded by Apellis Pharmaceuticals, Inc. The analysis was funded by Swedish Orphan Biovitrum AB. Medical writing support was provided by Miriam Souto of nspm ltd (Meggen, Switzerland) and funded by Swedish Orphan Biovitrum AB and Apellis Pharmaceuticals, Inc.
Authorship
Contribution: The study was designed by the Apellis Pharmaceuticals and the academic authors; trial investigators collected the data; all authors contributed to the analysis or interpretation of the data as well as revision of the manuscript; the authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol; manuscript review was conducted by Swedish Orphan Biovitrum AB and Apellis Pharmaceuticals during the development process; full editorial control of the manuscript was maintained by the authors; and all authors provided their final approval for publication.
Conflict-of-interest disclosure: R.P.d.L. reports consultancy and honoraria from Alexion, Novartis, Pfizer, Apellis Pharmaceuticals, and Swedish Orphan Biovitrum AB; and research funding from Alexion, Novartis, Pfizer, and Amgen. M.G. reports consultancy with BioCryst, Regeneron, and Swedish Orphan Biovitrum AB; speaker’s fees from Alexion, AstraZeneca, Novartis, and Swedish Orphan Biovitrum AB; and serves as a scientific advisory board member for Alexion, AstraZeneca, BioCryst, and Novartis. R.J.K. reports research support from Novartis and Swedish Orphan Biovitrum AB; consultancy with Swedish Orphan Biovitrum AB; speaker’s fees from Alexion, Swedish Orphan Biovitrum AB, Jazz, Astellas, Otsuka, Amgen, Novartis, and Biologix; and serves as a scientific advisory board member for Alexion, Swedish Orphan Biovitrum AB, Jazz, AbbVie, Novartis, and Roche. J.S. reports research support from Alexion, Apellis Pharmaceuticals, Novartis, R.A. Pharmaceuticals, and Sanofi-Genzyme; consultancy with BioCryst; speaker’s fees from Alexion, Novartis, and Swedish Orphan Biovitrum AB; and serves as a scientific advisory board member for Link Pharmaceuticals, Novartis, Sanofi-Genzyme, and Swedish Orphan Biovitrum AB. C.d.C. reports research funding from Alexion Pharmaceuticals and Apellis Pharmaceuticals; consultancy with Apellis Pharmaceuticals; and honoraria from Novartis, Alexion Pharmaceuticals, BioCryst, and Apellis Pharmaceuticals. R.H. is currently employed by Swedish Orphan Biovitrum AB and holds company shares. L.T. is a consultant to Swedish Orphan Biovitrum AB. M.Y. is currently employed by Apellis Pharmaceuticals, Inc and holds company shares. J.P. reports consultancy, honoraria, and membership on an entity’s board of directors or advisory committees with Apellis Pharmaceuticals, Blueprint Medicines, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, and Grunenthal, and Merck Sharp & Dohme; consultancy and membership on an entity’s board of directors or advisory committees with Amgen; speakers bureau with Chugai and Pfizer; and membership on an entity’s board of directors or advisory committees, and speakers bureau with Alexion, Boehringer Ingelheim, and Novartis.
Correspondence: Régis Peffault de Latour, French Reference Center for Aplastic Anemia and Paroxysmal Nocturnal Hemoglobinuria, Université Paris Cité, Saint-Louis Hospital, 1 Ave Claude Vellefaux, 75010 Paris, France; email: regis.peffaultdelatour@aphp.fr.
References
Author notes
All authors analyzed the data and had full access to all clinical trial data. Data access will be granted in response to qualified research requests. Individual participant data will not be shared. All requests are evaluated by a cross-functional panel of experts within Sobi, and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity, and commitment to publication of the results. To request access to study data, a data sharing request form (available on www.sobi.com) should be sent to medical.info@sobi.com.
The full-text version of this article contains a data supplement.