Rurioctocog alfa pegol expressed a low immunogenicity profile when tested in previously treated patients with severe hemophilia A.
Only 1 of 360 patients developed transient low-titer FVIII inhibitors, which were not associated with serious adverse events.
Visual Abstract
Rurioctocog alfa pegol is an extended–half-life full-length recombinant factor VIII (FVIII) bound to 20-kDa polyethylene glycol (PEG) that has been shown to be well tolerated and efficacious in the treatment and prevention of bleeding events in previously treated patients with severe hemophilia A. Here, we present a comprehensive analysis of immunogenicity data collected during 6 clinical studies of rurioctocog alfa pegol, including a total of 360 unique previously treated patients with severe hemophilia A. The analysis included treatment-emerging FVIII-neutralizing antibodies (FVIII inhibitors); preexisting and treatment-emerging antibodies binding to FVIII, PEG-FVIII, or PEG; and treatment-emerging antibodies binding to Chinese hamster ovary host cell proteins. Moreover, the potential association between the presence of these binding antibodies and adverse events (AEs) observed in patients was investigated, and the potential impact of these antibodies on the incremental recovery of rurioctocog alfa pegol in patients was analyzed. Overall, the data indicate that rurioctocog alfa pegol is not associated with any unexpected immunogenicity characteristics. Of 360 patients, 1 patient developed a transient FVIII inhibitor with a titer of 0.6 Bethesda units per mL, which was not associated with any serious AEs. Antibodies binding to FVIII, PEG-FVIII, or PEG were not detected at the time when the inhibitor was present. Moreover, 54 of 360 patients either entered the clinical studies with preexisting binding antibodies or developed these antibodies after exposure to rurioctocog alfa pegol. These antibodies were transient in most patients and did not show any causal relationship to either AEs or spontaneous bleeding episodes.
Introduction
Hemophilia A is a congenital bleeding disorder caused by a deficiency of biologically active factor VIII (FVIII). Despite the recent availability of alternative therapies such as emicizumab,1 many patients with severe hemophilia A are still treated with FVIII replacement therapies. A major complication of FVIII replacement therapies is the development of FVIII neutralizing antibodies (FVIII inhibitors), rendering treatment less effective or even ineffective.2 FVIII inhibitors are observed in ∼30% of previously untreated patients with severe hemophilia A2 and usually develop within the first 20 exposure days (EDs).3-5 In contrast, the rate of FVIII inhibitor development in previously treated patients (PTPs) with a history of at least 150 EDs is very low.6 The reasons for FVIII inhibitor development are poorly understood although there is evidence that both genetic and nongenetic risk factors influence their development.7-11
FVIII inhibitors are not the only antibodies to develop in patients who receive FVIII replacement therapies. We, and others, have presented evidence that FVIII-binding antibodies are found in some patients who do not have FVIII inhibitors.5,12-15 In these cases, FVIII-binding antibodies can be considered as nonneutralizing antibodies. Similar FVIII-binding antibodies can be found in some healthy individuals.14,15 The biological significance of these antibodies is not clear. Hofbauer et al and Cannavò et al suggested that they might be early indicators of emerging neutralizing antibodies.15,16 Alternatively, they could contribute to the maintenance of immune homeostasis as described for some self-reactive antibodies in healthy individuals.17 It is important to monitor these antibodies during the clinical development of new FVIII products to better understand their potential biological significance.
A major limitation of FVIII replacement therapies is the short half-life of FVIII. Previous studies have reported significant interindividual heterogeneity in FVIII clearance rates in patients with hemophilia A, with the FVIII half-life varying from a minimum of 5.3 hours to a maximum of 28.8 hours.18-24 In recent years, different technologies have been used to increase the half-life of FVIII concentrates, including the conjugation of FVIII with different sizes of polyethylene glycol (PEG), or fusion with the Fc-fragment of immunoglobulin GI (IgG1).25-28
The first PEGylated FVIII product, approved in 2015, was rurioctocog alfa pegol (ADYNOVATE [US]/ADYNOVI [Europe]; Baxalta US Inc, a Takeda company, Lexington, MA; and Baxalta Innovations GmbH, a Takeda company, Vienna, Austria). Rurioctocog alfa pegol is a PEGylated, full-length, recombinant FVIII with an extended half-life expressed in Chinese hamster ovary (CHO) cells that was developed from unmodified octocog alfa (ADVATE; Baxalta US Inc, a Takeda company, Lexington, MA).29-31 A 20-kDa branched PEG is conjugated to primary amine residues consisting of 2 10-kDa arms per attachment site.25,32,33 Most of the amine residues are located at the surface of the FVIII molecule, mainly within the B-domain.25 Compared with octocog alfa, the mean half-life of rurioctocog alfa pegol is 1.3- to 1.5-fold longer in children aged <12 years with severe hemophilia A, and 1.4- to 1.5-fold longer in patients aged ≥12 years with severe hemophilia A.25,34 Rurioctocog alfa pegol has been shown to be effective and well tolerated in the prevention and control of bleeding events in PTPs with severe hemophilia A.25,34-37
In this article, we present a comprehensive analysis of immunogenicity data collected during 6 clinical studies of rurioctocog alfa pegol in patients with severe hemophilia A who had previously received treatment with either plasma-derived or recombinant FVIII for ≥50 EDs (patients aged <6 years) or ≥150 EDs (patients aged ≥6 years) without FVIII inhibitors.25,34-37 The immunogenicity data include FVIII inhibitors as well as IgM and IgG antibodies binding to PEG-FVIII, FVIII, or PEG. Additionally, we analyzed antibodies against a total protein preparation of CHO host cells, which are used for the expression of octocog alfa, the source material for rurioctocog alfa pegol.
Methods
Clinical studies included in the analysis
FVIII inhibitors and antibodies binding to FVIII, PEG-FVIII, PEG, or CHO host cell proteins were monitored in all patients enrolled across 6 clinical studies of rurioctocog alfa pegol: ClinicalTrials.gov identifier: NCT01599819, NCT01736475, NCT02210091, NCT01913405, NCT01945593, and NCT02585960.25,34-37 An overview of the 6 studies is provided in Table 1. Details of the study designs, enrollment criteria, and treatment schedules have been published previously.25,34-37
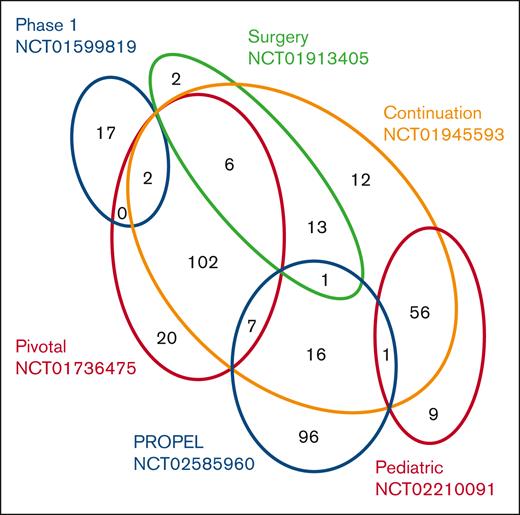
All 6 studies enrolled patients with severe hemophilia A (FVIII activity level of <1%) who had previously received treatment with either plasma-derived or recombinant FVIII for ≥50 EDs (patients aged <6 years) or ≥150 EDs (patients aged ≥6 years).25,34-37 Details of the 1-stage clotting assay and the chromogenic assay used to measure FVIII activity levels are provided in supplemental Data. Patients were excluded from the studies if they had a history of FVIII inhibitors or had FVIII inhibitors at screening.25,34-37 In total, 360 unique patients were enrolled. Some patients were enrolled in >1 study, as indicated in Figure 1. All patients included in the studies received treatment with rurioctocog alfa pegol as indicated in Table 1 and previously described in detail.25,34-37 All 6 studies were conducted in compliance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonization. Institutional review board/independent ethics committee approval was obtained for the study protocols and informed consent forms. Written informed consent was obtained from each patient or their legally authorized representative.
Euler diagram indicating number and distribution of patients participating in each of the 6 clinical studies. In total, 360 unique patients with severe hemophilia A (FVIII activity level of <1%) who had previously received treatment with either plasma-derived or recombinant FVIII for ≥50 EDs (patients aged <6 years) or ≥150 EDs (patients aged ≥6 years) were enrolled in the 6 clinical studies NCT01599819, NCT01736475, NCT02210091, NCT01913405, NCT01945593, and NCT02585960, as described in Table 1. Some patients were enrolled in >1 study as indicated.
Euler diagram indicating number and distribution of patients participating in each of the 6 clinical studies. In total, 360 unique patients with severe hemophilia A (FVIII activity level of <1%) who had previously received treatment with either plasma-derived or recombinant FVIII for ≥50 EDs (patients aged <6 years) or ≥150 EDs (patients aged ≥6 years) were enrolled in the 6 clinical studies NCT01599819, NCT01736475, NCT02210091, NCT01913405, NCT01945593, and NCT02585960, as described in Table 1. Some patients were enrolled in >1 study as indicated.
Monitoring of AEs
Adverse events (AEs) were captured from the first exposure to rurioctocog alfa pegol until the study completion visit. Unresolved AEs at the study completion visit were followed-up until resolution. For all 6 clinical studies, AEs were described on the AE-case report form using the medical diagnosis or, if a diagnosis was not established at the time of AE reporting, signs and symptoms in standard medical terminology were described. Each AE was evaluated by the investigator and the sponsor for seriousness, severity, and causality to exposure with rurioctocog alfa pegol.
Blood sampling schedule for immunogenicity assessment
Immunogenicity was assessed by analyzing FVIII inhibitors and antibodies binding to FVIII, PEG-FVIII, PEG, or CHO host cell proteins. Blood samples for immunogenicity assessments were always collected before infusion of rurioctocog alfa pegol, after a minimum of a 72-hour to 96-hour washout period after the last infusion of either octocog alfa or rurioctocog alfa pegol. Based on the study design and duration of study participation, blood samples for immunogenicity testing were collected at screening, at the baseline visit, during scheduled study visits, and at the study completion visit. Testing was performed on citrated plasma samples in a central laboratory.
Study NCT02585960 included an assessment of the pharmacokinetic (PK) profile of the study drug (single infusion of 60 ± 5 IU/kg rurioctocog alfa pegol) between screening and baseline to enable subsequent PK-guided prophylaxis targeting FVIII trough levels of either 1% to 3% or 8% to 12%.37 Therefore, patients were already exposed to the study drug before baseline sampling for immunogenicity assessment. Baseline sampling in all other studies was done before the first administration of study drug.
FVIII inhibitors
FVIII inhibitor analysis for study NCT01599819 was performed at the Medical University of Vienna, Austria, using a Nijmegen-modified Bethesda assay with a lower limit of inhibitor detection of 0.6 Bethesda units (BU) per mL. The assay included Siemens Actin FS as the activated partial thromboplastin time (APTT) reagent. For all other studies, FVIII inhibitor analysis was performed by Esoterix (Englewood, CO) using a Nijmegen-modified Bethesda assay with a lower limit of inhibitor detection of 0.4 BU/mL. The inhibitor assay used by Esoterix included Siemens Dade Actin FSL as the APTT reagent. Further details of the inhibitor assays used by both laboratories are provided in supplemental Data.
A patient was confirmed as being FVIII inhibitor positive if neutralizing antibodies were detected at ≥0.6 BU/mL for at least 2 consecutive exposure samples. A positive inhibitor that peaked at ≤5 BU/mL was considered a low-titer inhibitor, and a positive inhibitor of >5 BU/mL was considered a high-titer inhibitor. Transient inhibitors were defined as those that are low titer (≤5 BU/mL) and disappear within 6 months, with the patient able to remain on FVIII therapy for the treatment of hemorrhages.
Detection of antibodies binding to FVIII, PEG-FVIII, PEG, or CHO host cell proteins
The antibodies binding to FVIII, PEG-FVIII, PEG, or CHO host cell proteins were detected using validated enzyme-linked immunosorbent assays following the principles described by Whelan et al14 and the relevant regulatory guidelines that applied at the time the clinical studies were conducted.38,39 The relative sensitivities of the different assays are summarized in supplemental Table 2. Details of all assays are provided in supplemental Data.
Temporal association between the detection of binding antibodies and AEs
Potential temporal associations between the detection of preexisting or treatment-emerging antibodies binding to FVIII, PEG-FVIII, PEG, or CHO host cell proteins and the appearance of AEs were assessed. For this purpose, the time period between the last negative antibody assessment before, and the first negative antibody assessment after, the occurrence of positive data for binding antibodies was considered in this assessment.
IR
Blood samples for incremental recovery (IR) were collected after a washout period of at least 72 hours and up to 96 hours after the last infusion of rurioctocog alfa pegol or octocog alfa. A preinfusion sample was drawn 30 minutes before infusion and the postinfusion sample was drawn 15 to 30 minutes after infusion of rurioctocog alfa pegol. The typical time points for evaluation of IR were at baseline, at the PK visit (for patients who underwent PK assessment), at scheduled study visits, and at the study completion visit. Refer to individual study designs for scheduled study visits because IR assessments varied based on study design and duration. FVIII assays for IR included the 1-stage clotting assay (primary assay) and the chromogenic assay (supportive assay). All testing was conducted on citrated plasma samples at a designated central laboratory.
Statistical analysis
Data were analyzed in a descriptive manner, mostly by counts, frequencies, or Euler diagrams. IR was calculated as an increase of the FVIII level in IU/dL after infusion from the value before infusion divided by the dose per body mass in IU/kg. Box-and-whiskers plots of IR before, during, and after any positive results for binding antibodies were drawn with the boxes featuring medians and quartiles whereas the whiskers extended to 1.5 times the interquartile range from the boxes.
In this article, we present a comprehensive analysis of immunogenicity data collected during 6 clinical studies of rurioctocog alfa pegol in patients with severe hemophilia A.
Results
Development of neutralizing antibodies (FVIII inhibitors)
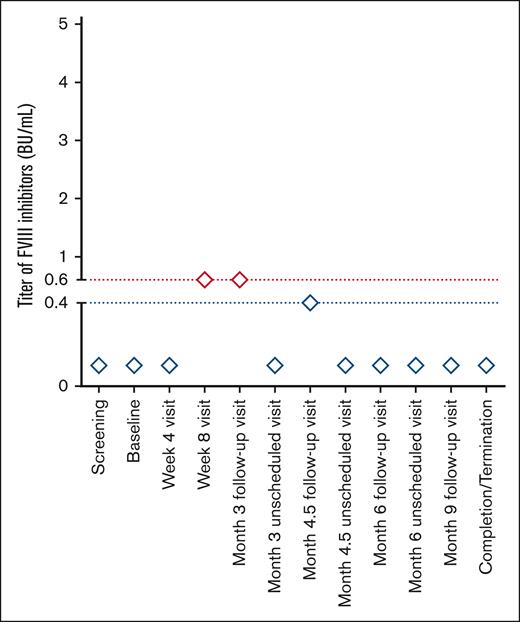
None of the 360 unique patients enrolled in the 6 clinical studies developed persistent FVIII inhibitors. One of the patients who participated in study NCT02585960 developed transient FVIII inhibitors. The transient inhibitors were detected at the week-8 visit and confirmed at the subsequent month-3 follow-up visit. FVIII inhibitors had a titer of 0.6 BU/mL, which corresponds to the lowest limit of positivity. Samples taken at all subsequent time points tested negative (<0.6 BU/mL; Figure 2). Samples tested for IgM and IgG antibodies binding to FVIII, PEG-FVIII, or PEG were negative at all time points investigated. PK parameters and IR were not assessed during the period in which the transient FVIII inhibitors were detected. The IR for this patient was assessed at baseline (IR: 2.681), at the month-6 follow-up visit (IR: 2.579), at the month-9 follow-up visit (IR: 2.557), and at the completion/termination visit (IR: 2.446).
Kinetics of transient FVIII inhibitors in the only patient who developed FVIII inhibitors. The patient participated in study NCT02585960. The transient FVIII inhibitor was detected at the week-8 visit and was confirmed at the subsequent month-3 follow-up visit. The FVIII inhibitor had a titer of 0.6 BU/mL, which corresponds to the lowest limit of positivity. Samples taken at all subsequent time points tested negative (<0.6 BU/mL). At the time when the inhibitor was detected, the patient had experienced 176 EDs to rurioctocog alfa pegol, 174 EDs were spent receiving prophylaxis.
Kinetics of transient FVIII inhibitors in the only patient who developed FVIII inhibitors. The patient participated in study NCT02585960. The transient FVIII inhibitor was detected at the week-8 visit and was confirmed at the subsequent month-3 follow-up visit. The FVIII inhibitor had a titer of 0.6 BU/mL, which corresponds to the lowest limit of positivity. Samples taken at all subsequent time points tested negative (<0.6 BU/mL). At the time when the inhibitor was detected, the patient had experienced 176 EDs to rurioctocog alfa pegol, 174 EDs were spent receiving prophylaxis.
The development of transient low-titer FVIII inhibitors was considered a serious AE. Inhibitor development was not associated with any other serious AEs. The patient experienced 3 nonserious AEs (headache, oropharyngeal pain, and productive cough) that were not considered related to study treatment but were in temporal association with the presence of the transient low-titer FVIII inhibitors.
IgM and IgG antibodies binding to FVIII, PEG-FVIII, or PEG
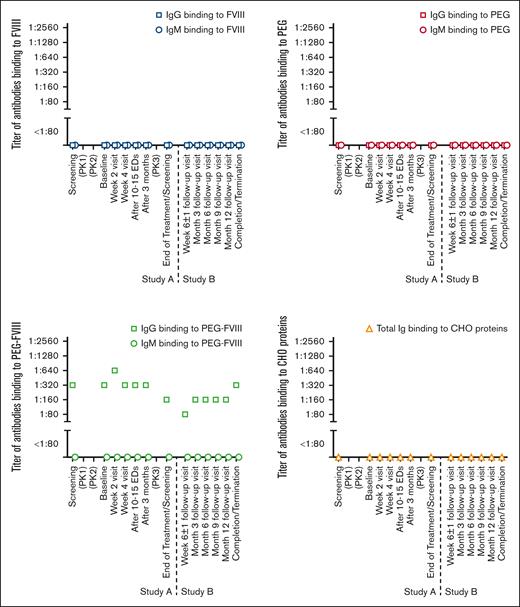
Of the 360 patients who were included in the 6 clinical studies, 54 tested positive for either preexisting or treatment-emerging antibodies binding to FVIII, PEG-FVIII, or PEG (Table 2). These 54 patients were included in ≥1 of the 6 clinical studies, as indicated in Table 3. Of 54 patients, 34 had preexisting binding antibodies before their first exposure to rurioctocog alfa pegol. The preexisting antibodies disappeared over the course of the clinical studies in 33 of 34 patients. One patient (patient 8) tested positive for preexisting IgG antibodies binding to PEG-FVIII from screening in study NCT01736475 until completion of study NCT01945593 (Figure 3). FVIII inhibitors were not detected in this patient at any time point throughout the 2 studies. Additional information for patient 8 is provided in supplemental Data.
Preexisting persistent IgG antibodies binding to PEG-FVIII. The patient (patient 8) presented participated in studies NCT01736475 (study A) and NCT01945593 (study B). This patient had preexisting IgG antibodies binding to PEG-FVIII and tested positive from screening in study A until completion in study B.
Preexisting persistent IgG antibodies binding to PEG-FVIII. The patient (patient 8) presented participated in studies NCT01736475 (study A) and NCT01945593 (study B). This patient had preexisting IgG antibodies binding to PEG-FVIII and tested positive from screening in study A until completion in study B.
Overall, 22 patients who tested negative at screening developed binding antibodies after exposure to rurioctocog alfa pegol (Table 2). Antibodies were transient and not detectable at subsequent visits or at completion of the study in 21 of 22 patients. One patient (patient 35) tested positive for antibodies binding to PEG-FVIII (titer 1:80) at the completion/termination visit of study NCT02210091/screening for study NCT01945593. No subsequent data on binding antibodies were available for this patient who was withdrawn from the study because of continued noncompliance with the protocol. Therefore, no conclusions could be drawn as to whether these binding antibodies were transient or persistent.
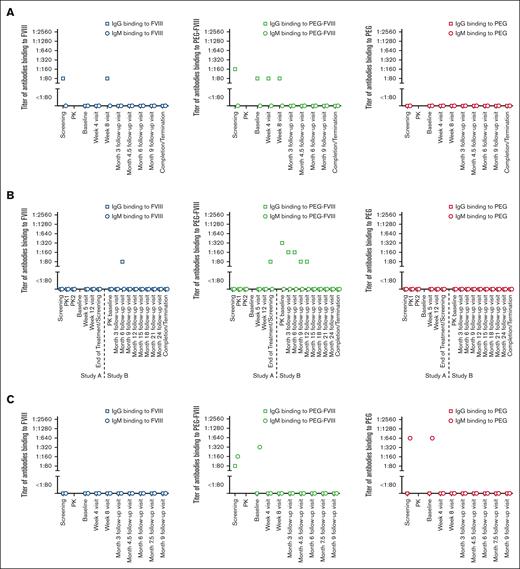
Two patients (patients 24 and 40) presented with preexisting IgG antibodies binding to PEG-FVIII, which disappeared over the course of the clinical studies. In addition, both patients developed transient IgG antibodies binding to FVIII after exposure to rurioctocog alfa pegol. Results for patient 24 are shown in Figure 4.
Preexisting transient IgG antibodies binding to PEG-FVIII, and treatment-emerging transient IgG antibodies binding to FVIII. The patient (patient 24) participated in studies NCT02210091 (study A) and NCT01945593 (study B). They are 1 of 2 patients who presented with preexisting IgG antibodies binding to PEG-FVIII and, in addition, developed IgG antibodies binding to FVIII after exposure to rurioctocog alfa pegol. Both antibody populations were transient and disappeared over the course of the clinical studies.
Preexisting transient IgG antibodies binding to PEG-FVIII, and treatment-emerging transient IgG antibodies binding to FVIII. The patient (patient 24) participated in studies NCT02210091 (study A) and NCT01945593 (study B). They are 1 of 2 patients who presented with preexisting IgG antibodies binding to PEG-FVIII and, in addition, developed IgG antibodies binding to FVIII after exposure to rurioctocog alfa pegol. Both antibody populations were transient and disappeared over the course of the clinical studies.
IgM and IgG antibodies binding to FVIII
Preexisting antibodies binding to FVIII were found in 5 patients: 1 patient presented with preexisting IgM antibodies and 4 patients presented with preexisting IgG antibodies (Table 2). A representative example is shown in Figure 5A. Ten patients developed IgG antibodies binding to FVIII after treatment with rurioctocog alfa pegol (Table 2). A representative example is shown in Figure 5B.
IgM and IgG antibodies binding to PEG-FVIII
Preexisting antibodies binding to PEG-FVIII were found in 29 patients: 6 patients tested positive for IgM antibodies, 22 patients tested positive for IgG antibodies, and 1 patient tested positive for both IgM and IgG antibodies (Table 2). Representative examples are shown in Figure 5A,C. After treatment with rurioctocog alfa pegol, 14 patients developed IgG antibodies binding to PEG-FVIII (Table 2). A representative example is shown in Figure 5B.
IgM and IgG antibodies binding to PEG
Preexisting antibodies binding to PEG were found in 6 patients, and all of them presented with preexisting IgM antibodies (Table 2). A representative example is shown in Figure 5C. One patient developed transient IgM antibodies binding to PEG after treatment with rurioctocog alfa pegol (Table 2; Figure 5D).
Total Ig (IgM + IgG + IgA) antibodies binding to CHO host cell proteins
Rurioctocog alfa pegol could potentially contain residual levels of CHO host cell proteins as minor impurities. Therefore, all samples taken for the testing of antibodies binding to FVIII, PEG-FVIII, and PEG were also tested for antibodies binding to CHO host cell proteins. Of 360 patients, none tested positive for either preexisting or treatment-emerging antibodies binding to CHO host cell proteins.
Temporal association between binding antibodies and AEs
Potential temporal associations between the detection of preexisting or treatment-emerging antibodies and the appearance of AEs were assessed considering the time period between the last negative antibody assessment before, and the first negative antibody assessment after, the occurrence of positive data for binding antibodies.
Nineteen patients (Table 2) showed a temporal association between the detection of binding antibodies and AEs. For 17 patients, based on the assessment of the investigators and the sponsor, the AEs were not considered related to treatment with rurioctocog alfa pegol. Two patients (patients 43 and 44; Table 2) who tested positive for binding antibodies experienced AEs that were considered related to rurioctocog alfa pegol. Patient 43 experienced a mild headache ∼5 hours after the previous treatment with rurioctocog alfa pegol. The event resolved overnight, after ∼18 hours, without any change in rurioctocog alfa pegol treatment. Patient 44 experienced a nonserious infusion-related reaction and increased blood pressure 15 minutes after administration of rurioctocog alfa pegol, both of which were considered related to study treatment. Both events resolved after 1 hour 40 minutes without any change in rurioctocog alfa pegol treatment.
Based on the assessment of the investigators and the sponsor, no causal relationships were identified between detected binding antibodies and any AEs to which they were temporally associated.
Potential association between the occurrence of binding antibodies and spontaneous bleeding events
There was no causal relationship observed between the detection of antibodies binding to FVIII, PEG-FVIII, or PEG and any potentially increased occurrence of spontaneous bleeding episodes.
Potential modulation of IR by antibodies binding to FVIII, PEG-FVIII, or PEG
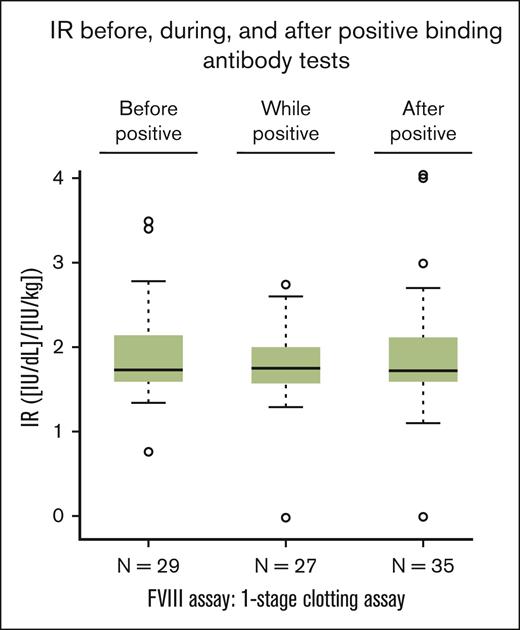
Samples for the analysis of a potential modulation of the IR by antibodies binding to FVIII, PEG-FVIII, or PEG were available for ∼50% of the patients who tested positive for any of these antibodies (Figure 6). The IR was determined before, during, and/or after the first positive test result. The box plots shown in Figure 6 do not indicate a modulation of the IR by antibodies binding to FVIII, PEG-FVIII, or PEG.
IR in patients with positive results of antibodies binding to FVIII, PEG-FVIII, or PEG. IR was calculated as an increase of FVIII level in IU/dL after infusion from the value before infusion divided by the dose per body mass in IU/kg. Box-and-whiskers plots of IR before (“before positive”), during (“while positive”), and after (“after positive”) any positive results for binding antibodies were drawn with the boxes featuring medians and quartiles whereas the whiskers extended to 1.5 times the interquartile range from the boxes. One patient each in the “while positive” group and in the “after positive” group had an apparent IR close to 0. FVIII level by the 1-stage clotting assay changed from 25.4 IU/dL to 24.4 IU/dL in the former, and from 1.3 IU/dL to 1.0 IU/dL in the latter. Both patients had IRs in the expected range both before and after reporting an apparent IR close to 0; therefore, it is likely that these values close to 0 represent preanalytical mistakes, such as errors in sample labeling or sample preparation.
IR in patients with positive results of antibodies binding to FVIII, PEG-FVIII, or PEG. IR was calculated as an increase of FVIII level in IU/dL after infusion from the value before infusion divided by the dose per body mass in IU/kg. Box-and-whiskers plots of IR before (“before positive”), during (“while positive”), and after (“after positive”) any positive results for binding antibodies were drawn with the boxes featuring medians and quartiles whereas the whiskers extended to 1.5 times the interquartile range from the boxes. One patient each in the “while positive” group and in the “after positive” group had an apparent IR close to 0. FVIII level by the 1-stage clotting assay changed from 25.4 IU/dL to 24.4 IU/dL in the former, and from 1.3 IU/dL to 1.0 IU/dL in the latter. Both patients had IRs in the expected range both before and after reporting an apparent IR close to 0; therefore, it is likely that these values close to 0 represent preanalytical mistakes, such as errors in sample labeling or sample preparation.
Discussion
Here, we present the results of a comprehensive analysis of immunogenicity data generated from 360 PTPs with severe hemophilia A who were treated with rurioctocog alfa pegol, a full-length, extended half-life, recombinant FVIII, PEGylated with a branched 20-kDa PEG,25,30,31 during 6 clinical studies.25,34-37 These studies were part of the clinical development program for rurioctocog alfa pegol in which the patient inclusion criteria followed the relevant regulatory guidelines; therefore, these studies do not reflect the entire range of patients encountered in clinical practice.
The immunogenicity assessment focused on the analysis of treatment-emerging FVIII inhibitors; preexisting and treatment-emerging IgM and IgG antibodies binding to FVIII, PEG-FVIII, or PEG; and treatment-emerging antibodies binding to CHO host cell proteins. Moreover, the potential association of these antibodies with treatment outcomes (IR and spontaneous bleeding events) and AEs observed during the 6 studies was analyzed.
A limitation of this analysis is that data were pooled from 6 clinical studies of different durations in which rurioctocog alfa pegol was administered at different doses and dosing regimens. However, it is important to acknowledge that the data across all 6 studies were generated in central laboratories using the same assay platforms for the analysis of FVIII inhibitors and the same fully validated enzyme-linked immunosorbent assay technologies for the analysis of binding antibodies. Another limitation is that the FVIII inhibitor analysis was conducted at 2 different central laboratories. The Medical University of Vienna analyzed all samples from study NCT01599819, a phase 1 study, whereas all samples from the other 5 studies were analyzed by Esoterix.
None of the 360 PTPs included in the study developed persistent FVIII inhibitors. One of the patients developed low-titer transient FVIII inhibitors. The FVIII inhibitor titer was at the lowest limit of positivity. Surprisingly, samples from the same patient that were tested for IgM and IgG antibodies specifically binding to FVIII, PEG-FVIII, or PEG were negative at all investigated time points. This apparent discrepancy could be due to plasma constituents causing a false-positive result in the FVIII inhibitor assay but a negative result in the assay for the detection of antibodies specifically binding to FVIII. The inhibitor analysis for this patient was conducted by Esoterix using Siemens Dade Actin FSL as the APTT reagent, which is known to be lupus-anticoagulant sensitive. Lupus anticoagulants have been shown to interfere with the FVIII inhibitor assay and cause a low-titer positive result in the absence of FVIII inhibitors40,41; therefore, we cannot exclude the possibility that low-titer inhibitors of 0.4 to 0.6 BU/mL could be false positive results owing to the presence of lupus anticoagulants. An alternative explanation could be the presence of nonspecific antibodies binding to FVIII, which could cause a positive result in the inhibitor assay but a negative result in the assay for the detection of FVIII-specific antibodies.41 We also cannot exclude the possibility that the patient had low-titer FVIII-specific binding antibodies (eg, antibodies with titers of 1:20 or 1:40), which were below the limit of confirmation (titer of 1:80) for FVIII specificity of the detected antibodies in the assay platform.
Fifty-four patients either had preexisting antibodies binding to FVIII, PEG-FVIII, or PEG before first exposure to rurioctocog alfa pegol or developed the binding antibodies after exposure to the study drug. Although there was a temporal association between the detection of binding antibodies and AEs in a number of patients, we did not observe any causal relationship between the detection of these antibodies and any AEs or an increased occurrence of spontaneous bleeding episodes. Moreover, we did not observe a significant impact of binding antibodies on the in vivo IR of rurioctocog alfa pegol. However, due to the limited availability of samples, the potential effect of binding antibodies on the IR could only be investigated in approximately half of the patients who developed such binding antibodies. Although available data indicating no effect are reassuring, the large amount of missing data precludes the drawing of any final conclusions from this analysis.
Recently, several papers indicated that antibodies binding to PEG could potentially affect the in vivo IR of PEGylated FVIII given to patients42-44 and could even induce inhibitory activities resulting in the neutralization of PEGylated FVIII in vitro and in vivo.32 Pezeshkpoor et al32 presented data indicating that antibodies against the PEG moiety of a 40PEG-BDDFVIII drug (B-domain–deleted FVIII PEGylated with a 40-kDa PEG) abolished the efficacy of the drug. The authors characterized antibodies binding to PEG, which were observed in 2 of 46 patients with mild hemophilia A who were treated with the 40PEG-BDDFVIII drug. One of the patients developed antibodies against both the FVIII and the PEG moiety, and the other patient developed antibodies only against the PEG moiety.32 No FVIII inhibitors were detected; however, anti-PEG antibodies isolated from these 2 patients inhibited the activity of the 40PEG-BDDFVIII both in vivo and in vitro. Interestingly, the anti-PEG antibodies found in these 2 patients also bound to other PEGylated therapeutics such as 20PEG-FLFVIII rurioctocog alfa pegol, 40PEG-BDDFVIII turoctocog alfa pegol (N8-GP; Novo Nordisk), 60PEG-BDDFVIII damoctocog alfa pegol (BAY 94–9027; Bayer), and 40PEGFIX nonacog beta pegol (N9-GP; Novo Nordisk).32 Although FVIII compounds PEGylated with 40 kDa PEG or 60 kDa PEG were completely inhibited by the antibodies, with an inhibition kinetic typical for type 1 inhibitors, FVIII PEGylated with a 20 kDa PEG was only partially inhibited, with an inhibition kinetic typical for type 2 inhibitors.32
In the studies presented in this article, preexisting antibodies binding to PEG were found in 6 patients, and all of them were IgM antibodies. Moreover, 1 patient developed transient IgM antibodies binding to PEG during treatment with rurioctocog alfa pegol. Preexisting antibodies binding to PEG-FVIII were found in 29 patients: 6 patients tested positive for IgM antibodies, 22 patients tested positive for IgG antibodies, and 1 patient tested positive for both IgM and IgG antibodies. Moreover, 14 patients developed IgG antibodies binding to PEG-FVIII during their treatment. None of the patients who presented with either preexisting or treatment-emerging antibodies binding to PEG or PEG-FVIII had detectable FVIII inhibitors at any time during the studies.
We did not adapt the assay for FVIII inhibitors to include PEG-FVIII as the target protein. Therefore, we cannot completely exclude that some of the antibodies against PEG or the PEG moiety of PEG-FVIII might have had some inhibitory activity against PEG-FVIII. However, we did not observe any causal relationship between the detection of antibodies binding to PEG-FVIII or PEG and any potentially increased occurrence of spontaneous bleeding episodes in patients. Therefore, we believe that the patients included in our studies did not develop any antibodies binding to PEG that caused a clinically relevant inhibition of rurioctocog alfa pegol. In contrast to the 40PEG-BDDFVIII used in the study presented by Pezeshkpoor et al,32 rurioctocog alfa pegol is a full-length FVIII PEGylated with a 20 kDa branched PEG conjugated to primary amine residues that are mostly located at the surface of the FVIII molecule, mainly within the B-domain.25,30-32 Therefore, rurioctocog alfa pegol might have a reduced propensity to induce inhibitory anti-PEG antibodies in patients compared with 40PEG-BDDFVIII. Moreover, the smaller PEG size in rurioctocog alfa pegol provides fewer available PEG polymer binding sites for antibodies against PEG, which might result in less inhibition of the PEG-FVIII molecule by anti-PEG antibodies. This hypothesis is supported by the in vitro data reported by Pezeshkpoor et al indicating that the inhibitory anti-PEG antibodies completely inhibited the biological activity of FVIII compounds PEGylated with a 40-kDa PEG or a 60-kDa PEG but only partially inhibited PEGylated FVIII with a 20-kDa PEG.32
Another difference is that the patient population used in the different studies. Pezeshkpoor et al32 included patients with mild hemophilia A who had either no or limited exposure to FVIII products before the study, whereas the studies presented in our article included patients with severe hemophilia A (FVIII activity level of <1%) who had previously received treatment with either plasma-derived or recombinant FVIII for ≥50 EDs (patients aged <6 years) or ≥150 EDs (patients aged ≥6 years).25,34-37
In conclusion, the data presented in this article indicate that rurioctocog alfa pegol has a low immunogenicity profile and is not associated with any unexpected immunogenicity characteristics when administered to 360 PTPs with severe hemophilia A.
Acknowledgments
The authors thank all the patients and their caregivers who took part in the clinical studies as well as the study investigators and sites.
The studies were funded by Baxalta Innovations GmbH, a Takeda company, Vienna, Austria; and by Takeda Development Center Americas, Inc, Lexington, MA. Under the direction of the authors, editorial support was provided by Excel Scientific Solutions, Inc., a member of Envision Pharma Group (Fairfield, CT), and was funded by Takeda Development Center Americas, Inc, Lexington, MA.
Authorship
Contribution: F.M.H. designed and supervised the immunological program of the study, reviewed and interpreted data, created the figures with antibody data, and wrote, reviewed, and revised the manuscript; B.M.R. designed the immunological program of the study, reviewed and interpreted data, created the figures with antibody data, and wrote, reviewed, and revised the manuscript; P.A. designed and established the antibody assays, supervised the immunological data analysis, reviewed and interpreted the immunological data, and reviewed and revised the manuscript; W.E. contributed to the conception and design of the clinical studies as well as to the analysis and interpretation of the clinical study data, and wrote and revised the manuscript; L.P. interpreted the clinical study data, and reviewed and revised the manuscript; S.T. contributed to the design of some of the clinical studies as well as to the analysis and interpretation of clinical study data, and wrote and revised the manuscript; and all authors gave their final approval for the manuscript to be published.
Conflict-of-interest disclosure: F.M.H. was previously an employee of Baxalta Innovations GmbH, a Takeda company, Vienna, Austria. F.M.H. is currently an employee of BioAgilytix Europe GmbH. B.M.R. was previously an employee of Baxalta Innovations GmbH, a Takeda company, Vienna, Austria. P.A.’s institution operated as a contract research organization for Baxalta Innovations GmbH, a Takeda company, Vienna, Austria. W.E. was an employee of Baxalta Innovations GmbH, a Takeda company, Vienna, Austria, at the time of the study; and was a Takeda stock owner at the time of the study. L.P. is an employee of Takeda Development Center Americas, Inc, Cambridge, MA; and is a Takeda stock owner. S.T. is an employee of Takeda Development Center Americas, Inc, Cambridge, MA; and is a Takeda stock owner.
Correspondence: Srilatha Tangada, Takeda Development Center Americas, Inc., 650 East Kendall Street, Cambridge, MA, 2142; email: srilatha.tangada@takeda.com.
References
Author notes
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available, within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its deidentification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
The full-text version of this article contains a data supplement.