Of requested URDs, only half are available for CT, and availability for workup of those requested is <70%.
Availability is markedly worse for non-European ancestry patients, especially those of African, non-Black Hispanic, and Asian heritage.
Visual Abstract
Despite the global unrelated donor (URD) registry size, the degree to which URD availability is a transplant barrier is not established. We evaluated the availability of 3,843 URDs requested for 455 diverse adult patients (predominantly with acute leukemia). URDs for non-Europeans were more likely to be domestic and had markedly lower Donor Readiness scores. Of URDs requested for confirmatory HLA-typing (CT) alone (ie, without simultaneous workup), 1,894 of 3,529 (54%) were available. Availability of domestic URDs was 45%. Donor Readiness score was highly predictive of CT availability. More non-European patients (n = 120) than Europeans (n = 335) had >10 URDs requested and <5 available. Of workup requests (after CT or CT-workup), <70% (604/889 [68%]) were available. More non-Europeans had <2 URDs available. URD availability for CT was markedly worse for non-Europeans, with availabilities for African, non-Black Hispanic, and Asian patients being 150/458 (33%), 120/258 (47%), and 119/270 (44%), respectively, with further decrements in URD workup availability. Our data suggest the functional size of the URD pool is much smaller than appreciated, mandating major operational changes for transplant centers and donor registries. Likelihood of donor availability should have a high priority in donor selection. Considering patient ancestry and URD Donor Readiness scores, centers should pursue, and registries permit, simultaneous pursuit of many URDs and abandon futile searches. Patients should be informed about their likelihood of donor availability and alternative options. Finally, although registries should address high URD attrition and speed procurement, use of all HLA-disparate graft types is needed to facilitate timely transplant for all.
Introduction
Although allogeneic transplantation can be curative for patients with high-risk hematologic malignancies, the majority of transplant candidates lack a human leukocyte antigen (HLA)-identical sibling donor. Unrelated donor (URD) transplants are the most common alternative,1 and their use is increasing with the advent of posttransplant cyclophosphamide or abatacept-based graft-versus-host disease prophylaxis.2-4 This trend may be enhanced by the emerging data that could support the use of younger 8/8 URDs over older sibling donors,5 the recognition of the limitations associated with older haploidentical donors6,7 or those against whom the patient has donor-specific HLA antibodies,8-10 and the decline in cord blood transplant activity.1
For these reasons, prompt URD availability is critically important, especially as acute leukemia and other aggressive myeloid malignancies are the most common transplant indications.1 Traditionally, the priority in URD selection has been optimizing the HLA match. More recently, the advantage of using a younger URD (ie, age ≤35 years) has been demonstrated.7 However, donor availability has received much less emphasis. Therefore, to further investigate whether donor availability is a major barrier to URD transplantation, we evaluated real-world donor availability in a patient population of diverse ancestry. Our hypothesis was that disparities in URD availability exist for underserved racial/ethnic patient populations. A second hypothesis was that the limited donor availability for these populations has not improved in the postpandemic era.
Methods
Patient inclusion
We evaluated availability of requested URDs during the period from January 2020 to December 2022, overall and by patient ancestry, for 455 consecutive adults with acute leukemia, myelodysplastic syndrome, or myeloproliferative neoplasms who had a formal URD search and for whom at least 1 URD was requested for confirmatory typing (CT) or simultaneous CT-workup.
Donor prioritization and identification
During the study period, in the absence of an HLA-identical sibling donor, an 8/8 HLA allele-matched URD was prioritized followed by HLA-disparate grafts including mismatched (5-7/8) URD, double unit cord blood, or haploidentical grafts. All patients underwent URD search before being considered for either a cord blood or haploidentical donor transplant.
Definitions
Requested URDs were considered “available for CT” if they were contacted by their donor registry and provided a sample for confirmatory high-resolution HLA typing. Requested URDs were considered “available for workup” if they agreed to proceed with the donation process, underwent workup, and were cleared for donation. “Simultaneous CT-workup” URDs were requested for patients who were especially urgent. These donors were considered available if they completed the requirements defining availability for both CT and workup. The number of donors initially pursued (for either CT or simultaneous CT-workup) for each patient was based on patient urgency, patient ancestry, and the URD search prognosis11, as well as the preferred graft source if an 8/8 URD could not be identified. An individually assigned transplant coordinator closely monitored each patient’s search and pursued additional URDs as needed.
Patient ancestry was based on detailed kinship history recorded by transplant staff during the pretransplant evaluation. This evaluation of the patient’s ancestors’ countries of origin and whether the patient self-identified as Black and/or Hispanic was performed as previously described.12 The most recent Donor Readiness score13 (the prediction of a donor’s likelihood of availability for CT) logged by the registry was recorded when available. URD race/ethnicity was not available for many, and therefore, this information was not collected. For the analysis of URD availability by era, the years 2020 and 2021 were considered pandemic and 2022, postpandemic.
Statistical methods
Descriptive statistics were reported using median and range for continuous variables and frequency and percentages for categorical variables. Differences in baseline characteristics by ancestry were assessed using Wilcoxon rank-sum test for continuous variables and Pearson χ2 test or Fisher's exact test for categorical variables. All analyses were performed in R version 4.0.5. This study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center and conducted in accordance with the Declaration of Helsinki.
Results
Patient and donor characteristics
Of the 455 patients (median age, 63 years), approximately one-quarter (26%) had non-European ancestry, and more than half (55%) had acute leukemia (Table 1).
For these 455 patients, a total of 3,843 URDs (median age, 27 years) were requested for CT (n = 3,529 [92%]) or simultaneous CT-workup (n = 314 [8%]; Table 2). Overall, 2,730 URDs (71%) were requested for European patients and 1,113 (29%) for non-European patients (including 458 URDs for 40 African, 258 for 33 non-Black Hispanic, and 270 for 31 Asian patients). Additionally, 56% (n = 2,140) of URDs were from domestic and the remainder from international registries. URDs requested for non-European ancestry patients had higher proportions with age >35 years and female sex, although these differences were modest. Notably, URDs requested for non-European ancestry patients were more likely to be domestic (Europeans, 1,411/2,730 [52%] vs non-Europeans, 729/1,113 [65%]; P < .001), with 71% of URDs requested for African ancestry and non-Black Hispanic patients being domestic.
Of 2,775 URDs with an assigned Donor Readiness score, the median score was 68% (range, 16-94), Table 3. URDs for non-European ancestry patients had markedly lower median Donor Readiness score (Europeans 71% vs non-Europeans 52%) with 12 times the proportion with Donor Readiness score ≤30% (Europeans, 32/2,073 [2%] vs non-Europeans, 168/702 [24%]; P < .001). One-third of URDs requested for African ancestry patients had a Donor Readiness score of ≤30%.
URD availability: overall and by donor demographics
Overall, of those requested for CT alone (ie, excluding those requested for simultaneous CT-workup), only approximately half (1,894/3,529 [54%]) of URDs were available for CT (Table 4). Of donors who were confirmatory typed, approximately three-quarters (459/575 [80%]) of those subsequently requested for workup were available, whereas less than half (145/314 [46%]) of requested donors were available for simultaneous CT-workup.
Donor age (≤35 vs >35 years) had no impact on URD availability. As shown in Table 4, donor sex had a modest impact, with a higher proportion of male donors available for CT or simultaneous CT-workup. Notably, donor location was strongly associated with CT availability. Less than half of domestic URDs were available. Although international URDs had a nearly 20% greater proportion of being available for CT than domestic URDs (64% vs 45%; P < .001), this was driven by donor location with marked variability according to country of origin. Of the countries from where >50 URDs were requested for CT, URD availability was as follows: Germany 71%, Israel 69%, Poland 69%, United Kingdom 55%, and Brazil 41%.
Donor Readiness score was strongly associated with URD availability for CT (Table 5). Of those URDs with a score >70%, 70% were available for CT. CT availability progressively declined with lower scores, with only 15% of URDs with a score ≤30% being available (P < .001).
URD availability for CT per patient
URD availability for CT per patient (3,843 URDs for 455 patients) is shown in Table 6. A median of 7 URDs (range, 0-30) were requested for CT per patient (excluding simultaneous CT-workup), with >70% (328/455) of patients having at least 5 URDs requested and 21% (96/455) with >10. Additionally, for 132 of 455 patients (29%), at least 1 donor was requested for simultaneous CT-workup. Taken together, for >90% of patients, at least 4 URDs were initially pursued simultaneously. A greater percentage of non-European patients had >10 URDs requested, including, for example, 40% of African ancestry patients.
Although the 455 patient cohort had a median of 4 (range, 0-11) URDs available for CT, a median of 3 (range, 0-23) requested URDs were unavailable, with nearly a third of patients (136/455 [30%]) having at least 5 URDs unavailable. Higher proportions of non-European ancestry patients had at least 10 URDs requested (P = .045) and less than 5 available for CT (P < .001). Moreover, nearly double the proportion of non-Europeans had at least 5 URDs unavailable (54/114 [47%] vs 82/331 [25%]; P < .001). Notably, African ancestry patients had the highest proportion with two-thirds having at least 5 URDs unavailable (25/38 [66%]).
URD availability for workup per patient
For nearly a quarter of patients (109/455 [24%]), no URDs were requested for workup or simultaneous CT-workup, with a higher proportion of these patients having non-European ancestry (P = .005). Furthermore, for more than one-quarter of patients (134/455 [29%]), 3 to 5 URDs were all requested for workup or simultaneous CT-workup.
Among 281 patients with at least 1 URD requested for workup after CT, a median of 2 URDs (range, 0-5) were available (Table 7). Non-European ancestry patients had fewer URDs available for workup (P = .007). They also had more URDs unavailable (P = 0.017), with greater than twice the proportion with at least 2 unavailable (Europeans, 15/217 [7%] vs non-Europeans, 10/63 [16%]). African ancestry patients had the highest proportion (14/19 [74%]) with at least 1 URD unavailable for workup.
Among 132 patients for whom at least 1 URD was requested for simultaneous CT-workup, the majority (n = 78 [59%]) had only 1 URD who was available, with 22 (17%) having none and 32 (24%) having 2 to 3. Of these patients, more than a fifth (n = 30 [23%]) had ≥2 URDs unavailable.
Overall URD availability
Overall URD availability is shown visually in Sankey diagrams in Figure 1 and summarized in Table 8. Of URDs requested for CT (either alone or simultaneous CT-workup), only half (2,039/3,843 [53%]) were available, and of the total 889 URDs requested for workup (either after CT or for simultaneous CT-workup), <70% (604/889 [68%]) were available (Figure 1A). URDs requested for non-European patients were less likely to be available for CT or workup (P < .001 for both). An additional 20 URDs became unavailable for donation after workup.
African and non-Black Hispanic patients had markedly worse URD availability. Only one-third of URDs (150/458 [33%]) requested for African patients were available for CT, with less than half (32/77 [42%]) available for workup (Figure 1B). Less than half of URDs (120/258 [47%]) requested for non-Black Hispanic patients were available for CT, with less than two-thirds (37/61 [61%]) available for workup (Figure 1C). Patients of Asian ancestry also had limitations in URD availability as shown in Figure 1D.
Additionally, among 339 patients for whom at least 1 5-8/8 URD was activated for workup or simultaneous CT-workup and collection dates were requested, nearly a quarter of patients (77/339 [23%]) had no URDs who could collect within 7 days of the first proposed date, and 39 of 339 (12%) had no URDs who could collect within 14 days of the first proposed date.
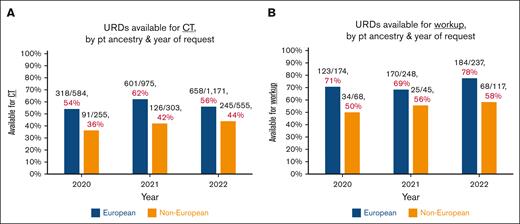
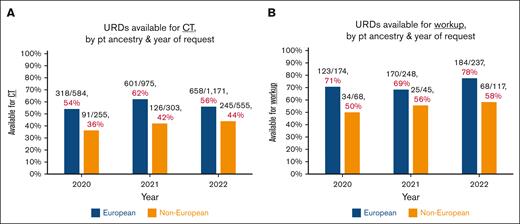
URD availability by era
To investigate the impact of the pandemic during the 2020 to 2022 study period, URD availability for CT (Figure 2A) and workup (Figure 2B) was analyzed by year. For European ancestry patients, there was no improvement in availability for CT and a modest improvement in URD availability workup in 2022 (postpandemic) relative to 2021. Availability rates for non-Europeans in 2022 vs 2021 for both CT and for workup were essentially unchanged.
Donor search outcomes by patient ancestry over time. (A-B) Donors available for CT (A) or workup (B), by year. As URDs who were activated for simultaneous CT-workup were evaluated for availability of both, these URDs are included in both figures. The marked disparities in URD availability by patient ancestry are demonstrated. Notably, in 2022 (considered postpandemic), there was no appreciable improvement as compared with 2021.
Donor search outcomes by patient ancestry over time. (A-B) Donors available for CT (A) or workup (B), by year. As URDs who were activated for simultaneous CT-workup were evaluated for availability of both, these URDs are included in both figures. The marked disparities in URD availability by patient ancestry are demonstrated. Notably, in 2022 (considered postpandemic), there was no appreciable improvement as compared with 2021.
Analysis of patients who underwent transplant
Of 304 8/8 URD or mismatched graft recipients, the majority received 8/8 URD grafts (219/304 [72%]); others (85/304, 28%) received HLA-disparate grafts (20 cord blood, 18 haploidentical, and 47 with 5-7/8 URD; supplemental Table 1). Eight patients ultimately received an HLA-identical sibling donor. In these patients, URD search had been pursued due to a significant delay in related donor availability. As expected,7,14-16 non-European ancestry patients were less likely to receive 8/8 URDs (P < .001), and for those who did, their URDs were older (P = .018), with non-Europeans receiving nearly double the proportion of >35 years (20/181 [11%] vs 8/38 [21%]). Furthermore, the 5-7/8 URDs for non-Europeans were more HLA mismatched (P < .001). Overall, 143 of 455 (31%) patients did not undergo transplant, predominantly due to disease progression and/or prohibitive comorbidities.
Next, we evaluated 263 patients who underwent transplant for whom a most preferred 5-8/8 URD was selected by the transplant team at the time of search formalization. Notably, of these patients, more than one-third (103/263, 39%) did not undergo transplantation with their most preferred URD. This was due to donor availability or collection center scheduling delays or a combination of these factors.
Discussion
In this real-world analysis, the first of its kind to the best of our knowledge, we demonstrate significant ongoing disparities in URD access that have major implications for transplant center and registry operations. Our data suggest that the functional inventory of young, healthy, and readily available URDs is likely to be modest and potentially critically small for specific underserved racial/ethnic populations. Importantly, for non-European ancestry patients and especially those of African, non-Black Hispanic, or Asian origins, it must be assumed that, regardless of match grade, the majority of requested URDs will not be available. Accordingly, search strategy and pursuit of more mismatched URDs or other donor types need to be considered. Notably, the comparison of donor availability during the year of 2022 vs 2021 suggests that our findings cannot be purely explained by the adverse impact of the COVID-19 pandemic.17
Another finding of our study is that, when available, the Donor Readiness Score revealed markedly lower scores for donors requested for non-European ancestry patients. Registries must address the high donor attrition and work to improve Donor Readiness scores and URD availability especially for US domestic donors, younger donors,18 and those of non-European ancestry,19-22 given their higher attrition rates and lower availability. These interventions include regular engagement with listed URDs to renew their commitment and reassess eligibility.23 Such work should proceed in collaboration with advocates from underserved/underrepresented populations24-26 and alongside efforts to address donor discrimination27 and overcome donor mistrust of the health care system.
Recently, Auletta et al28 have described structural factors within the health care system that result in disparities in allogeneic transplantation care delivery. Our analysis emphasizes that the disparities in volunteer donor availability must also be addressed. This challenge, together with the lack of representation of ancestrally diverse populations across US and global donor pools, will become increasingly problematic given the rapid diversification of the US population combined with ongoing socioeconomic and cultural challenges that affect donor availability. Short staffing is also ongoing in many centers adding additional capacity challenges for stem cell collections.
From a practical standpoint, incorporating careful assessment of patient ancestry and URD Donor Readiness scores, transplant centers should pursue, and registries should permit, simultaneous pursuit and evaluation of multiple URDs, especially for non-European ancestry patients. For many patients, concurrent activation of multiple URDs should proceed despite the additional cost incurred (accepting that searches for many non-European ancestry patients will, therefore, be more labor intensive and expensive). Efforts are needed to ensure reimbursement of these increased costs including through Medicare/Medicaid.29 Lack of addressing this barrier to URD availability will disproportionately adversely affect underserved patient populations. Centers need internal guidelines outlining the maximum degree of URD HLA mismatch that will be acceptable for their program. Physicians should guide coordinators in the degree of transplant urgency, with search strategy adjusted accordingly. Centers should also determine the prioritization of haploidentical donors relative to URDs and whether cord blood grafts will be considered if no adult donor can be secured in the time required for appropriate care, especially given use of cord blood greatly speeds transplantation as we have previously reported.15 Moreover, centers are ethically obliged to inform patients whether timely URD procurement is unlikely,11,30,31 especially because these patients are commonly from underserved racial/ethnic groups. The futility of pursuing a matched donor and the potential need for alternative donors should also be explained. There may be a role for patient advocates to counsel transplant candidates at formal search initiation to discuss these challenges and treatment alternatives. Registries also need to clearly communicate to potential donors the rationale for securing backup donors and the possibility that another donor may be used instead.
Our analysis also highlights the importance of prospective transplant center analyses of URD procurement and not relying on modeling and a pure focus on HLA match and other donor demographics such as age.32-36 Now that mismatched URDs are feasible, donor availability becomes 1 of the highest priorities in URD selection. Furthermore, our study not only emphasizes the importance of real-world data but also begs the question of how many patients by recipient ancestry develop disease progression precluding transplantation during lengthy URD searches and whose responsibility it is to abandon futile URD searches.
Finally, prospective37 efforts are needed to mitigate donor attrition and speed donor identification (including the simultaneous activation for CT/workup of multiple URDs as appropriate). We have recently refined an URD search prognosis tool11 that represents an advance from prior reports.30,31 It facilitates rapid triage to alternative donors if the estimated likelihood of identifying an 8/8 URD is poor. Transplant center donor selection algorithms should incorporate careful examination of search prognosis as well as Donor Readiness scores. Futile searches, defined as those that will not yield a guaranteed URD of the minimal acceptable attributes in the time required for appropriate clinical care, should be abandoned. Utilization of all alternative donors (ie, 5-7/8 URD,3,38 haploidentical,39 and cord blood40-45) is needed to facilitate donors for all in the time required for optimized transplantation. These efforts as well as addressing the intersectional impacts of patient socioeconomic status,16 including insurance coverage,46 language barriers,47 and health literacy, will be critical to address structural barriers to care48 and advance equity for allograft candidates across populations.
Acknowledgments
The authors thank the Memorial Sloan Kettering Cancer Center transplant coordinators for assisting in detailed patient ancestral evaluations and their diligent work in performing searches and securing donors.
This work was supported in part by the National Cancer Institute P30 CA008748.
Authorship
Contribution: W.B.F., E.D., A.A., and J.N.B. designed the study, assembled and analyzed the data, and wrote the manuscript; S.B. and S.D. performed the statistical analysis; E.D., A.A., M.N., C.R., S.C., A.K., and D.W. maintained the patient database and provided data; and all authors read and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: A.S. serves as a consultant at the scientific advisory board of ExCellThera. B.G. has received research funding from Actinium Pharmaceuticals; and serves on the data and safety and monitoring board for Synthetic Biologics, Inc. I.P. has received research funding from Merck and serves as a member on a data and safety monitoring board for ExCellThera. The remaining authors declare no competing financial interests.
Correspondence: Warren B. Fingrut, Department of Medicine, Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: wfingrut@gmail.com; and Juliet N. Barker, Department of Medicine, Bone Marrow Transplant and Cellular Therapy Program, Weill Cornell Medicine, New York, NY; email: jub2029@med.cornell.edu.
References
Author notes
W.B.F. and E.D. contributed equally.
Original data from the study are available upon reasonable request from the author, Eric Davis (davise@mskcc.org).
The full-text version of this article contains a data supplement.

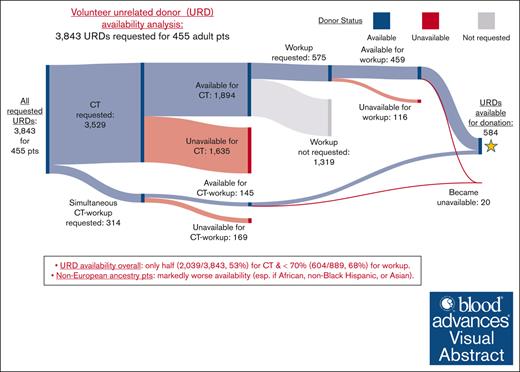

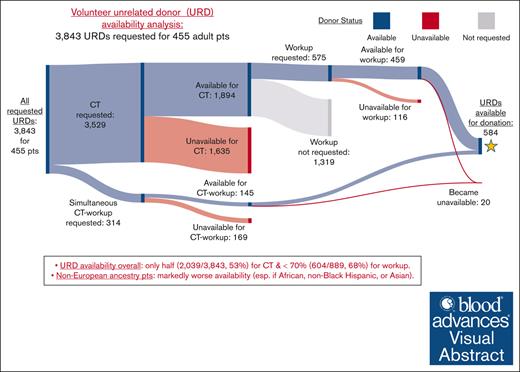
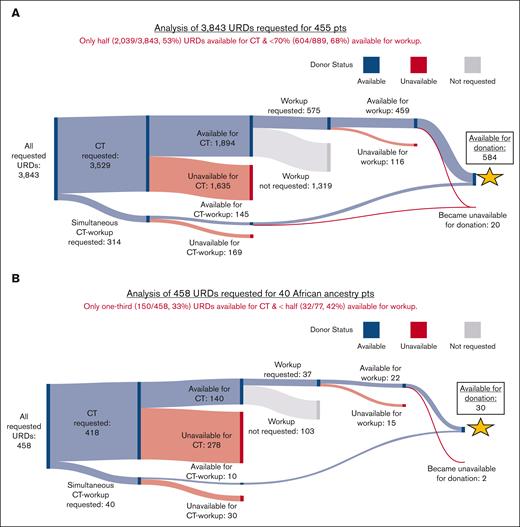
![Sankey diagrams showing availability of requested URDs for CT, workup, and donation. URDs were predominantly requested first for CT, and suitable URDs were then selected for workup. When transplants were urgent, URDs were requested for simultaneous CT-workup. Available URDs at each step of this process are shown in blue, unavailable URDs in red, and URDs who were available for CT but not requested for workup in gray. (A) Results for 3,843 URDs requested for all 455 patients. Of 3,529 URDs requested for CT, 1,894 URDs were available. Of 314 for simultaneous CT-workup, 145 were available. Taken together, only half (2,039/3,843 [53%]) of the requested URDs were available for CT. Of those selected for workup, <70% (604/889 [68%]) were available. Most of those who underwent workup (584/604 [97%]) were available for donation. (B) Results for 458 URDs requested for 40 African ancestry patients. (C) Results for 258 URDs requested for 33 non-Black Hispanic ancestry patients. (D) Results for 270 URDs requested for 31 Asian ancestry patients. Pts, patients.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/11/10.1182_bloodadvances.2023012385/2/m_blooda_adv-2023-012385-gr1b.jpeg?Expires=1768250735&Signature=h5joo8PQUDFWdkdQHmrU7yIo14ju79GcgTiPgQBs2KuaF4ZzFglCIhi6lqdkxzcwImXjXRjnRyNFSO8uHSwjtHViXGOcpav-pLVIYunjQFLvkkqSIZTm1341ARgUsjNpJL8O5X9o42G3oiltkJEFoBiew2p1LquAcnZxM6wS3cEegcebbJiAM3pykPGSN9SpPmUaNXT3cn28T5gSNXa~IcWFFmlyQRNkPtwJl4PP3vbiy4CaMOoUz3iz2YTpTTXqpgR4Q0~MWg-DZSdfSwAo3okWL60EoNpRKq65jGPqsoIq6AZ7i0OKCEPxBbO5baytTBNZ5d7exnxw5BefQKXT3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)