Outcomes for patients who received cellular therapy appeared better when the therapy was given in 2L vs 3L.
Further study is needed to determine whether 3L cellular therapy is a viable option for those with initial response to 2L chimeric antigen receptor T-cell therapy.
Visual Abstract
The optimal management of patients with relapsed/refractory large B-cell lymphoma (LBCL) after disease progression or lack of response to second-line (2L) therapy remains unclear. Here, we report outcomes among patients who received subsequent antilymphoma therapy per investigator discretion separately by their randomized 2L arm in ZUMA-7, namely axicabtagene ciloleucel (axi-cel) vs standard of care (SOC). Progression-free survival (PFS) and overall survival (OS) were calculated from 3L therapy initiation. In the SOC arm, 127 of 179 randomized patients (71%) received 3L therapy. Median PFS among those who received 3L cellular immunotherapy (n = 68) vs those who did not (n = 59) was 6.3 vs 1.9 months, respectively; median OS was 16.3 vs 9.5 months, respectively. In the axi-cel arm, 84 of 180 randomized patients (47%) received 3L therapy. Median PFS among those who received 3L chemotherapy (n = 60) vs cellular immunotherapy (n = 8) was 1.7 vs 3.5 months, respectively; median OS was 8.1 months vs not reached, respectively. Of the 60 patients who received 3L chemotherapy, 10 underwent stem cell transplantation (SCT) after salvage chemotherapy. Median PFS was 11.5 vs 1.6 months, and median OS was 17.5 vs 7.2 months for those who did vs did not reach SCT, respectively. Eight patients received 3L cellular immunotherapy after 2L axi-cel. Of these, 6 patients received subsequent SCT in any line; all 6 were alive at data cutoff. These findings help inform subsequent treatment choices after 2L therapy failure for relapsed/refractory LBCL. The trial was registered at www.clinicaltrials.gov as #NCT03391466.
Introduction
For nearly 30 years, the standard second-line (2L) treatment for large B-cell lymphoma (LBCL) with curative intent was salvage chemotherapy, followed in patients with a response by high-dose chemotherapy and autologous stem cell transplantation (HDT-ASCT).1 However, outcomes for patients who cannot proceed to HDT-ASCT are poor.2
Anti-CD19 chimeric antigen receptor (CAR) T-cell therapies have shown marked benefit for relapsed/refractory (R/R) LBCL in the 2L and third-line (3L) settings.1,3-8 Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 CAR T-cell therapy approved for the treatment of adults with R/R LBCL after ≥2 lines of systemic therapy and for patients refractory to or who relapsed within 12 months of first-line (1L) chemoimmunotherapy.6,9-11 In ZUMA-1 (NCT02348216), axi-cel demonstrated an 83% objective response rate (ORR), a 58% complete response (CR) rate, and a 5-year overall survival (OS) rate of 43% among patients with refractory LBCL.3,12 The global, randomized, phase 3 ZUMA-7 study (NCT03391466) assessed axi-cel vs standard of care (SOC) as 2L therapy in patients with early R/R LBCL.6 The primary analysis of event-free survival demonstrated superiority of axi-cel (n = 180) vs SOC (n = 179; hazard ratio [HR], 0.398; stratified log-rank P < .0001; median follow-up, 24.9 months).6 ORR per-blinded central review was 83% in the axi-cel arm and 50% in the SOC arm, with a CR rate of 65% and 32%, respectively.6 The primary OS analysis of ZUMA-7 (median follow-up, 47.2 months) demonstrated a statistically significant improvement in OS with axi-cel over SOC (HR, 0.73; stratified 2-sided log-rank P < .03); median OS was not reached in the axi-cel arm (95% confidence interval [CI], 28.6 months to not estimable [NE]) and was 31.1 months (95% CI, 17.1 to NE) in the SOC arm.13
CAR T-cell therapy has been proposed as the new SOC for 2L treatment based on ZUMA-7 and TRANSFORM.6-8 However, the question of optimal management after 2L therapy remains for patients who require additional therapy due to lack of response or disease progression.8,14 Here, we describe outcomes of patients who received subsequent antilymphoma therapy in ZUMA-7.
Methods
Patients and study design
Full ZUMA-7 study details were previously reported.6 Patients aged ≥18 years with histologically confirmed LBCL15 were eligible for enrollment if they had R/R disease after adequate 1L chemoimmunotherapy. Patients were randomized 1:1 to axi-cel or SOC and stratified based on response to 1L therapy and 2L age-adjusted International Prognostic Index.6 Patients in the axi-cel arm were treated with a single infusion of 2 × 106 CAR T cells/kg after 3 days of lymphodepleting chemotherapy. After leukapheresis and before axi-cel, patients could receive bridging therapy (limited to glucocorticoids) at the investigator’s discretion. Patients in the SOC arm received 2 to 3 cycles of an investigator-selected, protocol-defined, platinum-based chemoimmunotherapy regimen; those with partial response (PR) or CR proceeded to HDT-ASCT. There was no protocol-specified crossover between the 2 arms; however, patients in both arms could receive subsequent therapy off protocol per investigator discretion.
The study protocol was approved by the institutional review board at each site, all patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Subsequent antilymphoma therapy
In ZUMA-7, subsequent therapies and disease assessments after new lymphoma therapy, not defined in the study protocol, were per investigator discretion. For this intention-to-treat analysis, subsequent therapy was defined as any new, off-protocol lymphoma therapy, regardless of whether randomized protocol therapy was given. Thus, for randomized patients who did not receive on-study 2L axi-cel or SOC, the next administered treatment was classified as 3L therapy (see Figure 1 for subsequent 3L therapy categorization).
In the SOC arm, 3L therapy was categorized as either cellular immunotherapy or other treatments (no cellular immunotherapy in 3L, which included patients who did not receive cellular immunotherapy in any line, as well as patients who received cellular immunotherapy in fourth-line [4L] or beyond). In the axi-cel arm, 3L therapy was categorized as chemotherapy, cellular immunotherapy, or other. Specifically, 3L cellular immunotherapy in the axi-cel arm was limited to axi-cel retreatment on protocol among patients who initially achieved CR or PR at the first disease assessment but later experienced disease progression; the treatment schedule for axi-cel retreatment followed initial procedures, as aforementioned. Briefly, patients continued to meet original trial eligibility and received the same target dose of CAR T cells/kg as the initial infusion (2 × 106 CAR T cells/kg). As the intent of bridging therapy was unknown for patients who required subsequent therapy in this analysis, and to conduct the analyses in a uniform manner, a systematic approach was taken when classifying cellular immunotherapy as 3L or later treatment. Specifically, cellular immunotherapy was considered 3L if preceded by local treatment or temporizing measures, including lymphodepleting chemotherapy, steroids, radiation, or rituximab only (without other chemotherapy, including polatuzumab- or rituximab-containing chemotherapy) in the absence of disease progression. For patients who received chemotherapy bridging and then CAR T-cell therapy, the latter was considered 4L treatment.
Assessments
Progression-free survival (PFS), OS, and best response to subsequent therapy were assessed. All response assessments were per investigator and not confirmed by blinded central review, with the schedule of assessments not defined per protocol.
Statistical analysis
PFS was defined as the time from 3L therapy initiation to the date of disease progression or death from any cause, whichever was earlier. Patients who did not meet the criteria for a PFS event were censored at 4L treatment initiation, if applicable, or last known alive date. Patients who received subsequent SCT while in a response from 3L axi-cel retreatment were censored at the time of SCT. This approach is consistent with that taken for patients who received on-study 2L axi-cel and subsequently received SCT in the absence of any documented progression or new therapy, per the statistical analysis plan for the primary analysis.6 OS was defined as the time from 3L therapy initiation to death from any cause. Patients who had not died by the analysis data cutoff date were censored at their last known alive date. Kaplan-Meier estimates were calculated for PFS and OS. Median follow-up times were calculated using the reverse Kaplan-Meier estimator.
The associations between subsequent therapies in any-line setting and OS were assessed using a Cox model with the therapy types as the time-varying covariates. Specifically, in the SOC arm, cellular immunotherapy was compared with no cellular immunotherapy in any line of therapy. In the axi-cel arm, SCT, cellular immunotherapy, and chemoimmunotherapy were compared with any other type of subsequent therapies. HRs with 2-sided 95% CIs were calculated.
ORR was defined as the proportion of patients with CR or PR. Because responses to off-protocol treatments were per investigator assessment, the data herein are limited to those provided by investigators. Therefore, disease assessments were not available for all patients at all time points. No formal hypothesis was tested for this exploratory post hoc analysis.
Results
Overview of subsequent 3L+ therapy
Available data through 18 March 2021, the data cutoff date for the primary event-free survival analysis,6 were included herein. As previously reported, 359 patients were randomized to treatment in ZUMA-7 between 25 January 2018 and 4 October 2019, and 180 and 179 patients were assigned to the axi-cel and SOC arms, respectively (Figure 2).6 Patient and disease characteristics at start of 3L therapy in this analysis were not available.
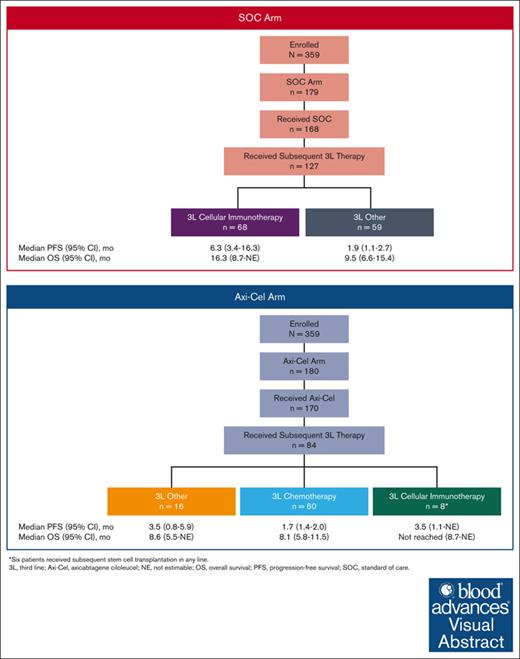
Patient disposition in the SOC arm and axi-cel arm for subsequent 3L therapy. (A) SOC arm and (B) axi-cel arm. ∗Patients received axi-cel (n = 51); other autologous anti-CD19 CAR T-cell therapy (n = 10); unspecified CAR T-cell therapy (n = 3); anti-CD19/CD22 CAR T-cell therapy, allogeneic CRISPR-Cas9 engineered T cells, anti-CD22 CAR T-cell therapy, and natural killer-cell infusion (n = 1 each). †Patients may have received >1 3L regimen. Other 3L regimens included radiation (n = 4), nivolumab (clinical trial, n = 4), pembrolizumab (n = 2 [with n = 1 on clinical trial]), ipilimumab (clinical trial, n = 1), R-lenalidomide (n = 2), varlilumab (clinical trial, n = 2), oral dihydroorotate (clinical trial, n = 1), CPI-613 (clinical trial, n = 1), dexamethasone (n = 1), HDT-ASCT (n = 1), and allogeneic SCT (n = 1). For the patient under “other 3L regimens” classified as receiving allogeneic SCT, the patient received cyclophosphamide and fludarabine as lymphodepleting chemotherapy without any other chemoimmunotherapy prior to alloSCT; for this reason, the patient was not categorized into the 3L chemotherapy group. For the patient under “other 3L regimens” classified as received HDT-ASCT, the patient received nivolumab with ipilimumab (without any chemoimmunotherapy) before HDT-ASCT and, therefore, was not categorized into the 3L chemotherapy group. ‡Among these 60 patients, only 1 received 3L polatuzumab vedotin plus bendamustine and rituximab. §Refers to SCT in any line. Three patients received allogeneic SCT after 3L axi-cel in the absence of progression. PD, progressive disease.
Patient disposition in the SOC arm and axi-cel arm for subsequent 3L therapy. (A) SOC arm and (B) axi-cel arm. ∗Patients received axi-cel (n = 51); other autologous anti-CD19 CAR T-cell therapy (n = 10); unspecified CAR T-cell therapy (n = 3); anti-CD19/CD22 CAR T-cell therapy, allogeneic CRISPR-Cas9 engineered T cells, anti-CD22 CAR T-cell therapy, and natural killer-cell infusion (n = 1 each). †Patients may have received >1 3L regimen. Other 3L regimens included radiation (n = 4), nivolumab (clinical trial, n = 4), pembrolizumab (n = 2 [with n = 1 on clinical trial]), ipilimumab (clinical trial, n = 1), R-lenalidomide (n = 2), varlilumab (clinical trial, n = 2), oral dihydroorotate (clinical trial, n = 1), CPI-613 (clinical trial, n = 1), dexamethasone (n = 1), HDT-ASCT (n = 1), and allogeneic SCT (n = 1). For the patient under “other 3L regimens” classified as receiving allogeneic SCT, the patient received cyclophosphamide and fludarabine as lymphodepleting chemotherapy without any other chemoimmunotherapy prior to alloSCT; for this reason, the patient was not categorized into the 3L chemotherapy group. For the patient under “other 3L regimens” classified as received HDT-ASCT, the patient received nivolumab with ipilimumab (without any chemoimmunotherapy) before HDT-ASCT and, therefore, was not categorized into the 3L chemotherapy group. ‡Among these 60 patients, only 1 received 3L polatuzumab vedotin plus bendamustine and rituximab. §Refers to SCT in any line. Three patients received allogeneic SCT after 3L axi-cel in the absence of progression. PD, progressive disease.
In the SOC arm, 168 of 179 randomized patients received ≥1 dose of 2L SOC treatment. Of these, 62 patients reached on-protocol HDT-ASCT and 106 did not (Figure 2A). In total, 127 of 179 (71%) randomized patients required subsequent 3L therapy in the SOC arm (Figure 2A; supplemental Table 1). The median time from randomization to 3L therapy was 2.8 months (range, 0.1-18.4). Sixty-eight patients received 3L cellular immunotherapy, which included axi-cel (n = 51), other autologous anti-CD19 CAR T-cell therapy (n = 10), unspecified CAR T-cell therapy (n = 3), and anti-CD19/CD22 CAR T-cell therapy, allogeneic CRISPR-Cas9 engineered T cells, anti-CD22 CAR T-cell therapy, and natural killer cell infusion (n = 1 each).
In the axi-cel arm, 170 of 180 randomized patients received 2L axi-cel treatment, and 84 of 180 (47%) randomized patients received 3L subsequent therapy (Figure 2B; supplemental Table 1). The median time from randomization to 3L therapy was 4.4 months (range, 0.2-22.3 months). Sixty and 8 patients received 3L chemotherapy and 3L cellular immunotherapy, respectively. All 8 patients who received 3L cellular immunotherapy received on-protocol axi-cel retreatment, which was allowed for patients who initially responded to axi-cel and subsequently relapsed. Sixteen patients received other 3L therapy (Figure 2). Subsequent therapies received in any line by therapy class and treatment arm are shown in supplemental Table 2.
In total, 14 patients (SOC arm, n = 8 [4%]; axi-cel arm, n = 6 [3%]) did not receive 3L therapy after documented disease progression. Thirteen of these patients had primary refractory disease, whereas 1 relapsed after 1L treatment. Patients had an age-adjusted International Prognostic Index score of either 1 (n = 5) or 2 (n = 9) at randomization. Per central laboratory, 10 patients (71%) had diffuse LBCL (9 germinal center B-cell subtype and 1 activated B-cell subtype), 3 (21%) had high-grade B-cell lymphoma (all double-/triple-hit), and 1 (7%) had other disease type. Three patients (21%) had double-expressor lymphoma. The best response to 2L therapy among these patients was CR (n = 3), stable disease (n = 1), and progressive disease (n = 9); 1 patient was randomized but did not receive 2L therapy. All 14 patients died, with a median survival from randomization of 2.1 months.
3L treatment outcomes in the SOC arm
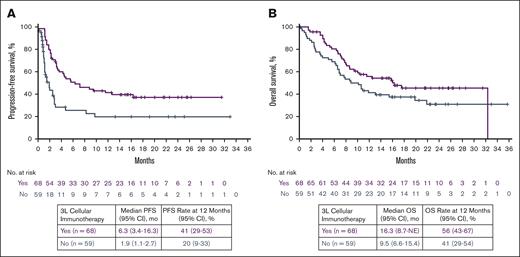
Median follow-up times since 3L treatment initiation for PFS and OS were 16.6 (range, 0-33.0) and 20.0 (range, 0.2-35.7) months, respectively, for the 127 patients in the SOC arm. For patients who received 3L cellular immunotherapy (n = 68; Figure 2A purple box), median PFS was 6.3 months (95% CI, 3.4-16.3) and median OS was 16.3 months (95% CI, 8.7 to NE; Figure 3). For the 59 patients who received other therapies in 3L (Figure 2A gray box), median PFS and median OS were 1.9 months (95% CI, 1.1-2.7) and 9.5 months (95% CI, 6.6-15.4), respectively (Figure 3). The 12-month PFS rate was 41% among the patients who received 3L cellular immunotherapy and 20% among those who did not. The corresponding 12-month OS rates were 56% and 41% for these patients, respectively.
PFS and OS by 3L cellular immunotherapy in the SOC arm since 3L treatment initiation. Kaplan-Meier estimates were calculated from 3L treatment initiation. No., number.
PFS and OS by 3L cellular immunotherapy in the SOC arm since 3L treatment initiation. Kaplan-Meier estimates were calculated from 3L treatment initiation. No., number.
Of the 68 patients who received 3L cellular immunotherapy, the ORR was 57% (95% CI, 45-69), with a CR rate of 34% (95% CI, 23-46; supplemental Figure 1).
3L treatment outcomes in the axi-cel arm
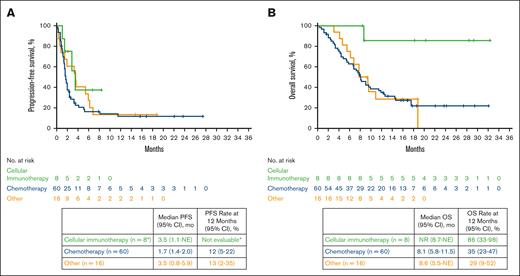
Median follow-up times since 3L treatment initiation for PFS and OS were 15.7 (range, 0-27.4) and 18.6 (range, 0.4-32.3) months, respectively, for the 84 patients in the axi-cel arm. For patients who received 3L chemotherapy (n = 60; Figure 2B blue boxes), median PFS was 1.7 months (95% CI, 1.4-2.0), and median OS was 8.1 months (95% CI, 5.8-11.5) since 3L treatment initiation (Figure 4), with an ORR of 25% (95% CI, 15-38) and CR rate of 13% (95% CI, 6-25) to chemotherapy (supplemental Figure 2). Of the 60 patients who received 3L chemotherapy, 34 had an initial response to 2L axi-cel; among these 34 patients, median PFS was 1.7 months (95% CI, 1.4-2.4), and median OS was 8.1 months (95% CI, 6.8-11.9), with an ORR of 32% (CR rate, 18%). Of the 26 patients who received 3L chemotherapy and did not respond to 2L axi-cel, median PFS was 1.6 months (95% CI, 1.1-2.0), and median OS was 6.9 months (95% CI, 3.3 to NE), with an ORR of 15% (CR rate, 8%). For patients who received 3L cellular immunotherapy (n = 8; Figure 2B green boxes), median PFS was 3.5 months (95% CI, 1.1 to NE), and median OS was not reached (95% CI, 8.7 to NE) since 3L treatment initiation. For patients who received other 3L therapies (n = 16; Figure 2B orange box), median PFS was 3.5 months (95% CI, 0.8-5.9), and median OS was 8.6 months (95% CI, 5.5 to NE).
PFS and OS by 3L treatment in the axi-cel arm since 3L treatment initiation. ∗Six of these 8 patients received subsequent SCT in any line. This analysis followed the statistical analysis plan for the ZUMA-7 primary event-free survival analysis,6 whereby patients who received subsequent SCT while in response from 3L axi-cel were censored at time of SCT. Because there were no available data for this subgroup at 12 months, the PFS rate is not evaluable. Kaplan-Meier estimates were calculated from 3L treatment initiation. No., number.
PFS and OS by 3L treatment in the axi-cel arm since 3L treatment initiation. ∗Six of these 8 patients received subsequent SCT in any line. This analysis followed the statistical analysis plan for the ZUMA-7 primary event-free survival analysis,6 whereby patients who received subsequent SCT while in response from 3L axi-cel were censored at time of SCT. Because there were no available data for this subgroup at 12 months, the PFS rate is not evaluable. Kaplan-Meier estimates were calculated from 3L treatment initiation. No., number.
Of the 60 patients who received 3L chemotherapy (Figure 2B blue boxes), 10 patients received SCT after chemotherapy (17%; 9 ASCT and 1 allogeneic SCT). Of note, chemotherapy and SCT were together considered 1 line of therapy; however, it is unknown how many patients who received 3L chemotherapy were intended for SCT. Among those who did not receive SCT (n = 50), median PFS was 1.6 months (95% CI, 1.2-1.8), and median OS was 7.2 months (95% CI, 4.8-9.1), with an ORR of 14% (95% CI, 6-27) and a CR rate of 4% (95% CI, 0.5-14) to 3L chemotherapy (supplemental Figure 3A). Among patients who went on to receive SCT, median PFS was 11.5 months (95% CI, 2.4 to NE), and median OS was 17.5 months (95% CI, 2.4 to NE), with an 80% ORR (95% CI, 44-97) and 60% CR rate (95% CI, 26-88) to 3L chemotherapy before SCT (supplemental Figure 3B). The median time from initiation of 3L chemotherapy to SCT was 2.7 months. Best response to SCT included 5 CR and 3 progressive diseases (2 patients were not evaluable).
Eight patients in the axi-cel arm received 3L cellular immunotherapy (supplemental Results), which was limited to axi-cel retreatment on protocol among patients who initially responded to 2L axi-cel but later experienced disease progression (Figure 2B green boxes); 1 patient had product manufactured from peripheral blood mononuclear cells from first apheresis, and the remaining 7 received a second bag of product produced from the original manufacturing process. Among these 8 patients, the median time from first axi-cel infusion to first assessment of progressive disease was 3.0 months (range, 2.6-17.1). ORR and CR rates to 3L axi-cel were 75% and 50%, respectively. Six of 8 patients received subsequent SCT (1 HDT-ASCT and 5 allogeneic SCT) in any line. After SCT, 5 of 6 patients remained in CR. All 6 patients who received SCT were alive at data cutoff (median follow-up since 3L treatment initiation, 24.4 months).
Any-line cellular immunotherapy outcomes
In the SOC arm, 100 patients received subsequent cellular immunotherapy in any line, of whom 32 received treatment in 4L or beyond. Among patients who received cellular immunotherapy in any line, the ORR was 54% (95% CI, 44-64), and the CR rate was 30% (95% CI, 21-40). Survival outcomes were better for patients who received cellular immunotherapy in any line vs those who did not, as evidenced by a lower hazard of death (HR, 0.33; 95% CI, 0.16-0.68).
Among patients in the axi-cel arm, patients who received subsequent cellular immunotherapy in any line demonstrated an ORR of 77% (95% CI, 46-95), a CR rate of 46% (95% CI, 19-75), and had better survival outcomes with lower hazard of death (HR, 0.22; 95% CI, 0.07-0.66) vs patients who received other therapy. Conversely, patients in the axi-cel arm who received chemotherapy in any line vs other therapy had a numerically higher hazard of death (HR, 1.50; 95% CI, 0.58-3.89). Few patients (19/76, excluding 6 who received SCT after 3L axi-cel retreatment) reached SCT in any line after chemotherapy or other therapies. However, for the select patients who did reach SCT (excluding those who received SCT after 3L axi-cel retreatment), survival outcomes were better for patients who received subsequent any-line SCT vs other therapy (HR, 0.39; 95% CI, 0.17-0.88).
Discussion
CAR T-cell therapy has been proposed as the new SOC for patients with primary refractory or early relapsed disease based on pivotal results from ZUMA-7 and TRANSFORM.6-8,16,17 However, for patients who require additional antilymphoma therapy, data are needed on optimal treatment sequencing after CAR T-cell therapy. This analysis of patients who enrolled in ZUMA-7 and received subsequent therapy provides insight into treatment sequencing approaches in the 3L setting and beyond.
Although no formal comparative statistical analyses were conducted, outcomes for patients who received subsequent 3L cellular immunotherapy in this analysis appeared numerically inferior to those who received 2L cellular immunotherapy in ZUMA-7.6 Specifically, median PFS was 14.7 months with 2L axi-cel13 vs 6.3 months for 3L cellular immunotherapy in the SOC arm. Median OS, which was not reached with 2L axi-cel,13 was 16.3 months with 3L cellular immunotherapy in the SOC arm. ORR and CR rates were numerically greater with 2L axi-cel treatment6 (ORR, 83%; CR rate, 65%) vs 3L cellular immunotherapy in the SOC arm (ORR, 57%; CR rate, 34%); however, it should be noted that although responses were stringently assessed in ZUMA-7 (per-blinded central review), all disease assessments reported for this post hoc analysis were per investigator and not performed at defined time points. Furthermore, because patient and disease characteristics at start of 3L therapy in this analysis are not available, it is not possible to determine the impact of these characteristics at start of 3L therapy on outcomes. Generally, patients who require 3L therapy do not respond to or progress after 2L therapy, necessitating further treatment. This suggests there is an element of the disease that is aggressive or associated with poor prognostic features and drives the need for further treatment. It is unknown to what extent patients who required subsequent 3L therapy in this analysis were comparable with those randomized to 2L treatment in ZUMA-7. Although formal comparative statistical analyses are not feasible, and noting the aforementioned limitations, the results from this analysis suggest that earlier CAR T-cell intervention may provide greater patient benefit; additional studies, including in the front-line setting,18 are in progress.
Furthermore, a very small number of patients who initially responded to axi-cel in 2L but later experienced disease progression received subsequent 3L anti-CD19 CAR T-cell therapy (n = 8). These patients achieved noteworthy responses that should be interpreted cautiously given the limited sample size available in this analysis. Foremost, most of these patients (n = 6 of 8) received SCT in a subsequent line; thus, it is not feasible to determine whether their promising outcomes were due to 3L CAR T-cell therapy or SCT. Because the 6 patients did not receive 2L chemotherapy, it is possible that they remain chemosensitive. Although these data suggest a potential treatment strategy for some patients after 2L CAR T-cell therapy, definitive conclusions are precluded, given available findings in this analysis, and further study with a larger sample is warranted. Notably, CAR22, an autologous CAR T-cell therapy that targets CD22 (vs CD19 for axi-cel), has shown promise in a phase 1 study of patients with R/R LBCL who progressed after anti-CD19 CAR T-cell therapy.19,20 In this anti-CD19 CAR-refractory population and with a median follow-up of 15.7 months, CAR22 demonstrated expected safety signals, a 72% ORR (53% CR rate), and median PFS of 9.4 months.20 These data, together with our findings, suggest that 3L CAR T-cell therapy after an initial response in 2L may provide a meaningful benefit to some patients. Further study is warranted to guide management and determine the optimal antigen target after failure of CD19-directed therapy. Of note, although not randomly assigned to these off-protocol therapies, patients in ZUMA-7 who received cellular immunotherapy in any line demonstrated improved survival and lower risk of death vs those who did not receive cellular immunotherapy in any line. These results support prior trials of cellular immunotherapy in patients with R/R LBCL, which have shown durable efficacy and promising outcomes with long-term follow-up.3,4,6,7,21
Understanding outcomes of SCT after 2L cellular therapy remains an area with limited evidence. In our analysis, although limited by small patient subsets, only 17% of patients who received 3L chemotherapy after 2L axi-cel therapy reached SCT. Although it is not possible to determine how many of these patients were intended to proceed to SCT, these findings reinforce prior observations that only a minority of patients who are potential candidates for transplantation reach definitive therapy.6-8,22 Low transplant rates among these patients could be due to low chance of response to salvage chemotherapy. Although definitive conclusions are limited by small numbers, patients in the axi-cel arm who received subsequent chemotherapy in 3L or later appeared to have inferior survival outcomes vs patients who received other subsequent therapies; however, those patients in the axi-cel arm who reached SCT after 3L or later chemotherapy appeared to have improved outcomes compared with those who did not receive SCT. This is not unexpected because those patients in the axi-cel arm who received SCT after 3L or later chemotherapy represent the minority of patients who respond to 3L chemotherapy and reach definitive therapy with SCT, thus selecting those with the best outcomes. This observation is consistent with findings observed in the 2L setting6 in which only a minority respond to salvage chemotherapy and reach SCT and suggests the consideration of SCT for patients who are still chemosensitive after 2L CAR T-cell therapy. However, it is not known a priori which patients will respond to salvage therapy. Finally, this analysis cannot distinguish outcomes with allogeneic SCT vs HDT-ASCT after 2L axi-cel because the number of patients in the axi-cel arm who received transplantation after 3L chemotherapy was very limited (n = 10), and, of those, only 1 patient received allogeneic SCT. Additional research is needed to guide preferential sequencing of ASCT or allogeneic SCT following progression after 2L axi-cel and 3L chemotherapy, as this remains a key question among clinicians.
This analysis is limited by real-world heterogeneity, including timing of disease assessments and selection of subsequent therapy that occurred off protocol per investigator on ZUMA-7. Because disease assessments were not protocol defined and were performed at the investigator’s discretion, data were not available for every patient at every time point. As aforementioned, blinded central review of responses was not conducted, which may have introduced variability in response assessments. Furthermore, for patients who required subsequent therapy, the intent of bridging was unknown; to conduct the analysis uniformly, a systematic approach was taken when classifying cellular immunotherapy as 3L or later treatment. Because subsequent 3L treatment was not predetermined, there may have been a bias for or against the use of certain therapies depending on individual patient or disease characteristics, physician preference, or availability of alternative therapies. For instance, the CD3×CD20 bispecific antibodies epcoritamab and glofitamab were approved in the first half of 2023 for the treatment of R/R LBCL in the 3L or later setting.23-25 However, bispecifics were in earlier stages of clinical development and not approved for use by regulatory agencies during the conduct of ZUMA-7; as such, they were not widely available as a potential 3L treatment option. Indeed, only 1 patient in our analysis received a bispecific antibody in 3L on a clinical trial, with 7 and 6 patients in the axi-cel and SOC arms, respectively, receiving bispecific antibodies in any line. The inclusion of a substantial proportion of patients who had received prior CAR T-cell therapy in clinical studies of bispecific antibodies suggests that bispecific antibody treatment after 2L CAR T-cell therapy may be a viable approach.24,26,27 These findings warrant further investigation in prospective clinical trials along with studies to specifically address optimal use of bispecific antibodies in the treatment sequence.
In summary, these results suggest that outcomes may be improved with CAR T-cell therapy earlier in the treatment sequence and warrant further investigation. Cellular immunotherapy may be a viable treatment option after progression after 2L axi-cel therapy for patients with an initial response. In contrast, outcomes with chemotherapy in later lines were poor; although, for the few patients who were able to reach SCT, outcomes were improved. Although the small number of patients reported herein preclude definitive conclusions, these results provide preliminary evidence to inform subsequent treatment choices that afford meaningful clinical benefit for patients after failure of 2L therapy for R/R LBCL.
Acknowledgments
The authors thank the patients who participated in this study and their families, caregivers, and friends; the ZUMA-7 investigators, coordinators, and health care staff at each site; and all employees of Kite, a Gilead company, who were involved over the course of the study for their contributions. Medical writing support was provided by Ashley Skorusa of Nexus Global Group Science LLC and funded by Kite, a Gilead company.
Authorship
Contribution: A.G., A.P., M.S., and C.T. designed the study; A.G., J. Munoz, J.R.W., F.L.L., D.B.M., A.P.R., M.-A.P., P.M.R., J. McGuirk, C.A.J., M.J.K., I.A., and O.O.O. enrolled and treated patients and gathered data; and all authors participated in the analysis and interpretation of the data, writing the manuscript, and approval of the final submitted version.
Conflict-of-interest disclosure: A.G. has received honoraria from Kite, a Gilead company; has provided consultancy for Amgen, Atara, Bristol Myers Squibb, CRISPR Therapeutics, Kite, and Wugen Inc; and has received research funding from Amgen, Genentech, and Kite. J. Munoz has received honoraria from Curio Science, Kyowa, OncView, Physicians' Education Resource, Targeted Oncology, and Seattle Genetics; has provided consultancy for ADC Therapeutics, Alexion, Bayer, BeiGene, Bristol Myers Squibb, Debiopharm, Epizyme, Fosun Kite, Genmab, Innovent, Janssen, Juno/Celgene, Karyopharm, Kite, Kyowa, Lilly/Loxo, MEI Pharma, MorphoSys/Incyte, Novartis, Pfizer, Pharmacyclics/AbbVie, Seattle Genetics, Servier, TG Therapeutics, and Zodiac; has provided speakers’ bureau participation for Acrotech/Aurobindo, AstraZeneca, Bayer, BeiGene, Celgene/Bristol Myers Squibb, Genentech/Roche, Kite, Kyowa, Pharmacyclics/Janssen, Seattle Genetics, and Verastem; and has received research funding from Bayer, Celgene, Genentech, Incyte, Janssen, Kite, Merck, Millennium, Pharmacyclics, Portola, and Seattle Genetics. J.R.W. has provided consultancy for Bristol Myers Squibb, Genentech, and Kite; and has received research funding from ADC Therapeutics, AstraZeneca, Bristol Myers Squibb, Calithera, Genentech, Kite, Kymera, MorphoSys/Incyte, and Novartis. F.L.L. has provided consultancy for Allogene, Amgen, bluebird bio, Bristol Myers Squibb/Celgene, Calibr, Cellular Biomedicine Group, Cowen, EcoR1, Emerging Therapy Solutions, Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite, Legend Biotech, Novartis, Umoja, and Wugen; has received research funding from Allogene, Kite, and Novartis; and has received patents, royalties, other intellectual property from several patents held by the institution in author's name (unlicensed) in the field of cellular immunotherapy. D.B.M. has received honoraria and travel support from Janssen; has provided consultancy for Adaptive Biotechnologies, Bristol Myers Squibb, Janssen, Kite, and Miltenyi; has received research funding from 2Seventy Bio, Adicet, Allogene, Fate Therapeutics, Kite, and Miltenyi; and has received patents, royalties, or other intellectual property for cGVHD patent holder for ibrutinib as cGVHD therapy but no compensation. A.P.R. has no relevant financial relationships to disclose. M.-A.P. has received honoraria from Adicet, Allogene, AlloVir, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Syncopation, VectivBio AG, and Vor Biopharma; has served on data safety monitoring boards for Cidara Therapeutics, Medigene, and Sellas Life Sciences, and the scientific advisory board for NexImmune; has ownership interests in NexImmune, Omeros, and OrcaBio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. P.M.R. has provided consultancy for Kite; and has received research funding from Genentech and Seagen. J. McGuirk has received honoraria from AlloVir, Bristol Myers Squibb, Caribou, CRISPR Therapeutics, Nektar, Novartis, and Sana Biotechnologies. C.A.J. has received honoraria from Kite, Novartis, Bristol Myers Squibb/Celgene, Instil Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, bluebird bio, and Daiichi Sankyo; has provided consultancy for Kite, Novartis, Bristol Myers Squibb/Celgene, Instil Bio, ImmPACT Bio, Lonza, Ipsen, Epizyme, bluebird bio, and Daiichi Sankyo; and received research funding from Kite and Pfizer. M.J.K. has received honoraria from and provided consultancy for Bristol Myers Squibb/Celgene, Kite, Miltenyi Biotech, Novartis, Adicet Bio, and Roche; has received research funding from Kite, Roche, Takeda, and Celgene; and has received travel support from Kite, Miltenyi Biotech, Novartis, and Roche. I.A. has provided speakers’ bureau participation for Kite and Novartis. A.P., M.S., and C.T. are employees of Kite and have stock or other ownership in Gilead Sciences. O.O.O. has received honoraria from Gilead and Pfizer; has provided consultancy for AbbVie, ADC, Curio Science, Epizyme, Gilead, Janssen, Kite, Pfizer, Nektar, Novartis, Syncopation, and TGR Therapeutics; and has received other research funding to institution from Allogene, Daichi Sankyo, Kite, and Pfizer.
Correspondence: Armin Ghobadi, Washington University School of Medicine, 4921 Parkview Place, St. Louis, MO 63110; email: arminghobadi@wustl.edu.
References
Author notes
Kite is committed to sharing clinical trial data with external medical experts and scientific researchers in the interest of advancing public health. As such, Kite shares anonymized individual patient data (IPD) upon request or as required by law and/or regulation. Qualified external researchers may request IPD for studies of Kite or Gilead compounds approved in the United States and the European Union with a marketing authorization date on or after 1 January 2014 and are publicly listed on ClinicalTrials.gov or the European Union Clinical Trials Register. For studies of newly approved compounds or indication, the IPD will be available for request 6 months after US Food and Drug Administration and European Medicines Agency approval. Such requests are at Kite’s discretion and are dependent on the nature of the request, the merit of the research proposed, availability of the data, and the intended use of the data. If Kite agrees to the release of clinical data for research purposes, the requestor will be required to sign a data sharing agreement to ensure protection of patient confidentiality before the release of any data. Data will be available for request 6 months after US Food and Drug Administration and European Medicines Agency approval. Access can be requested by contacting medinfo@kitepharma.com and requests will be addressed within 60 days.
The full-text version of this article contains a data supplement.

![Patient disposition in the SOC arm and axi-cel arm for subsequent 3L therapy. (A) SOC arm and (B) axi-cel arm. ∗Patients received axi-cel (n = 51); other autologous anti-CD19 CAR T-cell therapy (n = 10); unspecified CAR T-cell therapy (n = 3); anti-CD19/CD22 CAR T-cell therapy, allogeneic CRISPR-Cas9 engineered T cells, anti-CD22 CAR T-cell therapy, and natural killer-cell infusion (n = 1 each). †Patients may have received >1 3L regimen. Other 3L regimens included radiation (n = 4), nivolumab (clinical trial, n = 4), pembrolizumab (n = 2 [with n = 1 on clinical trial]), ipilimumab (clinical trial, n = 1), R-lenalidomide (n = 2), varlilumab (clinical trial, n = 2), oral dihydroorotate (clinical trial, n = 1), CPI-613 (clinical trial, n = 1), dexamethasone (n = 1), HDT-ASCT (n = 1), and allogeneic SCT (n = 1). For the patient under “other 3L regimens” classified as receiving allogeneic SCT, the patient received cyclophosphamide and fludarabine as lymphodepleting chemotherapy without any other chemoimmunotherapy prior to alloSCT; for this reason, the patient was not categorized into the 3L chemotherapy group. For the patient under “other 3L regimens” classified as received HDT-ASCT, the patient received nivolumab with ipilimumab (without any chemoimmunotherapy) before HDT-ASCT and, therefore, was not categorized into the 3L chemotherapy group. ‡Among these 60 patients, only 1 received 3L polatuzumab vedotin plus bendamustine and rituximab. §Refers to SCT in any line. Three patients received allogeneic SCT after 3L axi-cel in the absence of progression. PD, progressive disease.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/11/10.1182_bloodadvances.2023011532/1/m_blooda_adv-2023-011532-gr2.jpeg?Expires=1768862870&Signature=wRFJvpTo89GGiOZpVec3lV2BX9EzhkLkW4CDNCPviU9hlOfEQbXTY4pNPb1g-6v5JAqcuXCewSa6Or9ecV22~8xeMZS0Sj11Rsw8LVTJDKWeEZusXyf5p~pdPuH1pldIJeC38s5~iyjqoH4oFDQqHWxv9KDZ8T8nIokvNhklrynmYknCF~oim1X5nVUHcsuMBxB7KTaQFW3TN2Aelas4O8ist6YLneobrS-uCzEq-HY4L8lrrLhiDodb8ddOt4krBksWG1gt~aW-gnzm6mSAgPHHZhDWWENSscxxbDROfOd3-Bl7wlPHvJ71G8YcLBaQHwwWI8UkdxeAaR6CY1OJ-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)