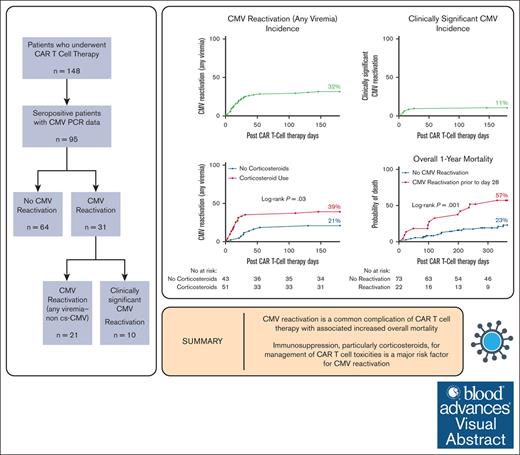
Key Points
CMV reactivation is a common complication of CAR T-cell therapy and is associated with increased overall mortality.
Immunosuppression, particularly corticosteroids, for management of CAR T-cell toxicities is a major risk factor for CMV reactivation.
Visual Abstract
Cytomegalovirus (CMV) reactivation is a major complication among seropositive allogeneic hematopoietic cell transplantation recipients; however, data on CMV reactivation after chimeric antigen receptor (CAR) T-cell therapy are limited. We report the incidence and outcomes of 95 adult CMV-seropositive patients who received CAR T-cell therapy between February 2018 and February 2023. CMV outcomes were CMV reactivation (any viremia) and clinically significant CMV infection (cs-CMV). Thirty-one patients (33%) had evidence of CMV reactivation (any viremia), and 10 patients (11%) had cs-CMV. The median time from CAR T-cell infusion to CMV reactivation was 19 days (interquartile range [IQR], 9-31). The cumulative incidence of CMV (any viremia) was significantly higher among patients with grade 3 to 4 cytokine release syndrome (67 vs 28%; P = .01), and those who received corticosteroids (39 vs 21%; P = .03), anakinra (56 vs 28%; P = .02), or ≥2 immunosuppressants (41 vs 21%; P = .02). Receipt of corticosteroids (18 vs 0%; P = .004), tocilizumab (14 vs 0%; P = .04), anakinra (33 vs 7%; P = .008), and ≥2 immunosuppressants (20 vs 0%; P = .001) were all associated with cs-CMV. Receiving ≥2 immunosuppressants was associated with a twofold increase in CMV reactivation in multivariate analyses (adjusted odds ratio [aOR], 2.27; 95% confidence interval, 1.1-4.8; P = .03). Overall, the 1-year mortality was significantly higher in those with CMV reactivation (57% vs 23%; P = .001). Immunosuppression, particularly with corticosteroids, for the management of CAR T-cell toxicities, is a major risk factor for CMV reactivation.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has been a pivotal advance in the management of patients with resistant/refractory malignancies, including lymphoma, multiple myeloma, and acute lymphoblastic leukemia.1-3 In general, CAR T-cell recipients are at increased risk of infection due to various factors, including underlying malignancy, lymphodepleting therapy, multiple previous lines of chemotherapy, and prolonged cytopenias.4 Additionally, immunosuppressive agents, including corticosteroids and tocilizumab, which are often used to manage CAR T-cell therapy–related toxicities, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), also put these patients at risk of infection.4-6 Bacterial and viral infections are the most common infections and usually occur within the first month after CAR T-cell infusion.4,5,7-11
More than 60% of allogeneic hematopoietic cell transplantation (HCT) recipients have detectable cytomegalovirus (CMV) DNA levels in the first 100 days after transplantation.12-14 However, data on CMV reactivation in patients undergoing CAR T-cell therapy are limited. Some studies have reported a low incidence of CMV reactivation in this population; however, a number of factors, including a lack of routine testing, may have contributed to the lower incidence.1,2,4,15-17 In this study, we assessed the incidence and outcomes of CMV reactivation in CAR T-cell therapy recipients treated at our center.
Methods
Study subjects
We report on a single-center retrospective study of 95 adult CMV-seropositive patients who received CAR T-cell therapy between February 2018 and February 2023. The study was approved by the institutional review board and conducted consistently according to the principles of the Declaration of Helsinki.
Antiviral prophylaxis and CMV therapy
All patients received antiviral prophylaxis with acyclovir 400 to 800 mg orally twice daily. At our institution, preemptive therapy is initiated in HCT recipients with a CMV viral load >200 international unit per milliliter (IU/mL) as the cutoff for initiation of therapy.14 However, during the study period, no institutional protocol for the prevention of CMV was implemented in CAR T-cell therapy recipients; therefore, patients with CMV reactivation received antivirals at the discretion of the treating physician. In the absence of leukopenia or thrombocytopenia, valganciclovir 900 mg orally twice daily (or ganciclovir 5 mg/kg IV twice daily, if oral intake was not tolerated) was the anti-CMV agent of choice. Patients who did not tolerate or in whom (val)ganciclovir was contraindicated received foscarnet 90 mg/kg every 12 hours.
Measurement of CMV viral load
The CMV viral load was monitored at the discretion of the treating physician. Plasma CMV viral load was measured at the University of Miami laboratory using the Food and Drug Administration–approved COBAS AmpliPrep/COBAS TaqMan test (Roche Diagnostics, Indianapolis, IN). The quantitative range of this assay was 137 IU/mL (2.14 log10) to 9 100 000 IU/mL (6.96 log10). All CMV values in this study are reported in international units per milliliter (IU/mL). For statistical analyses, CMV levels reported as “detected” but below the quantitative assay range (eg, <137 IU/mL) were considered equivalent to the lowest level of quantitation (ie, 137 IU/mL).13,14 Throughout the article, the term CMV viremia corresponds to CMV DNA levels in the plasma or CMV DNAemia.
Outcomes and statistical analysis
CMV outcomes were CMV reactivation (any viremia) and clinically significant CMV (cs-CMV; CMV viremia or end-organ disease that required antiviral treatment), as described previously.13,14 Mann-Whitney U, Fisher exact, or log-rank tests were used, where appropriate. For risk factor analysis, we first conducted univariate analysis; to develop a multivariate model, we included variables whose P values were <.2. For multivariate analysis, we conducted Cox proportional hazard regression analysis with stepwise backward elimination. A P value of <.05 was considered statistically significant. Statistical analyses were performed using SPSS version 26.0 and GraphPad Prism Software, version 10.1.1.
Results
Patient characteristics
During the study period, there were a total of 148 patients who received CAR T-cell therapy. A flowchart of the patient selection is shown in supplemental Figure 1. Patients who were CMV seronegative, had a history of allogeneic HCT, those who received letermovir prophylaxis, or had missing CMV data were excluded. In total, 95 adult CMV-seropositive patients who had CMV polymerase chain reaction (PCR) data available were analyzed. The patient characteristics are presented in Table 1. The distribution of CAR T-cell products administered was as follows: 71% axicabtagene ciloleucel (Yescarta), 16% brexucabtagene autoleucel (Tecartus), 7% tisagenlecleucel (Kymriah), 4% idecabtagene vicleucel (ABECMA), and 2% lisocabtagene maraleucel (Breyanzi).
CAR T-cell toxicities
The study subjects were followed up for a median of 352 days (interquartile range [IQR], 144-548) after CAR T-cell infusion. Seventy-four patients (78%) developed any grade of CRS, with a median time to CRS onset of 4 days (IQR, 2-6). Forty-two patients (44%) developed any grade ICANS. Among the patients who developed CRS, 66 (89%) received tocilizumab, 52 (70%) received corticosteroids, and 9 (12%) received anakinra. In the corticosteroid group, the median total dose of the dexamethasone equivalent was 110 mg (IQR, 63-160). Among those receiving tocilizumab, the median number of doses was 2 (IQR, 1-4), and the standard dosage was 8 mg/kg.
Timing and frequency of CMV testing
The median number of days from CAR T-cell infusion to first CMV PCR testing was 14 days (IQR, 9-25) for the entire cohort; 13 days (IQR, 7-16) for those with CMV reactivation, and 17 days (IQR, 10-36) for the group of patients with no CMV reactivation. Among patients with CMV reactivation, the median number of tests before detectable CMV DNA levels was 1 (IQR, 1-3), whereas among patients without CMV reactivation, the median number of tests was 2 (IQR, 1-5).
Incidence of CMV reactivation and viral kinetics
Among the 95 CAR T-cell recipients analyzed, 70 patients had CMV levels drawn at any time before CAR T-cell infusion, and only 1 of them had a single measurement with detected CMV before CAR T-cell therapy and it was at the lowest detectable DNA level (<137 IU/mL). However, after CAR T, 31 patients (33%) had evidence of CMV reactivation (any viremia), and 10 patients (11%) had cs-CMV reactivation requiring antiviral therapy.
The median time from CAR T-cell infusion to CMV reactivation was 19 days (IQR, 9-31). The median peak viremia was 137 (IQR, 137-1217) for 31 patients with CMV reactivation (any viremia). In the 21 patients with CMV reactivation who were not treated, the median peak viral load was 137 IU/mL, and all had detected plasma CMV DNA values <137 IU/mL, except for 1 patient who had a CMV DNA level of 199 IU/mL. In patients with cs-CMV reactivation, the median peak viral load was 2388 IU/mL (IQR, 1091-6841); the pretreatment viremia, defined as the last CMV viral load available immediately before initiation of antiviral therapy, was 1088 IU/mL (IQR, 672-2369). Six of the patients with cs-CMV reactivation (60%) met classificatory criteria for CMV syndrome,18,19 and there were no cases of end-organ disease. Nine patients (90%) had documented clearance of viremia, defined as documentation of a negative PCR value (ie, below the level of detection) in response to antiviral therapy, and 1 patient had plasma CMV DNA levels trending down in response to therapy but died before clearance of viremia.
Risk factors for CMV
Among patients with cs-CMV reactivation, 100% received corticosteroids with a dexamethasone equivalent median dose of 130 mg (IQR, 74-263), compared with the group of patients who did not have evidence of CMV reactivation, in which 31 patients (48%; P = .003) received corticosteroids with a median dose of 100 mg (IQR, 30-150). The proportion of patients who received tocilizumab and anakinra was also significantly higher among those with cs-CMV reactivation (100% vs 66%, P = .05; and 33% vs 6%, P = .04, respectively). There were no significant differences in the demographics, CAR T-cell product used, hypogammaglobulinemia (defined as serum immunoglobulin G [IgG] level <400 mg/dL), or number of previous lines of chemotherapy between those with and without CMV reactivation (Table 1).
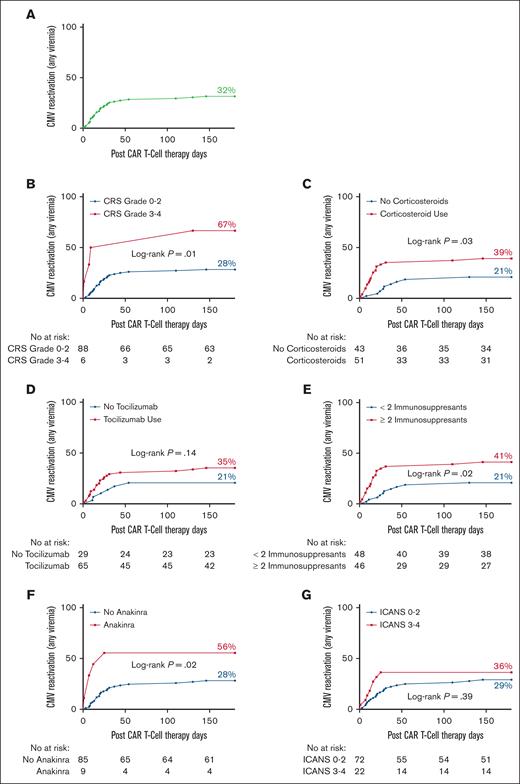
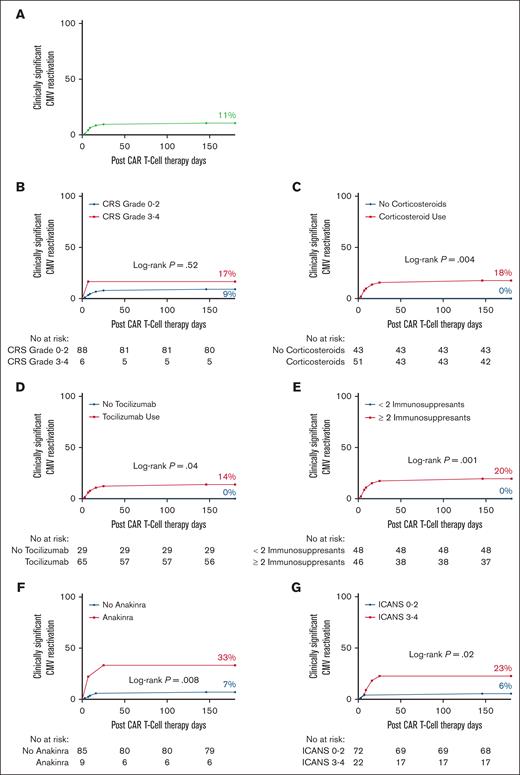
In time-to-event analyses, the 180-day cumulative incidence of CMV (Figure 1; any viremia) was significantly higher among patients with grade 3 to 4 CRS (67 vs 28%; P = .01) and those who received corticosteroids (39 vs 21%; P = .03), anakinra (56 vs 28%; P = .02), or ≥2 immunosuppressants (41 vs 21%; P = .02). Receipt of corticosteroids (18 vs 0%; P = .004), tocilizumab (14 vs 0%; P = .04), anakinra (33 vs 7%; P = .008), ≥ 2 immunosuppressants (20 vs 0%; P = .001), and grade 3 to 4 ICANS (23 vs 6%; P = .02) were all associated with cs-CMV (Figure 2).
Increased risk of CMV reactivation in CAR T-cell therapy recipients receiving immunosuppression. (A) The 180-day incidence of CMV reactivation (any viremia) in the entire cohort. (B-G) The 180-day incidence of CMV reactivation (any viremia) by (B) degree of CRS, (C) corticosteroid use, (D) tocilizumab use, (E) use of ≥2 immunosuppressants, (F) anakinra use, and (G) degree of ICANS. The number of subjects was 94.
Increased risk of CMV reactivation in CAR T-cell therapy recipients receiving immunosuppression. (A) The 180-day incidence of CMV reactivation (any viremia) in the entire cohort. (B-G) The 180-day incidence of CMV reactivation (any viremia) by (B) degree of CRS, (C) corticosteroid use, (D) tocilizumab use, (E) use of ≥2 immunosuppressants, (F) anakinra use, and (G) degree of ICANS. The number of subjects was 94.
Increased risk of clinically significant CMV reactivation in CAR T-cell therapy recipients receiving immunosuppression. (A) The 180-day incidence of clinically significant CMV reactivation in the entire cohort. (B-G) The 180-day incidence of clinically significant CMV reactivation in patients receiving CAR T-cell therapy by (B) degree of CRS, (C) corticosteroid use, (D) tocilizumab use, (E) use of ≥2 immunosuppressants, (F) anakinra use, and (G) degree of ICANS. The number of subjects was 94.
Increased risk of clinically significant CMV reactivation in CAR T-cell therapy recipients receiving immunosuppression. (A) The 180-day incidence of clinically significant CMV reactivation in the entire cohort. (B-G) The 180-day incidence of clinically significant CMV reactivation in patients receiving CAR T-cell therapy by (B) degree of CRS, (C) corticosteroid use, (D) tocilizumab use, (E) use of ≥2 immunosuppressants, (F) anakinra use, and (G) degree of ICANS. The number of subjects was 94.
In the univariate analysis, there was a nonstatistically significant trend for corticosteroid use and the risk of CMV viremia (odds ratio [OR], 2.01; 95% confidence interval [CI], 0.9-4.3; P = .07). The use of ≥2 immunosuppressants was associated with a twofold increase in CMV viremia (OR, 2.17; 95% CI, 1.0-4.5; P = .04), a finding that remained significant in the adjusted analysis (adjusted odds ratio [aOR], 2.27; 95% CI, 1.1-4.8; P = .03) (supplemental Table 1). For cs-CMV, the use of anakinra was associated with a fourfold increased risk (OR, 4.3; 95% CI, 1.1-17; P = .04; supplemental Table 2). For cs-CMV, multivariate analysis was precluded as all variables were excluded except anakinra after stepwise backward elimination. Notably, cs-CMV was exclusively seen in patients who received both corticosteroids and tocilizumab. In total, 44 patients received both corticosteroids and tocilizumab, and 20% of them developed cs-CMV.
Mortality and progression/relapse rates
There was a significant increase in the 1-year overall mortality among CAR T-cell recipients with evidence of CMV reactivation (any viremia) by day 28 compared with those without detectable CMV viremia by day 28 after CAR T-cell infusion (57 vs 23%, P = .001; Figure 3A). The increased overall mortality among those with CMV reactivation by day 28 remained significant at 3 years (100 vs 52%, P = .001; supplemental Figure 2A). There was no association between CMV reactivation and the rate of nonrelapse mortality, but the 1-year and 3-year cumulative incidence of progression/relapse disease was higher among those with CMV reactivation by day 28 (Figure 3B-C; supplemental Figure 2B-C). Likewise, we observed an increased overall 1-year and 3-year mortality among those with cs-CMV by day 28 compared with those that had no CMV reactivation by day 28 or had viremia that required no antiviral therapy (61 vs 28%, P = .04; and 100 vs 60%, P = .03, respectively; supplemental Figure 3). Among those with cs-CMV reactivation, there were 2 deaths, but none were attributed to CMV reactivation; 1 was due to fungemia, whereas the other was attributed to CAR T-cell therapy–related toxicities causing multiorgan failure. Cs-CMV by day 28 was also associated with an increased incidence of progression/relapse at 1- and 3 years after CAR T-cell therapy (supplemental Figure 3).
CAR T-cell outcomes by CMV reactivation. (A) 1-year overall mortality, (B) 1-year nonrelapsed mortality, and (C) 1-year progression/relapse rates after CAR T-cell therapy among patients with no CMV reactivation and those with CMV reactivation by day 28. The number of subjects was 95.
CAR T-cell outcomes by CMV reactivation. (A) 1-year overall mortality, (B) 1-year nonrelapsed mortality, and (C) 1-year progression/relapse rates after CAR T-cell therapy among patients with no CMV reactivation and those with CMV reactivation by day 28. The number of subjects was 95.
Discussion
In pivotal clinical trials, ZUMA-2 and TRANSCEND, the incidence of CMV infection was <3%.1,2 However, CMV testing was not conducted routinely in the participating subjects, which could have led to a falsely low incidence. In this study, the incidence of CMV reactivation (any viremia) and cs-CMV after CAR T-cell therapy was 33% and 11%, respectively, and there were no cases of end-organ disease due to CMV reactivation. Our findings are consistent with prior reports.8,9,11,20-22 CMV was the most common viral infection in the first month of therapy in a study that included 60 anti-CD19 CAR T-cell recipients, with CMV reactivation and cs-CMV rates of 17% and 10%, respectively (median viremia, 6307 IU/mL; range, 310-44 250; none had CMV disease).8 Trando et al reported an incidence of CMV viremia of 40% among 57 CD19-targeted CAR T-cell recipients with available CMV data.23 In a recent single-center retrospective study, CMV reactivation (any viremia) occurred in 42 of 95 (44%) CD19-directed CAR T-cell recipients who were CMV seropositive, with 22% of the patients experiencing a viral load >1000 IU/mL.9 Chen et al also reported a cumulative incidence of CMV reactivation >400 IU/mL of 44% by day 100 after CD19-targeted CAR T.10 In a recent study by Kampouri et al, the incidence of CMV reactivation (any viremia) and cs-CMV among 72 adult CMV-seropositive CD19-, CD20-, and B-cell maturation antigen (BCMA)-targeted CAR T-cell recipients was 27% and 10%, respectively,11 with most cases corresponding to low-grade viremia (median 127 IU/mL) and no cases of end-organ disease.11 Khawaja et al also reported a cs-CMV rate of 10% among 230 CD19-targeted CAR T-cell recipients at a major cancer center.22 The fact that we observed very similar rates of CMV (any viremia) and cs-CMV compared with the incidence reported by other groups is quite remarkable, considering the different CMV PCR platforms used and different institutional cutoffs for initiation of therapy across studies.
CMV reactivation occurs relatively early after CAR T, with a median time to reactivation of 19 days in our study and 21 days in the reports by Chen et al10 and Marquez-Algaba et al.9 This could be related to the fact that CMV-specific cell–mediated immunity, as measured by virus-specific T-cell responses to IE-1 and pp65 antigens using a CMV enzyme-linked immunospot assay, reaches a nadir 2 weeks after infusion and recovers to baseline levels by week 4.11 We have previously shown that the production of interferon-γ alone does not fully capture the risk of CMV in the allogeneic HCT setting;13 therefore, further characterization of the immune reconstitution of the CMV-specific polyfunctional T-cell compartment after CAR T-cell therapy is needed.
Although cases of end-organ disease, including CMV pneumonitis, enteritis, and retinitis, have been reported after CAR T-cell infusion,10,22,24-27 our data, along with previous cohort studies, indicate that most CMV reactivation episodes after CAR T-cell infusion do not require antiviral therapy and that end-organ disease is uncommon.8-11,18,28 Yet, the impact of CMV reactivation (any viremia) after CAR T-cell therapy should not be underestimated. Sixty percent of patients with cs-CMV in this cohort met the criteria for CMV syndrome, as described in solid organ transplant recipients.19 One of the 2 deaths in the group of patients with cs-CMV was due to fungemia. CMV infection is known to be associated with indirect effects, including an increased risk of bacterial and fungal infections,14,29,30 and reactivation of CMV at relatively low viral load levels has been associated with increased mortality in other cellular therapy settings.12 Further supporting this notion, we observed increased 1-year overall mortality among patients with CMV reactivation by day 28, similar to Khawaja et al who reported a higher overall mortality rate 1-year after CAR T-cell therapy in patients with cs-CMV.22 We also observed a higher incidence of progression/relapse disease among those with CMV reactivation by day 28, which could reflect enrichment of patients with more aggressive lymphoma in this group, therefore not only at higher risk of relapse but also at higher risk of CAR T-cell toxicity requiring immunosuppression with associated infectious complications.
There are currently no clear guidelines or consensus on the optimal strategy for the prevention of CMV in patients receiving CAR T-cell therapy. Our findings indicate that CMV reactivation is a common complication after CAR T-cell therapy and that individuals who receive immunosuppression or immunomodulation, particularly corticosteroids and tocilizumab, for the management of CAR T–associated toxicities carry a higher risk. In a study by Marquez-Algaba et al, the sole risk factor for CMV reactivation was the administration of high-dose corticosteroids, and among those who had not received corticosteroids, none of them had cs-CMV.9 Similarly, in the present report, cs-CMV was exclusively seen in patients who received both corticosteroids and tocilizumab. In a study by Kampouri et al, the group of patients receiving >3 days of corticosteroids or BCMA CAR T-cell therapy had an incidence of CMV reactivation close to 50%.11 Marquez-Algaba et al reported an eightfold increase in the risk of CMV viremia ≥1000 IU/mL with corticosteroid treatment (OR, 8.4; 95% CI, 2.4-37).9 In patients treated with axicabtagene ciloleucel, there was a significant association between corticosteroid use and CMV viremia, which occurred within 2 weeks of corticosteroid initiation.31 Thus, our findings and those of others strongly suggest the potential benefit of routine early monitoring of CMV using a PCR assay (1-3 months) after CAR T-cell therapy and/or antiviral prophylaxis in CAR T-cell recipients who receive corticosteroids. We excluded 5 patients who had received letermovir prophylaxis, 4 of whom experienced CMV reactivation with 2 requiring antiviral therapy. Although the role of letermovir in the prevention of CMV in adult-seropositive allogeneic HCT recipients is well established,32,33 clinical trials specifically addressing the efficacy of letermovir prophylaxis in CMV-seropositive CAR T-cell recipients are needed.
Limitations of our study include those of a retrospective study conducted at a single center. In addition, the heterogeneity of the CAR T-cell therapies, most targeting CD19, prevent us from drawing conclusions specific to a given product, and multivariate analyses were restricted due to the small sample size. Lastly, CMV viremia was monitored at the discretion of the treating physician; therefore, it is conceivable that if routine monitoring had been undertaken, the incidence of CMV reactivation would be higher than that reported here. In addition, individual physician preferences could have influenced the testing frequency, yet the time to initiation of testing and the number of tests were comparable between the CMV and no CMV reactivation groups.
Despite these limitations, our observations are in alignment with recent reports and provide novel insights into the incidence and outcomes of CMV reactivation in this clinical setting. Our center has now implemented routine CMV monitoring in CAR T-cell recipients following the preemptive approach used in allogeneic HCT recipients not receiving letermovir prophylaxis,14 although the optimal duration and frequency of CMV PCR testing and the optimal viral load threshold for the initiation of preemptive therapy in this patient population remains to be established.18,28 Until more data are available, the clinical decision-making on when to initiate antiviral therapy might need to be individualized and guided by viral kinetics (eg, doubling time of CMV DNA levels, which may be as short as 2-3 days in high-risk allogeneic HCT recipients),14 risk factor stratification of the host (eg, corticosteroids, ≥2 immunosuppressants), type of CAR T-cell product (eg, BCMA), anticipated toxicity of antiviral agents (eg, if underlying cytopenia or renal disease), and the presence of symptoms. CMV-specific cell–mediated immunity monitoring using interferon-γ release assays or flow cytometry assessment of virus-specific polyfunctional T cells might also play an important role in the risk stratification of CAR T-cell recipients and further work in this area is needed.11,13
We conclude that CMV reactivation is a common complication of CAR T-cell therapy in CMV-seropositive individuals, which can be associated with increased overall mortality, even in the absence of end-organ disease. Although larger confirmatory studies are needed, our data indicate that administration of immunosuppression, particularly corticosteroids and tocilizumab, for the management of CAR T-cell toxicities is a major risk factor for CMV reactivation, and preventive interventions such as antiviral prophylaxis or routine monitoring for preemptive therapy may be indicated in this setting.
Acknowledgment
The authors thank all the patients who participated in the study.
Authorship
Contribution: R.Y.L., J.F.C., and K.V.K. conceived and designed the study; R.Y.L. and J.F.C. acquired the data; R.Y.L., J.F.C., Y.N., A.N., and K.V.K. analyzed the data; R.Y.L. and J.F.C. prepared the first draft of the manuscript; and all authors were involved in the revision of the draft manuscript and have agreed to the final content.
Conflict-of-interest disclosure: M.I.M received funding from Eurofins Viracor. The remaining authors declare no competing financial interests.
Correspondence: Rick Y. Lin, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32608; email: ricklin@medicine.ufl.edu.
References
Author notes
Original data will be available upon reasonable request from the corresponding author, Rick Y. Lin (ricklin@medicine.ufl.edu).
The full-text version of this article contains a data supplement.