Key Points
The combined mutation of D519V/E665V and K1813A in FVIII increased FVIIIa stability and the association between FVIIIa and FIXa.
The FVIII-D519V/E665V/K1813A mutant exhibited an approximate eightfold enhanced coagulation potential compared with that of WT-FVIII.
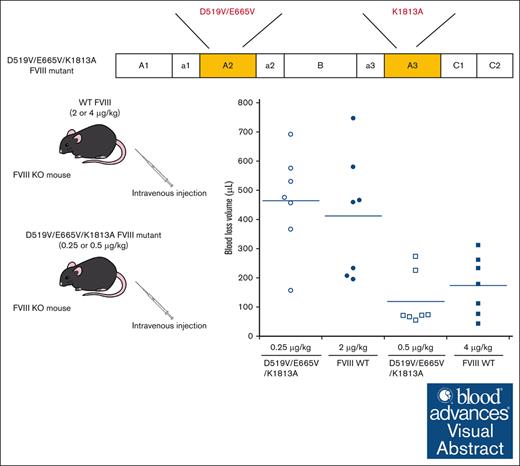
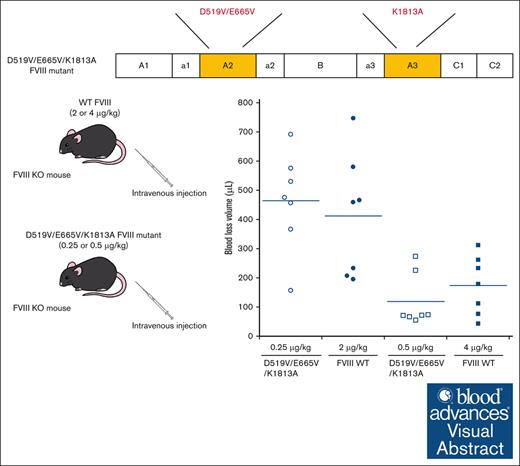
Visual Abstract
A2 domain dissociation in activated factor VIII (FVIIIa) results in reduced activity. Previous studies demonstrated that some FVIII mutants (D519V/E665V and K1813A) with delayed A2 dissociation enhanced coagulation potential. We speculated, therefore, that FVIII encompassing a combination of these mutations might further enhance coagulant activity. The aim was to assess the D519V/E665V/K1813A-FVIII mutation as a gain of function. The FVIII mutants, D519V/E665V/K1813A, D519V/E665V, and K1813A were expressed in a baby hamster kidney cell system, and global coagulation potential of these mutants was compared with wild-type (WT) FVIII in vitro and in hemophilia A mice in vivo. Kinetic analyses indicated that the apparent Kd for FIXa on the tenase assembly with D519V/E665V and D519V/E665V/K1813A mutants were lower, and that the generated FXa for D519V/E665V/K1813A was significantly greater than WT-FVIII. WT-FVIII activity after thrombin activation increased by ∼12-fold within 5 minutes, and returned to initial levels within 30 minutes. In contrast, The FVIII-related activity of D519V/E665V/K1813A increased further with time after thrombin activation, and showed an ∼25-fold increase at 2 hours. The A2 dissociation rate of D519V/E665V/K1813A was ∼50-fold slower than the WT in a 1-stage clotting assay. Thrombin generation assays demonstrated that D519V/E665V/K1813A (0.125 nM) exhibited coagulation potential comparable with that of the WT (1 nM). In animal studies, rotational thromboelastometry and tail-clip assays showed that the coagulation potential of D519V/E665V/K1813A (0.25 μg/kg) was equal to that of the WT (2 μg/kg). FVIII-D519V/E665V/K1813A mutant could provide an approximately eightfold increase in hemostatic function of WT-FVIII because of increased FVIIIa stability and the association between FVIIIa and FIXa.
Introduction
Factor VIII (FVIII), a plasma protein deficient or defective in the inherited bleeding disorder hemophilia A (HA), functions as a procofactor for the serine protease FIXa in phospholipid (PL) surface–dependent conversion of FX to activated FX (FXa).1 FVIII is synthesized as a multidomain, single-chain molecule (A1-A2-B-A3-C1-C2) consisting of 2332 amino acid residues with a molecular mass of ∼300 kDa,2,3 and is processed during secretion by proteolytic cleavage generating a variably sized heavy chain consisting of A1-A2-B domains linked to a light chain consisting of A3-C1-C2 domains.2-4 FVIII is activated by limited thrombin cleavage at 3 sites, Arg372, Arg740, and Arg1689, generating FVIIIa.5 In the intrinsic tenase assembly, FVIIIa interacts with FIXa and increases the kcat for FXa formation by ∼106-fold compared with FIXa alone.6,7 The modulation of FVIIIa-FIXa association, therefore, augments the potential for intrinsic tenase activity.
It is generally understood that FVIIIa is inactivated by 2 mechanisms: proteolytic cleavage by activated protein C (APC) and spontaneous dissociation of the A2 domain.8 Resistance to APC is known to be a major risk factor for venous thromboembolism.9,10 The genetic defect, FVLeiden (FV-Arg506Gln), is associated with APC resistance, due to inefficient cleavage at Arg506, and results in a high risk of venous thrombosis.9,10 APC also inactivates FVIIIa by cleavage at Arg336 and Arg562, although cleavage at Arg336 appears to play a more dominant role than that at Arg562 in these reactions.11 Previous studies have suggested that APC plays a marginal role in FVIIIa regulation,12,13 and there is no known phenotype associated with altered APC cleavage of FVIII. A more recent study in a murine model of HA demonstrated, however, that an APC-resistant FVIII mutant enhanced hemostasis,14 indicating that resistance to APC could mediate a procoagulant effect. The instability of the FVIIIa molecule is attributed to very weak electrostatic interactions (Kd, ∼260 nM) between the A2 subunit and the A1/A3C1C2 dimer,15 leading to reduced intrinsic tenase activity.12 The dissociation of A2 in FVIIIa appears to contribute more to reduced activity than APC-mediated inactivation.12,13
Wakabayashi et al16 reported that FVIII mutations at Asp519 (D519), Glu665 (E665), and Glu1984 (E1984) to Ala or Val resulted in twofold, and fourfold to eightfold higher thermal stability, respectively, of FVIII relative to wild-type (WT) FVIII (WT-FVIII). Moreover, later studies using a mouse HA model of vascular injury demonstrated that the FVIII-D519V/E665V mutation contributed to noncovalent stabilization of the A2 domain and enhanced coagulation potential.17 In addition, our previous studies demonstrated that the FVIII-Lys1813Ala (K1813A) mutation was associated with slower A2 dissociation and increased global coagulation potential in in vitro and in vivo models.18 The findings indicated, therefore, that suppression of FVIIIa inactivation could lead to improvements in hemostatic mechanisms.
In this context, our current investigations focused on D519V, E665V, and K1813A FVIII residues, to assess the additive impact of a combination of these mutations (D519V/E665V/K1813A) on the procoagulant activity of FVIIIa.
Materials and methods
Ethics
All animal experiments were approved by the animal care and use committee of Nara Medical University (no. 13138), and all procedures followed the Policy on the Care and Use of Laboratory Animals of Nara Medical University.
Reagents
Human purified FIXa, FX, and FXa (Hematologic Technologies, Essex Junction, VT), α-thrombin and recombinant hirudin (Calbiochem, San Diego, CA), chromogenic substrate S-2222 and Coagpia APTT-N (Sekisui Medical, Tokyo, Japan), Thrombocheck APTT-SLA and FVIII-deficient plasma (Sysmex, Kobe, Japan), plasma from patients with HA (George-King, Overland Park, KS), recombinant tissue factor (Innovin; Dade, Marburg, Germany), and thrombin-specific fluorogenic substrate (Bachem, Bubendorf, Switzerland) were purchased from the indicated vendors. PL vesicles (phosphatidylserine/phosphatidylcholine/phosphatidylethanolamine 10/60/30%; Sigma-Aldrich; St Louis, MO) were prepared using N-octylglucoside.19
Mutagenesis, expression, and purification of mutated FVIII
B domain–deleted lacking Q744-S1637 (BDD), WT-FVIII, and BDD-FVIII mutants (single mutated K1813A, double mutated D519V/E665V, and triple mutated D519V/E665V/K1813A) were stably expressed in baby hamster kidney cells and purified.20 The “legacy” numbering for FVIII residues was used for consistency with earlier studies. The resultant FVIII forms were typically >90% pure (supplemental Figure 1), as determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and staining with GelCode Blue-Stain Reagent (Pierce). Purified FVIII concentrations were measured by enzyme-linked immunosorbent assay using 2 anti-FVIII monoclonal antibodies (mAbs; anti-FVIII C2; ESH8, and anti-FVIII A2; R8B12).21 Concentrations were verified by measurements of absorbance at 280 nm, as previously described.18 FVIII activity (FVIII:C) was determined using a chromogenic FXa generation assay (Chromogenix). Samples were rapidly frozen and stored at −80°C.
FXa generation assays
The rate of FX conversion to FXa was monitored using a purified system.22 To calculate apparent Kd and Vmax for the FIXa-FVIIIa association, FVIII (0.5 nM) in buffer (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1 M NaCl, 5 mM CaCl2, pH 7.2, 0.01% Tween 20) containing PL vesicles (20 μM) was activated by the addition of thrombin (30 nM). Thrombin activity was terminated after 30 seconds by the addition of hirudin (10 U/mL), and FXa generation was initiated by the addition of FX (300 nM) and various concentrations of FIXa (0-5 nM). To calculate the Km and kcat for FX-FVIIIa association, FVIII (1 nM) in buffer containing PL vesicles (20 μM) was activated by the addition of thrombin (30 nM). Thrombin activity was terminated after 30 seconds by the addition of hirudin (10 U/mL), and FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (0-400 nM). As for the A2 dissociation in FVIIIa, FVIII (1 nM) in buffer containing PL vesicles (20 μM) was activated by the addition of thrombin (30 nM) and thereafter terminated after 30 seconds by the addition of hirudin (10 U/mL). At various time intervals (0-15 minutes), FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (300 nM). The reactions were quenched after 1 minute by adding 50 mM ethylene-diamine-tetraacetic acid. The amount and velocity rate of FXa generation were determined using the chromogenic substrate S-2222 (final concentration [FC]: 0.46 mM). All assays were performed at 23°C.
FVIII:C measurements
FVIII:C was measured in a 1-stage clotting assay using commercial FVIII-deficient plasma, Thrombocheck APTT-SLA and Coagpia APTT-N, with a STart4 hemostasis Analyzer (Diagnostica Stago, Asnieres, France). All reactions were performed at 37°C.
FVIII (10 nM) was activated by the addition of thrombin (0.4 nM) in HEPES-buffered saline (HBS buffer) containing 5 mM CaCl2 and 0.01% bovine serum albumin. Aliquots were removed from the mixtures at the indicated times, and thrombin was rapidly inactivated by the addition of hirudin (1 U/mL) and a 1500-fold dilution.20 The presence of thrombin and hirudin in the diluted samples did not affect FVIII:C levels in these assays (data not shown).
To assess spontaneous decay of FVIIIa activity, FVIII (1 nM) was activated by the addition of thrombin (30 nM) for 30 seconds, before termination of thrombin activity by the addition of hirudin, as previously described.18
FVIII cleavage by thrombin
Thrombin (2 nM) was added to FVIII (50 nM) at a 1:25 molar ratio in HBS buffer at 37°C. Samples were obtained at the indicated times, and the reactions were immediately terminated and prepared for SDS-PAGE by adding SDS under reducing conditions and boiling for 3 minutes.
Electrophoresis and western blotting
SDS-PAGE was performed at 150 V for 1 hour, using 8% gels followed by western blotting using a Bio-Rad mini-transblot apparatus (BIO-RAD, Hercules, CA) at 100 V for 1 hour. Protein bands were probed using an anti-A1 mAbC5, followed by goat anti-mouse peroxidase-linked secondary antibody. Signals were detected using enhanced chemiluminescence (PerkinElmer Life Science, Boston, MA). Densitometric scans were quantitated using Fusion Solo S (Vilber Lourmat, Collegien, France).
TGA
Thrombin generation assays (TGA) were performed as previously described.23 Briefly, samples (80 μL) were preincubated for 10 minutes with 20 μL of trigger reagent containing recombinant tissue factor and PL (FC: 1 pM and 4 μM, respectively) before the addition of 20 μL of reagent containing CaCl2 and fluorogenic substrate (FC: 16.7 mM and 2.5 mM, respectively). The development of fluorescent signals was monitored using a Fluoroskan Ascent microplate reader (Thermo Fisher Scientific, Waltham, MA). Data analyses were performed using the manufacturer's software to derive the standard parameters: peak thrombin, time-to-peak thrombin (time-to-peak), and endogenous thrombin potential.
Animals and tail-clip assay
HA mice with targeted destruction of exon16 on a 129×C57BL/6 background were kindly gifted by Yoichi Sakata (Jichi Medical University, Shimotsuke, Japan) and were backcrossed with C57BL/6 mice (CLEA Japan, Tokyo, Japan). The in vivo tail-clip assay was performed on male and female mice aged 8 to 12 weeks (body weight: 20-25 g). All the animals were maintained under specific pathogen-free conditions. The tail-clip assay was performed as previously reported.18 In outline, C57BL/6 and HA mice were anesthetized with a mixture of medetomidine, midazolam, and butorphanol and then placed on a hot plate (Tokyo Garasu Kikai, Tokyo, Japan) at 37°C for at least 10 minutes. After prewarming, HA mice were intravenously infused with the D519V/E665V/K1813A (0.25 and 0.5 μg/kg) and D519V/E665V (1.0 μg/kg) FVIII mutant, and WT-FVIII (2 and 4 μg/kg), or saline (Otsuka Pharmaceutical, Tokyo, Japan). At 5 minutes after administration, the tail was cut 5 mm from the tip and immediately placed in a conical tube containing 10 mL of saline (prewarmed to 37°C). The volume of blood loss collected at 40 minutes was quantified gravimetrically.
ROTEM
Rotational thromboelastometry (ROTEM) (nonactivated TEM) was used to assess coagulation potential in HA mice infused with the D519V/E665V (1.0 μg/kg), D519V/E665V/K1813A mutant (0.25, 0.5, 1, and 2 μg/kg) and WT-FVIII (2 μg/kg) using a whole-blood hemostasis analyzer (Pentapharm, Munich, Germany). The whole-blood samples were drawn from the inferior vena cava and collected into tubes containing 3.2% sodium citrate at a 9:1 ratio, 5 minutes after administration. Citrated whole-blood samples (280 μL) were incubated for 30 minutes at 25°C before the addition of 20 μL CaCl2 (FC: 12.5 mM) to initiate coagulation. Clot formation was evaluated using the parameters, clotting time (CT; time until reaching 2-mm amplitude), clot formation time (time until reaching 20-mm amplitude), α angle (the angle of the tangent between 0 mm and the curve when the clot firmness is 20 mm), and maximum clot firmness.
Computational structure modeling of FVIII
The 3-dimensional protein structures of both WT-FVIII and FVIII mutant (D519V/E665V/K1813A) were predicted using AlphaFold2.24 The amino acid sequence of the FVIII-BDD was input into a locally installed full AlphaFold2.3 system for PDB70 construction and an AlphaFold2 Colab-based approach was used for PDB100 construction to obtain structures.25,26 Structural models with higher predicted local distance difference test (pLDDT)scores, indicating better quality, were selected for further analysis and we removed signal peptide, small a1, a2, and a3 acidic domains because of the low pLDDT score indicating unreliable quality. These selected models were aligned with an X-ray crystal structure of FVIII from the RCB protein data bank (PDB reference: 6MF2)27 using the TM-align web server (https://zhanggroup.org/TM-score/help/).28 The predicted structures of FVIII were analyzed using PyMol molecular visualization software (version 2.5.5, Schrödinger, LLC).29 Electrostatic potential calculations of the molecular surface were analyzed using the PyMol APBS plugin with default settings, which implements the Adaptive Poisson–Boltzmann Solver.30 Color gradients were applied to depict regions of positive, negative, and neutral electrostatic potential, facilitating the interpretation of potential electrostatic interactions within the protein structure.
Data analysis
The Km and Vmax values for FVIIIa/FIXa-catalyzed activation of FX were calculated by fitting the data to a nonlinear least-squares regression using Equation 1.
The intermolecular or intramolecular stability of FVIII/FVIIIa activity was determined using Equation 2. The rate constant (k) for FVIII/FVIIIa activity was calculated using the following equation:
All animal experiments were approved by the animal care and use committee of Nara Medical University (no. 13138), and all procedures followed the Policy on the Care and Use of Laboratory Animals of Nara Medical University.
Results
Comparisons of the association between FIXa and K1813A, D519V/E665V, and D519V/E665V/K1813A mutants
Three mutated recombinant FVIII proteins were prepared and purified, and specific activities measured using 1-stage clotting assays and FXa generation assays. A direct-measured specific activity in 1-stage clotting assays demonstrated that the respective specific activities of WT-FVIII, K1813A, D519V/E665V, and D519V/E665V/K1813A was 4.15 ± 0.85, 9.00 ± 1.00, 4.95 ± 1.65, and 14.50 ± 2.50 U/μg, respectively. Similarly, the FXa generation assays identified that the specific activities of WT-FVIII, K1813A, D519V/E665V, and D519V/E665V/K1813A was 103 ± 6, 192 ± 11, 95 ± 23, and 268 ± 25 nM FXa/min per nM FVIII, respectively. These results suggested that the single mutation, K1813A, enhanced FVIII activity, and that this effect was potentiated further by the combined mutation, D519V/E665V/K1813A.
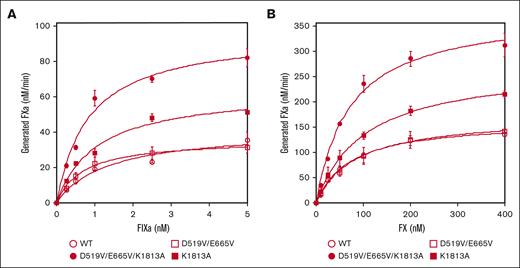
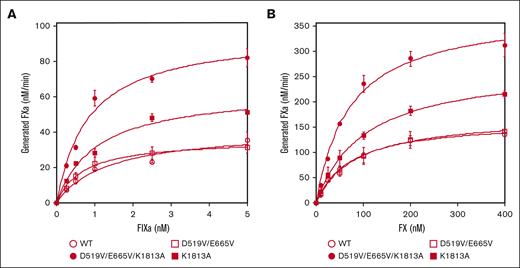
The interactions between mutated FVIII and FIXa or FX on PL surfaces in the intrinsic tenase complex were therefore assessed using purified FXa generation assays (Figure 1). Kinetic parameters derived in these experiments are summarized in Table 1. The apparent Kd for FIXa with D519V/E665V (0.78 ± 0.1 nM) or D519V/E665V/K1813A (0.84 ± 0.04 nM) was significantly lower than that with WT-FVIII (1.2 ± 0.2 nM; Figure 1A). Also, the generated FXa with K1813A (59 ± 5.1 nM FXa/min per nM FVIII) or D519V/E665V/K1813A (89 ± 6.0 nM FXa/min per nM FVIII) was significantly greater than that with WT-FVIII (38 ± 5.8 nM FXa/min per nM FVIII). Results with D519V/E665V, however, were not different from those with WT-FVIII. In contrast, the kinetic parameters of FXa generation with these mutants for FX appeared to be comparable with those of WT-FVIII except for that with D519V/E665V/K1813A (Figure 1B), and the data indicated that the interaction between FIXa and D519V/E665V/K1813A in the tenase complex was markedly increased compared with that of WT-FVIII.
The kinetics of FIXa-catalyzed FX activation with D519V/E665V/K1813A, D519V/E665V, and K1813A FVIII mutants. (A) FIXa association; WT-FVIII or FVIII mutants (0.5 nM) were activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FX (300 nM) and various concentrations of FIXa (0-5 nM) as described in “Methods.” (B) FX association; WT-FVIII or mutant FVIII (1 nM) was activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (0-400 nM). The symbols used are: open circles, WT; closed circles, D519V/E665V/K1813A; open squares, D519V/E665V; and closed squares, K1813A. Experiments were performed 3 times, and the average and standard deviation values are shown. The initial rates of FXa generation were plotted as a function of FIXa or FX concentration and fitted to Equation 1 by nonlinear least squares regression.
The kinetics of FIXa-catalyzed FX activation with D519V/E665V/K1813A, D519V/E665V, and K1813A FVIII mutants. (A) FIXa association; WT-FVIII or FVIII mutants (0.5 nM) were activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FX (300 nM) and various concentrations of FIXa (0-5 nM) as described in “Methods.” (B) FX association; WT-FVIII or mutant FVIII (1 nM) was activated by thrombin (30 nM) for 30 seconds in the presence of PL vesicles (20 μM). FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (0-400 nM). The symbols used are: open circles, WT; closed circles, D519V/E665V/K1813A; open squares, D519V/E665V; and closed squares, K1813A. Experiments were performed 3 times, and the average and standard deviation values are shown. The initial rates of FXa generation were plotted as a function of FIXa or FX concentration and fitted to Equation 1 by nonlinear least squares regression.
Comparisons of thrombin activation between K1813A, D519V/E665V, and D519V/E665V/K1813A mutants
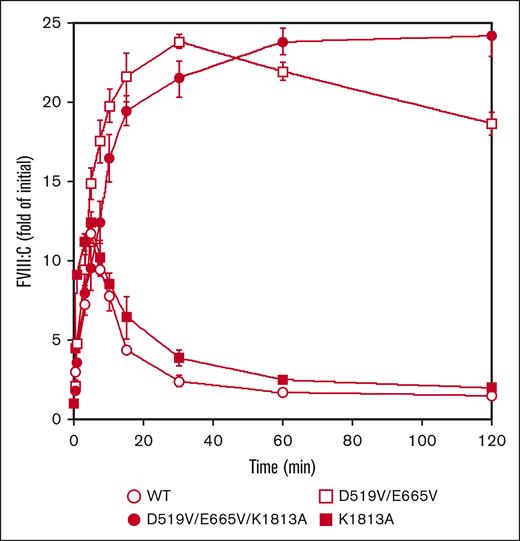
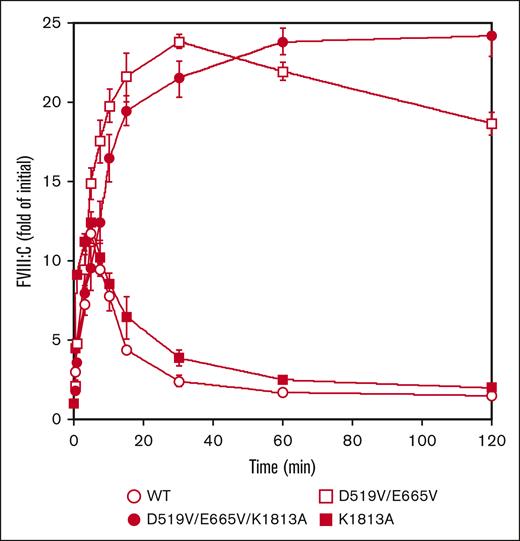
Our findings suggest that the higher FVIII-specific activity of D519V/E665V/K1813A might be attributed to enhanced association between FVIIIa and FIXa and/or slow dissociation of the FVIII A2 domain. In this context, therefore, FVIII mutants (10 nM) were first examined for activation by thrombin (0.4 nM) as described in “Methods.” The peak value of FVIII:C with K1813A after thrombin activation was increased by ∼12-fold at 5 minutes. This appeared to be slightly greater than that with WT-FVIII, and was followed by a time-dependent FVIII:C.18 In contrast, peak FVIII:C with the D519V/E665V was increased ∼24-fold at 30 minutes, and the time-dependent decrease in activity was significantly slower than that with the WT or the K1813A mutant. Moreover, FVIII:C with the combined D519V/E665V/K1813A mutant after thrombin activation continued to increase to ∼24-fold of the initial level at 120 minutes (Figure 2). As for thrombin-catalyzed cleavage, the time course of heavy chain cleavage determined by western blotting using anti-A1 mAbC5 was investigated. The D519V/E665V/K1813A, D519V/E665V, and K1813A mutants did not affect thrombin cleavage at Arg372, similar to WT-FVIII thrombin cleavage (supplemental Figure 2). The results suggest that the D519V/E665V and D519V/E665V/K1813A mutations considerably influence thrombin activation and inactivation of FVIII:C, although their thrombin-catalyzed cleavages were similar to that of the WT.
Thrombin-catalyzed activation of D519V/E665V/K1813A, D519V/E665V, and K1813A FVIII mutants. WT-FVIII or FVIII mutants (10 nM) were incubated with thrombin (0.4 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay. The symbols used are: open circles, WT; closed circles, D519V/E665V/K1813A; open squares, D519V/E665V; and closed squares, K1813A. The initial FVIII activities of the WT, and K1813A, D519V/E665V, and D519V/E665V/K1813A mutants were 6.1, and 12.8, 9.1, and 22.0 IU/mL, respectively, at t = 0. FVIII activity was expressed as a fold of initial (t = 0) and was plotted as a function of incubation time. Experiments were performed 3 times, and the average and standard deviation values are shown.
Thrombin-catalyzed activation of D519V/E665V/K1813A, D519V/E665V, and K1813A FVIII mutants. WT-FVIII or FVIII mutants (10 nM) were incubated with thrombin (0.4 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay. The symbols used are: open circles, WT; closed circles, D519V/E665V/K1813A; open squares, D519V/E665V; and closed squares, K1813A. The initial FVIII activities of the WT, and K1813A, D519V/E665V, and D519V/E665V/K1813A mutants were 6.1, and 12.8, 9.1, and 22.0 IU/mL, respectively, at t = 0. FVIII activity was expressed as a fold of initial (t = 0) and was plotted as a function of incubation time. Experiments were performed 3 times, and the average and standard deviation values are shown.
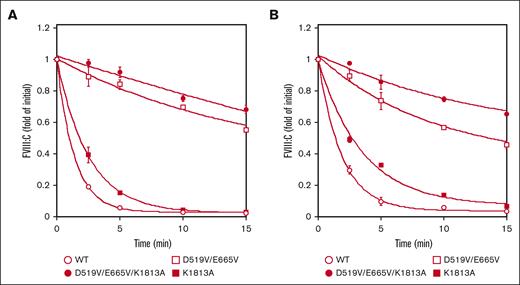
The stability of FVIIIa associated with the D519V/E665V/K1813A mutations
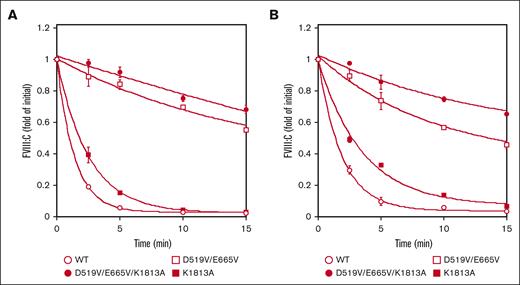
The spontaneous decay of the mutated FVIII preparations (1 nM) over time was assessed by measuring FVIII activity after activation by thrombin (30 nM) as described in “Methods.” The decay rate is believed to reflect dissociation of the A2 domain, and with the K1813A mutant estimate (0.40 min−1), appeared to be ∼1.8-fold lower than that of the WT (0.72 min−1) in a 1-stage clotting assay. These data were consistent with our earlier report.18 In contrast, the decline in FVIIIa activity with the D519V/E665V and D519V/E665V/K1813A mutants (0.06 and 0.014 min−1, respectively) was depressed ∼11-fold and 50-fold, respectively, compared with the WT (Figure 3A). As for the A2 dissociation in the intrinsic Xase assay, Figure 3B showed that the spontaneous decay in FVIIIa activity with the D519V/E665V and D519V/E665V/K1813A mutants (0.11 and 0.07 min−1, respectively) was depressed approximately fivefold and eightfold, respectively, compared with the WT (0.54 min−1) in a FXa generation assay, indicating that the stability of the A2 domains in these mutated proteins was enhanced relative to that of the WT. Similar data were also obtained using a different aPTT reagent (Coagpia APTT-N; data not shown). Taken together, FVIIIa with the combined mutation, K1813A and D519V/E665V, appeared to be markedly more stable than the other mutants (Figure 3).
Spontaneous decay on the stability of D519V/E665V/K1813A, D519V/E665V, and K1813A FVIIIa mutants. (A) WT-FVIII or FVIII mutants (1 nM) were incubated with thrombin (30 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay, as described in “Methods.” As for the A2 dissociation in the intrinsic Xase assay (B), FVIII (1 nM) in buffer containing PL vesicles (20 μM) was activated by the addition of thrombin (30 nM) and thereafter terminated after 30 seconds by the addition of hirudin (10 U/mL). At various time intervals (0-15 minutes), FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (300 nM). The symbols used are: open circles, WT; closed circles, D519V/E665V/K1813A; open squares, D519V/E665V; and closed squares, K1813A. FVIIIa activity was expressed as the fold-change of the initial value and was plotted as a function of incubation time. The experiments were performed 3 times, and the average and standard deviation calculations are shown. The data were fitted to the formula given in Equation 2.
Spontaneous decay on the stability of D519V/E665V/K1813A, D519V/E665V, and K1813A FVIIIa mutants. (A) WT-FVIII or FVIII mutants (1 nM) were incubated with thrombin (30 nM) before measuring FVIII activity at the indicated times in a 1-stage clotting assay, as described in “Methods.” As for the A2 dissociation in the intrinsic Xase assay (B), FVIII (1 nM) in buffer containing PL vesicles (20 μM) was activated by the addition of thrombin (30 nM) and thereafter terminated after 30 seconds by the addition of hirudin (10 U/mL). At various time intervals (0-15 minutes), FXa generation was initiated by the addition of FIXa (40 nM) and various concentrations of FX (300 nM). The symbols used are: open circles, WT; closed circles, D519V/E665V/K1813A; open squares, D519V/E665V; and closed squares, K1813A. FVIIIa activity was expressed as the fold-change of the initial value and was plotted as a function of incubation time. The experiments were performed 3 times, and the average and standard deviation calculations are shown. The data were fitted to the formula given in Equation 2.
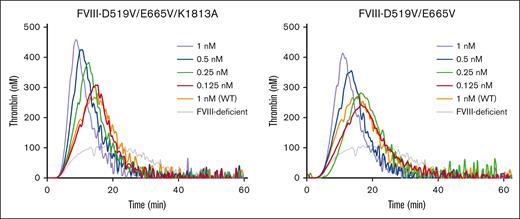
In vitro global coagulation function of the D519V/E665V/K1813A mutant in FVIII-deficient plasma
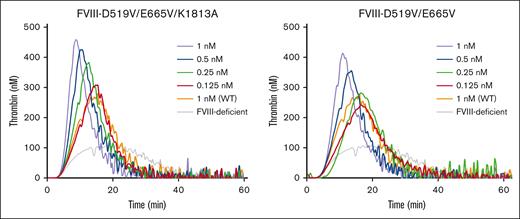
Some previous studies have shown that stabilization of A2 molecular configurations could enhance global coagulation function.16-18 We therefore assessed the effects of D519V/E665V/K1813A mutations on hemostasis by TF-triggered TGA, using FVIII-deficient plasma mixed with purified D519V/E665V and D519V/E665V/K1813A at a concentration of 0.125 to 1 nM. Representative TGA curves are illustrated in Figure 4, and the relevant parameters are summarized in supplemental Table 1. The time to peak in the presence of D519V/E665V/K1813A (≥0.5 nM) or D519V/E665V (1 nM) was significantly shorter than that with the WT at 1 nM, and the peak thrombin in the presence of D519V/E665V/K1813A at concentrations >0.25 nM was significantly higher than that with the WT at 1 nM. Also, the peak thrombin with D519V/E665V at 0.5 nM was significantly greater than that with the WT at 1 nM. The endogenous thrombin potential calculations with either D519V/E665V/K1813A or D519V/E665V were not different from that with the WT. These results demonstrate that thrombin generation in the presence of D519V/E665V and D519V/E665V/K1813A was increased by approximately fourfold and eightfold, respectively, of that of the WT, reflecting an approximate twofold increase in global coagulation potential with D519V/E665V/K1813A compared with D519V/E665V.
Thrombin generation potential of D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (1 nM) or FVIII mutants (D519V/E665V; 0.125-1 nM, and D519V/E665V/K1813A; 0.125-1 nM) were added to commercial FVIII-deficient plasma. These samples were incubated with recombinant tissue factor (rTF) (1 pM) and PL vesicles (4 μM) before the addition of fluorogenic substrate and CaCl2 at the start of the assay as described in “Methods.” Experiments were performed 3 times, and the mean values are shown. Thrombin generation curves are shown (gray, FVIII-deficient plasma; orange, WT 1 nM; red, mutants 0.125 nM; green, mutants 0.25 nM; blue, mutants 0.5 nM; and light purple, mutants 1 nM).
Thrombin generation potential of D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (1 nM) or FVIII mutants (D519V/E665V; 0.125-1 nM, and D519V/E665V/K1813A; 0.125-1 nM) were added to commercial FVIII-deficient plasma. These samples were incubated with recombinant tissue factor (rTF) (1 pM) and PL vesicles (4 μM) before the addition of fluorogenic substrate and CaCl2 at the start of the assay as described in “Methods.” Experiments were performed 3 times, and the mean values are shown. Thrombin generation curves are shown (gray, FVIII-deficient plasma; orange, WT 1 nM; red, mutants 0.125 nM; green, mutants 0.25 nM; blue, mutants 0.5 nM; and light purple, mutants 1 nM).
Global coagulation potential of the D519V/E665V/K1813A mutant in HA mice in vivo
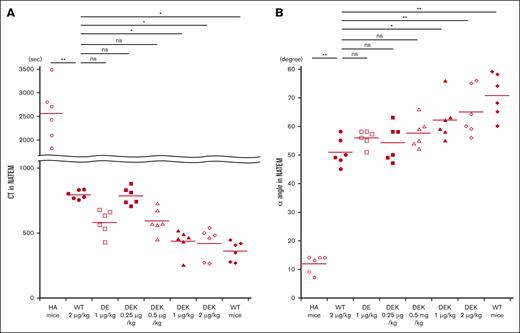
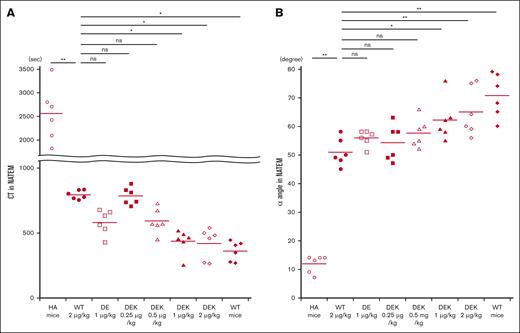
Coagulation potential in HA mice in vivo was investigated using whole blood–based ROTEM measurements after infusions of D519V/E665V or D519V/E665V/K1813A mutants as described in “Methods.” Representative thromboelastograms are illustrated in Figure 5, and the relevant coagulation parameters are summarized in Figure 6. Significantly shortened CT measurements were observed between HA mice and HA mice infused with WT-FVIII at 2 μg/kg (Figure 6A). There were no significant differences in CT values between WT-FVIII (2 μg/kg) and D519V/E665V (1 μg/kg) or D519V/E665V/K1813A (0.25 and 0.5 μg/kg). Shortened CT values in D519V/E665V/K1813A (1 and 2 μg/kg) were observed compared with in WT-FVIII (2 μg/kg). As for the α angle (Figure 6B), the α-angle value in HA mice infused with WT-FVIII at 2 μg/kg was significantly higher than that in HA mice, and that in D519V/E665V/K1813A at 0.25 and 0.5 μg/kg was comparable with that with WT-FVIII at 2 μg/kg. In contrast, we found higher α angles in D519V/E665V/K1813A (1 and 2 μg/kg) relative to WT-FVIII (2 μg/kg) with significant difference. No differences in maximum clot firmness were recorded in any of the mice (data not shown). Overall, these findings indicated that the coagulation function in D519V/E665V/K1813A was greater than in WT-FVIII and that D519V/E665V/K1813A at the same concentration as WT-FVIII may be a risk of thrombogenic potential.
Thromboelastograms obtained by ROTEM in HA mice infused with D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (2 μg/kg), mutant; D519V/E665V/K1813A (0.25, 0.5, 1 and 2 μg/kg), or D519V/E665V (1 μg/kg) was infused to the HA mice. Citrated whole-blood samples taken from these mice were assessed by a ROTEM triggered by CaCl2, as described in “Methods.” A representative thromboelastogram is shown. The average parameters (CT, clot formation time [CFT], CT + CFT, α angle, and maximum clot firmness [MCF]) in WT mice and HA mice infused with each FVIII mutant are shown below each figure.
Thromboelastograms obtained by ROTEM in HA mice infused with D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (2 μg/kg), mutant; D519V/E665V/K1813A (0.25, 0.5, 1 and 2 μg/kg), or D519V/E665V (1 μg/kg) was infused to the HA mice. Citrated whole-blood samples taken from these mice were assessed by a ROTEM triggered by CaCl2, as described in “Methods.” A representative thromboelastogram is shown. The average parameters (CT, clot formation time [CFT], CT + CFT, α angle, and maximum clot firmness [MCF]) in WT mice and HA mice infused with each FVIII mutant are shown below each figure.
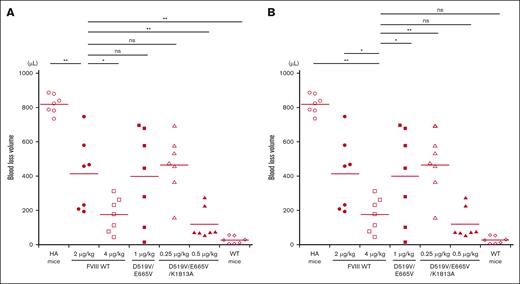
In vivo coagulation potential assessed by ROTEM in HA mice infused with D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (2 μg/kg) or mutant; D519V/E665V/K1813A (0.25, 0.5, 1, and 2 μg/kg) or D519V/E665V (1 μg/kg), or phosphate-buffered saline (PBS) were infused to the HA mice. CaCl2 was added to citrated whole-blood samples obtained from HA mice, followed by a ROTEM assay. The (A) CT and (B) α-angle parameters in WT mice and HA mice are shown. Statistical analysis between WT-FVIII (2 μg/kg) and FVIII mutants or no FVIII administration was performed using Dunnett multiple comparison test. Significant differences are defined as P < .05 (∗P < .05 and ∗∗P < .01). Each data point represents an individual mouse. The straight line in each graph represents the mean values. ns, not significant.
In vivo coagulation potential assessed by ROTEM in HA mice infused with D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (2 μg/kg) or mutant; D519V/E665V/K1813A (0.25, 0.5, 1, and 2 μg/kg) or D519V/E665V (1 μg/kg), or phosphate-buffered saline (PBS) were infused to the HA mice. CaCl2 was added to citrated whole-blood samples obtained from HA mice, followed by a ROTEM assay. The (A) CT and (B) α-angle parameters in WT mice and HA mice are shown. Statistical analysis between WT-FVIII (2 μg/kg) and FVIII mutants or no FVIII administration was performed using Dunnett multiple comparison test. Significant differences are defined as P < .05 (∗P < .05 and ∗∗P < .01). Each data point represents an individual mouse. The straight line in each graph represents the mean values. ns, not significant.
Hemostatic potential of the D519V/E665V/K1813A mutant in HA mice in vivo
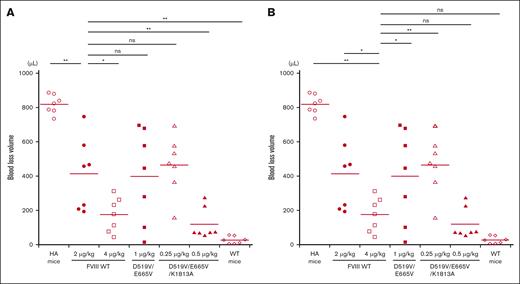
The in vivo hemostatic potential of WT-FVIII and the different mutant products was assessed by the blood loss volume in the tail-clip assay between WT-FVIII and FVIII mutants through Dunnett multiple comparison tests. The results are illustrated in Figure 7. The blood loss volume after D519V/E665V/K1813A infusions at 0.25 μg/kg was similar to that with WT-FVIII at 2 μg/kg (Figure 7A), and was significantly higher than that with WT-FVIII at 4 μg/kg (Figure 7B). The volume of blood loss in mice infused with D519V/E665V/K1813A at 0.5 μg/kg was significantly less than that in those given WT-FVIII at 2 μg/kg (Figure 7A), and was comparable to that in those given WT-FVIII at 4 μg/kg (Figure 7B). As for D519V/E665V (1 μg/kg), the blood loss volume after D519V/E665V infusions at 1 μg/kg was similar to that with WT-FVIII at 2 μg/kg (Figure 7A), and was significantly higher than that with WT-FVIII at 4 μg/kg (Figure 7B). The findings therefore indicate that overall hemostatic potential of the combined D519V/E665V/K1813A mutant in vivo could be enhanced eightfold relative to that of the WT, and fourfold relative to hat of D519V/E665V.
In vivo hemostatic effect assessed by tail clip assay in HA mice after infusion of D519V/E665V/K1813A and D519V/E665V FVIII mutants. The HA mice were infused with FVIII-WT (2 and 4 μg/kg) or mutant; D519V/E665V/K1813A (0.25 and 0.5 μg/kg) or D519V/E665V (1 μg/kg), or PBS. At 5 minutes after infusion, the terminal 5 mm of the tail was amputated, and shed blood was collected for 40 minutes, as described in “Methods.” The blood loss in (A) WT-FVIII at 2 μg/kg and (B) 4 μg/kg were compared with that in WT mice and HA mice infused with FVIII mutants. Statistical analysis between WT-FVIII and FVIII mutants or no FVIII administration was performed using the Dunnett multiple comparison test. Each point represents a mouse. The straight lines in each graph indicate the average values. Significant differences are defined as P < .05 (∗P < .05 and ∗∗P < .01). ns, not significant.
In vivo hemostatic effect assessed by tail clip assay in HA mice after infusion of D519V/E665V/K1813A and D519V/E665V FVIII mutants. The HA mice were infused with FVIII-WT (2 and 4 μg/kg) or mutant; D519V/E665V/K1813A (0.25 and 0.5 μg/kg) or D519V/E665V (1 μg/kg), or PBS. At 5 minutes after infusion, the terminal 5 mm of the tail was amputated, and shed blood was collected for 40 minutes, as described in “Methods.” The blood loss in (A) WT-FVIII at 2 μg/kg and (B) 4 μg/kg were compared with that in WT mice and HA mice infused with FVIII mutants. Statistical analysis between WT-FVIII and FVIII mutants or no FVIII administration was performed using the Dunnett multiple comparison test. Each point represents a mouse. The straight lines in each graph indicate the average values. Significant differences are defined as P < .05 (∗P < .05 and ∗∗P < .01). ns, not significant.
Discussion
Our earlier study demonstrated that the FVIII K1813A mutation could increase global FVIII coagulation potential compared with that of the WT because of better stability of the variant FVIII/FVIIIa. The spontaneous decay of FVIIIa for the K1813A mutant was 2.5-fold slower than that for the WT, and the coagulation potential of K1813A was twofold greater that that of the WT in in vitro and in vivo experiments.18 In addition, the cofactor activity of FVIIIa is mainly regulated by mechanisms involving dissociation of the FVIII A2 domain,12,13 and the D519V/E665V mutant appeared to provide enhanced coagulation potential by strengthening the interaction of the A2 domain in FVIIIa.17 Alternative studies have also reported that the decay rates of FVIIIa for 336(P4-P3′)562/D519V/E665V and 336(P4-P3′)562/A108I/D519V/E665V were reduced by ∼25-fold compared with the control D519V/E665V variant (∼14-fold),31 confirming that combined mutations led to enhanced FVIIIa stability. These data therefore provided novel evidence that a combination of K1813A and D519V/E665V could stabilize FVIIIa and enhance coagulation potential better than D519V/E665V or K1813A alone, indicating the synergistic effect of D519V/E665V and K1813A. This study further established that D519V/E665V/K1813A exhibited an approximate eightfold enhancement of global coagulation function compared with that of WT-FVIII due to reduced FVIIIa decay and increased the association between FVIIIa and FIXa.
The earlier study also demonstrated that the decay rate of FVIIIa in the D519V/E665V mutant was reduced by 14-fold compared with that in the WT, and the peak thrombin of TGA was fourfold higher than that of the WT,31 and the coagulation function of D519V/E665V in a tail-clip assay was twofold greater than that of the WT,17 consistent with our results. Resistance to APC cleavage with the FVIII variant (336(P4-P3′)562) combined with D519V/E665V mutation showed a twofold reduction of FVIIIa decay rate relative to D519V/E665V, however, thrombin generation potential of 336(P4-P3′)562/D519V/E665V was comparable with that of D519V/E665V. These results suggest that a stabilization of the A2 subunit did not necessarily enhance hemostatic potential. Nevertheless, these mechanisms could be especially pertinent for overall hemostasis in vivo, and our findings using the tail-clip murine model showed that the hemostatic potential of the combined D519V/E665V/K1813A mutant could be enhanced eightfold relative to that of the WT, and increased fourfold compared with that of D519V/E665V. Our computational modeling of FVIII revealed that the K1813A mutation altered the surface charge of the FIXa binding site to be negative, which may facilitate the binding of FIXa (supplemental Figure 3). Furthermore, the D519V/E665V mutations decreased the negative charge of the inner surface of the A2 domain, leading to a decrease in electrostatic repulsion and an enhancement in electrical stability of the A2 domain with the A1 and A3 domains (supplemental Figure 3). D519V/E665V mutations also increased the number of noncovalent bindings of A2 domain with A1 and A3 domains (supplemental Figure 3). These predicted structural changes strongly support the biochemical characteristics of the D519V/E665V/K1813A mutant.
Our kinetic analyses of FXa generation data demonstrated that apparent Kd values for the D519V/E665V/K1813A and D519V/E665V mutants were significantly lower than that for the WT, consistent with previous results for D519V/E665V,17 and highlighting the likely enhanced affinity for FIXa. Kao et al32 reported that the FIX triple mutant (FIX-V86A/E277A/R338A) exhibited a 13-fold higher specific activity than WT-FIX and that incorporation of the FIXPadua (R338L) mutation into FIX triple mutant promoted an ∼22-fold higher specific activity relative to that of WT-FIX. Moreover, these FIX mutants demonstrated a marked superior clotting function in in vitro and in vivo experiments. The studies also showed that FIXa-R338L exhibited a fivefold greater binding affinity and that FIXa-V86A/E277A/R338L expressed a ∼15-fold greater binding affinity to FVIIIa compared with FIXa-WT.32 The authors concluded that the enhanced FIXa-FVIIIa affinity led to increased coagulation potential. Therefore, the improved association between FIXa and FVIIIa in D519V/E665V/K1813A may be associated with additional modulation of hemostasis.
Regarding ROTEM data after 5-minute FVIII administration, we found no significant differences in CT and α-angle measurements between WT-FVIII (2 μg/kg) and D519V/E665V (1 μg/kg) or D519V/E665V/K1813A (0.25 and 0.5 μg/kg). CT represents clot initiation,33 and time to peak thrombin in TGA also exhibited a similar trend. In addition, the α angle represents the rate of fibrin production,33 indicating that the coagulation function in these FVIII mutants may be equal to that of WT-FVIII even if the dose of FVIII mutants was reduced. In contrast, shortened CT and high α-angle values in D519V/E665V/K1813A mutant (1 and 2 μg/kg) were observed compared with those of WT-FVIII (2 μg/kg), with significant differences. As for the tail-clip assays, the coagulation potential in D519V/E665V/K1813A mutant could be enhanced by approximately eightfold relative to the WT. These results demonstrated that the coagulation potential in the D519V/E665V/K1813A mutant was greater than that of WT-FVIII and that the D519V/E665V/K1813A mutant at the same concentration to WT-FVIII may be a risk of thrombogenic potential.
Advances with gene therapy in recent decades have had an extensive impact on therapeutic options for patients with hemophilia,34 and in August 2022, gene therapy was approved by the European commission for use in HA.35 A recent study reported that valoctocogene roxaparvovec (AAV5-hFVIII-SQ, that delivers a B-domain–deleted FVIII coding sequence with an adeno-associated virus [AAV] vector) was safe, sustained FVIII:C levels, and reduced bleeding for at least 2 years after gene transfer in HA.36 However, major limitations of gene therapy in HA are evident. The complementary DNA of FVIII is much longer than the packaging capacity of the AAV vector, low levels of expression are observed, and transaminases are elevated.34 In contrast, FIX-R338L, mediating an approximate eightfold increase in the specific activity of FIX,37 enabled highly effective gene therapy for patients with hemophilia B.38 More recently, in transgene expression studies, the APC-resistant FVIII R336Q/R562Q mutant (termed AAV-FVIII QQ) appeared to exhibit fivefold higher hemostatic function than that of the WT in vivo14 and blood loss assessed in the tail-clip assay was fivefold to 10-fold lower than that with AAV-FVIII-WT.39 These results indicate that the FVIII mutant could achieve hemostatic efficacy at lower AAV vector doses. The FVIII mutant, D519V/E665V/K1813A, used in our experiments could offer an approximate eightfold gain of function in coagulation potential and hemostatic efficacy relative to that of the WT in the murine model, although the precise mechanism(s) of how this mutation modulates A2 dissociation remain unclear.
There is the limitation of our study have to be discussed. We could not evaluate the direct binding affinity between FVIIIa and FIXa because the unstable FVIIIa is immediately inactivated. Nevertheless, to our knowledge, the FVIII-D519V/E665V/K1813A mutation appeared to offer the highest FVIII potency described to date, and further developments based on these findings could contribute to the future development of both recombinant FVIII products and gene-based therapies for patients with HA.
Acknowledgments
The authors are grateful to Kaoru Horiuchi for excellent technical assistance.
This research was supported by the Japan Agency for Medical Research and Development under grant number 21fk0410037, supported by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology (to K.N., 21K07804; and Y.N., 22K15928), and conducted, in part, by collaborative research with Takeda Pharmaceuticals Limited.
Authorship
Contribution: Y.N. carried out the experiments, analyzed the data, created the figure, wrote the manuscript, and approved the final version for publication; A.O. designed the study and carried out the experiments; N.B., Y.K., and T.O. conducted computational modeling of FVIII, and analyzed the structural data; and K.N. designed the study, interpreted the data, wrote the manuscript, and edited the manuscript.
Conflict-of-interest disclosure: Y.N. received a grant from Takeda Pharmaceutical Co. A.O. received a grant from Sanofi S.A. N.B. received speaker fees from Chugai Pharmaceutical and Novo Nordisk. Y.K. received a speaker fee from Novo Nordisk. T.O. received consultation fee from Chugai Pharmaceutical; received grants from Chugai Pharmaceutical, CSL Behring, Pfizer, and Novo Nordisk (not related to this research); and received speaker fees from Chugai Pharmaceutical, Sanofi S.A., Pfizer, Bayer, Daiichi Sankyo, Takeda Pharmaceutical, Novo Nordisk, Japan Blood Products Organization, Fujimoto Pharmaceutical, LSI Medienced, and CSL Behring. K.N. has received grants, personal fees, and nonfinancial support from Chugai Pharmaceutical Co, Ltd; received personal fees from F. Hoffmann-La Roche Ltd; reports grants and personal fees from Sysmex Co, SEKISUI MEDICAL, Takeda Pharmaceutical Co, Sanofi S.A., CSL Behring Co, KM Biologics Co, Novo Nordisk A/S, Bayer AG, and Fujimoto Seiyaku; and is an inventor of patents relating to emicizumab.
Correspondence: Yuto Nakajima, Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; email: nakajima-yamanashi@naramed-u.ac.jp.
References
Author notes
An account of this work was presented at the 85th annual meeting of the Japanese Society of Hematology 2023, Tokyo, Japan, 13 October 2023.
The data sets generated during and/or analyzed during this study are available, on reasonable request, from the corresponding author, Yuto Nakajima (nakajima-yamanashi@naramed-u.ac.jp).
The full-text version of this article contains a data supplement.

![Thromboelastograms obtained by ROTEM in HA mice infused with D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (2 μg/kg), mutant; D519V/E665V/K1813A (0.25, 0.5, 1 and 2 μg/kg), or D519V/E665V (1 μg/kg) was infused to the HA mice. Citrated whole-blood samples taken from these mice were assessed by a ROTEM triggered by CaCl2, as described in “Methods.” A representative thromboelastogram is shown. The average parameters (CT, clot formation time [CFT], CT + CFT, α angle, and maximum clot firmness [MCF]) in WT mice and HA mice infused with each FVIII mutant are shown below each figure.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2023012391/2/m_blooda_adv-2023-012391-gr5.jpeg?Expires=1765924723&Signature=fMLbT9njl8Ozd6Mku9g0OPngWgCtemQ9GRmdRKCkRCcw3XXI8fWxkRBfaWZO62B0QO3Pwb7H3bw51GMEN6BqiPfz0aEvBLGawGR88PJ8lnCk9g6Tck7Wke9hjKYGLhGbnHyoHeb-WX~-X3hhBu2qeB0smVWJEQ3FyudF65k1r2qNRsqgFnHrBfTQORqz6PQaJMxWAwBH~-Iw68LbI9Oxeo2sxxwWDJb3ggGRzq8KT1Zf4zNNCbhl7GVVy4P33v98KG01aqJeBnN6uTM8XRum6YznDAb6Mlv8XGy9m-fGroTVYhmls3UMbBg9Hu-NiR8WH9GY~MZq6ayz5uIPX4jtJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Thromboelastograms obtained by ROTEM in HA mice infused with D519V/E665V/K1813A and D519V/E665V FVIII mutants. WT-FVIII (2 μg/kg), mutant; D519V/E665V/K1813A (0.25, 0.5, 1 and 2 μg/kg), or D519V/E665V (1 μg/kg) was infused to the HA mice. Citrated whole-blood samples taken from these mice were assessed by a ROTEM triggered by CaCl2, as described in “Methods.” A representative thromboelastogram is shown. The average parameters (CT, clot formation time [CFT], CT + CFT, α angle, and maximum clot firmness [MCF]) in WT mice and HA mice infused with each FVIII mutant are shown below each figure.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/15/10.1182_bloodadvances.2023012391/2/m_blooda_adv-2023-012391-gr5.jpeg?Expires=1765994104&Signature=Vuqg8tD1XOkuNLm9gG7yKfWHE9HweOVyD8~aX5h~ybV28a~fJLoklYQ8jM-bKJvSaoFDFV2e-oqYS-6jHWek8kWXE9arL8tHrh3GzoRu8HXGC4Sf0Nw6P9Jcvgo94KFEiEGiQrOu-nWmGActeutCaOJ-cqZiZ2TxF-8UiLMlcx1qKl8vTrbzKguy5K9JT2d8xt10rrIl2UMWy86JJxWfUIj~guu8jybMhe5CCqlyQeHHRePjDFErIial73UrCq6WhRpBsmXXPWsC3bpS4D839tCEF5exSnIIQxhiICwpzi3sT765sQSHbjS1vOZsqabT-hPQ3UA8SCc~~ZfsJtPTHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)