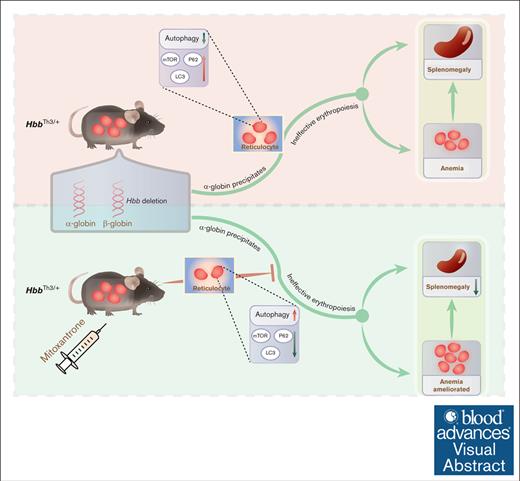
Key Points
Therapeutic effect of mitoxantrone on expanded and ineffective erythropoiesis in β-thalassemia was evaluated.
Significant preclinical evidence for targeting autophagy as a novel therapeutic approach for β-thalassemia has been provided.
Visual Abstract
β-thalassemia is a condition characterized by reduced or absent synthesis of β-globin resulting from genetic mutations, leading to expanded and ineffective erythropoiesis. Mitoxantrone has been widely used clinically as an antitumor agent considering its ability to inhibit cell proliferation. However, its therapeutic effect on expanded and ineffective erythropoiesis in β-thalassemia is untested. We found that mitoxantrone decreased α-globin precipitates and ameliorated anemia, splenomegaly, and ineffective erythropoiesis in the HbbTh3/+ mouse model of β-thalassemia intermedia. The partially reversed ineffective erythropoiesis is a consequence of effects on autophagy as mitochondrial retention and protein levels of mTOR, P62, and LC3 in reticulocytes decreased in mitoxantrone-treated HbbTh3/+ mice. These data provide significant preclinical evidence for targeting autophagy as a novel therapeutic approach for β-thalassemia.
Introduction
β-thalassemia results from mutations in the β-globin gene leading to reduced or absent synthesis of β-globin.1 Such mutations are more common in the Mediterranean, Middle East, and Southeast Asia, whereas the prevalence is increasing in other regions including China, primarily due to migration.2 In β-thalassemia, unpaired free α-globin precipitates on the cell membrane and impairs the maturation and viability of erythroid precursors, resulting in ineffective erythropoiesis and premature lysis of circulating red blood cells (RBCs).3,4 Deficiency of β-globin also leads to a decrease in hemoglobin (α2β2 tetramer) synthesis. The decreased RBCs and insufficient hemoglobin production induces a chronic stress erythropoiesis, manifested by progressive expansion of erythroid progenitors and precursors in β-thalassemia.
Chronic transfusion and iron-chelation therapy are a standard approach in the clinical treatment of patients with β-thalassemia.5,6 However, these therapies result in iatrogenic complications, such as iron deposition, infection, and allergic reactions to transfusion. There are also known potential side effects of iron-chelation therapy (specific to different chelators), in addition to limited compliance, despite some recently developed oral formulations, and the economic burden of chronically affording these agents. Thus, patients with β-thalassemia continue to experience a clinically unmet need for novel therapeutic agents. Mitoxantrone is a US Food and Drug Administration (FDA)–approved anthraquinone antitumor drug used as a first-line chemotherapy approach for acute myeloid leukemia, malignant lymphoma, breast cancer, and other diseases.7-11 Mitoxantrone exerts antitumor effects by disrupting DNA synthesis and repair mechanisms and triggers apoptosis in tumor cells. Interestingly, mitoxantrone was found to induce suicidal RBC death and myelosuppression, whereas clinically significant suppression of RBC was rare.12-14 Therefore, mitoxantrone could preferentially work on defective RBCs. Whether mitoxantrone affects expanded and ineffective erythropoiesis in β-thalassemia is largely unknown.
In this study, we sought to explore whether mitoxantrone influences pathogenic erythropoiesis to improve anemia in an Hbb gene–disrupted (HbbTh3/+) β-thalassemia intermedia mouse model. We demonstrate that mitoxantrone treatments decreased α-globin precipitates and improved anemia, splenomegaly, and ineffective erythropoiesis in HbbTh3/+ mice. Additionally, we identified correction in disordered autophagy in reticulocytes in mitoxantrone-treated HbbTh3/+ mice. Together, these novel preclinical data provide robust support for evaluating mitoxantrone as a repurposed agent for the management of ineffective erythropoiesis in patients with β-thalassemia.
Materials and methods
Mice
Mouse experimental protocols were approved by the Department of Laboratory Animals of Central South University. HbbTh3/+ mice were used as a β-thalassemia intermedia mouse model and originally purchased from Jackson Laboratories.15 Wild-type (WT) C57BL/6 mice were ordered from Hunan SJA Laboratory Animal Co Ltd. All mice were bred and maintained at Central South University Department of Laboratory Animals and had ad libitum access to food and water. Experiments were conducted with female mice aged 6 to 8 weeks, with WT female littermates used as normal controls.
Mice received intraperitoneal injection of mitoxantrone at a lower dose of 0.4 mg/kg (Selleck, China) twice a week for 2 weeks, and then the dose was adjusted upward to 0.8 mg/kg twice a week for another 4 weeks. Control group mice were injected with the same volume of phosphate-buffered saline (PBS) during this period. Mice were weighed weekly and euthanized on day 2 after the last injection. Blood samples obtained by orbital puncture were used for hematological analysis, globin precipitates assays, and reticulocyte isolation and analysis. Tissue samples were retained for subsequent experiments.
Detection of globin precipitates in RBCs
Globin precipitates from RBCs were analyzed as described previously.16,17 Briefly, RBCs were collected from 5 μL of peripheral blood by centrifugation (3500× rpm[revolutions per minute] for 5 minutes at room temperature), washed with PBS twice, and then lysed by repeated freezing and thawing with precooled 0.1% PBS. Precipitated globin was dissolved in loading buffer as previously described, fractioned by triton-acetic-acid-urea gel, and stained with Coomassie brilliant blue.
RNA extraction and qRT-PCR
Total RNA was extracted from mouse bone marrow cells and tissues using TRIzol reagent (Vazyme, Nanjing, China). The RNA purity and concentration were quantified with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Shanghai, China). Complementary DNA (cDNA) was synthesized by HiScript II Q RT SuperMix (Vazyme, Nanjing, China), and then quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis was performed using ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) and the primers (Erfe-F: 5'-ATGGGGCTGGAGAACAGC, Erfe-R: 5'-TGGCATTGTCCAAGAAGACA; Gapdh-F: 5'-ATCATCCCTGCATCCACT, Gapdh-R: 5'-ATCCACGACGGACACATT). qRT-PCR reactions were performed in triplicate and normalized against Gapdh messenger RNA (mRNA). The relative mRNA levels were calculated using the comparative ΔCt method.
Reticulocyte purification
After 2 washes with PBS, the blood cells were resuspended in 4 mL PBS, with 0.5% bovine serum albumin added. Diluted blood cell suspensions were carefully spread on the top layer of 2 mL Ficoll (TBDscience, Tianjing, China) in 15 mL centrifugal tube, then centrifuged at 300g for 20 minutes at 20°C, both ascending and descending slowly. The solution was divided into 4 layers from top to bottom: supernatant, single nucleated cells, reticulocytes, and erythrocytes. Reticulocytes were extracted and washed twice with PBS.
Western blot
Purified reticulocytes were homogenized in RIPA buffer with phosphatase inhibitors and protease inhibitor cocktail (Sigma-Aldrich, Shanghai, China) at 4°C. Large cellular debris was concentrated by centrifugation (13 800× rpm for 15 minutes at 4°C) and discarded. Extracted protein was then quantified with a Pierce BCA kit (Thermo Fisher Scientific, Shanghai, China), separated by 12% sodium dodecyl sulfate-PAGE, and transferred to a nitrocellulose membrane. Transferred membranes were then incubated with primary antibodies against β-actin (1:25 000; Invitrogen, CA), mTOR (1:1000, Santa Cruz Biotechnology, Shanghai, China), P62 (1:1000; Boster, Wuhan, China), and LC3B (1:1000; Boster, Wuhan, China), followed by secondary antibodies (horseradish peroxidase-conjugated; Thermo Fisher Scientific). Protein blots were estimated by WesternLumaxLight Enhance horseradish peroxidase substrate (ZETA, Shanghai, China) using the ChemiDoc XRS+ imaging system with Image Lab software (Bio-Rad, CA), and band intensity was quantified using the Gel-Pro software (Medica Cybernetics Inc, MD).
Flow cytometry
Bone marrow and spleen cells of WT and HbbTh3/+ mice were suspended in PBS added with 0.5% bovine serum albumin and passed through a 40 μM cell strainer (Falcon, NY) for flow cytometry analysis. For murine terminal erythroid differentiation analysis, 1 × 106 cells were incubated with antibodies of Ter119- fluorescein isothiocyanate (1:100; eBioscience, CA), CD45-APC-Cy7 (1:100; eBioscience), and CD44-APC (1:100; eBioscience).18 Incubated cells were stained with 7-amino-actinomycin D (AAD) (MultiSciences Biotech Co, Ltd, Hangzhou, China) for 5 minutes before detection by flow analyzer. For apoptosis analysis, 5 × 105 cells were incubated with Ter119- fluorescein isothiocyanate (1:100; eBioscience), CD45-APC-Cy7 (1:100; eBioscience), and Annexin V-PE/7-AAD apoptosis kit (MultiSciences Biotech Co, Ltd).
Electron microscopy
Ficoll-purified reticulocytes were fixed in glutaraldehyde (2.5%) and paraformaldehyde (2%), diluted in 0.1 M sodium arsenate (pH 7.4) at 4°C overnight.19,20 Cells were aggregated in clusters by gradient centrifugation and postfixed in 1% osmic acid, protected from light, and kept in ice for 1 hour. After uranyl acetate stained, sample were dehydrated through a graded series of ethanol/propylene oxide solutions, then infiltrated with and embedded in epoxy resin, which was polymerized at 65°C overnight. Ultrathin sections (80 nm) were cut and imaged using a JME-1400 plus transmission electron microscope with a RIO9 digital camera.
Statistical analysis
Statistical tests were performed using GraphPad Prism version 7 software. Data are shown as means ± standard deviation. P values of <.05 were considered statistically significant. All tests were 2 tailed.
Results
Mitoxantrone partially ameliorates anemia in β-thalassemic mice
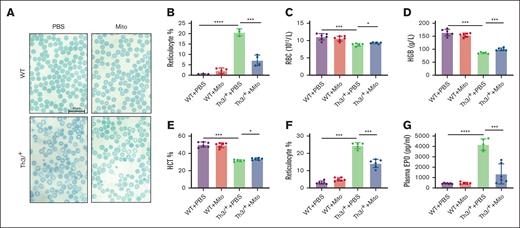
Mice were treated with mitoxantrone for 6 weeks, during which time there was no difference in body weight between WT and HbbTh3/+ mice (supplemental Figure 1). Compared with PBS-treated HbbTh3/+ mice, brilliant cresyl blue staining of peripheral blood reveals an improvement in erythrocyte morphology (Figure 1A) and a decrease in reticulocyte count in mitoxantrone-treated HbbTh3/+ mice (Figure 1B). Standard hematological tests demonstrate that mitoxantrone treatment increases RBC number (Figure 1C), hemoglobin level (Figure 1D), and hematocrit (Figure 1E) and significantly decreases the proportion of reticulocytes (Figure 1F) in HbbTh3/+ mice. Because erythropoietin (EPO) is induced by anemia or hypoxia, we evaluated EPO levels in mice. Mitoxantrone treatment significantly reduces plasma EPO protein levels in mitoxantrone-treated HbbTh3/+ mice (Figure 1G). In contrast, RBC parameters and EPO levels of WT mice do not appear to be affected by mitoxantrone regimens. The complete blood count data also show that mitoxantrone treatment increases platelets and decreases nucleated red cells in HbbTh3/+ mice but suppresses white blood cells (especially lymphocytes) in both WT and HbbTh3/+ mice (supplemental Table 1). These results demonstrate a partial resolution of anemia in HbbTh3/+ mice in response to mitoxantrone.
Mitoxantrone relieved anemia in β-thalassemic mice. (A) Representative pictures (scale bar, 20 μm) of circulating erythrocytes, stained with brilliant cresyl blue. (B) Statistics of reticulocyte identified by brilliant cresyl blue staining. Standard hematological tests of RBC counts (C), hemoglobin concentration (HGB; D), hematocrit (HCT; E), and reticulocyte ratio (F). (G) Plasma EPO concentration evaluated by enzyme-linked immunosorbent assay. The groups mean WT and HbbTh3/+ (Th3/+) mice treated with either mitoxantrone (Mito) or PBS. The bar plot represents the mean ± standard deviation (SD) of samples (n = 6-8 mice per group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Mitoxantrone relieved anemia in β-thalassemic mice. (A) Representative pictures (scale bar, 20 μm) of circulating erythrocytes, stained with brilliant cresyl blue. (B) Statistics of reticulocyte identified by brilliant cresyl blue staining. Standard hematological tests of RBC counts (C), hemoglobin concentration (HGB; D), hematocrit (HCT; E), and reticulocyte ratio (F). (G) Plasma EPO concentration evaluated by enzyme-linked immunosorbent assay. The groups mean WT and HbbTh3/+ (Th3/+) mice treated with either mitoxantrone (Mito) or PBS. The bar plot represents the mean ± standard deviation (SD) of samples (n = 6-8 mice per group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Mitoxantrone alleviates expanded erythropoiesis in β-thalassemic mice
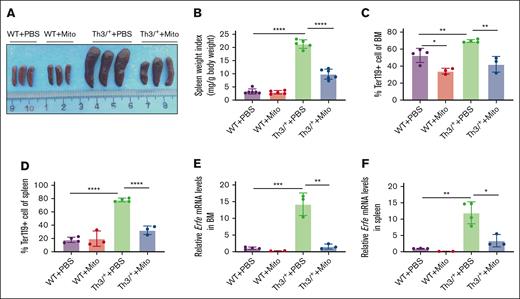
HbbTh3/+ mice develop splenomegaly due to compensatory expansion of erythropoiesis, leading to excessive proliferation of erythroid precursors in the spleen.1,21 In addition, erythroid expansion in the bone marrow and spleen increases erythroferrone synthesis by erythroid precursors.22-24 High levels of erythroferrone expression have been reported in β-thalassemic mice and human.22-25 After 6 weeks of mitoxantrone treatment, splenomegaly is significantly decreased in HbbTh3/+ mice (Figure 2A-B). Flow cytometry assays demonstrate a significant decrease in the percentage of erythroid cells (Ter119+) in the bone marrow and spleen in mitoxantrone-treated HbbTh3/+ mice relative to PBS-injected HbbTh3/+ mice (Figure 2C-D). Consistently, Erfe mRNA expression is significantly reduced in the bone marrow and spleens of mitoxantrone-treated HbbTh3/+ mice (Figure 2E-F). These results demonstrate reversal of erythroid expansion in mitoxantrone-treated HbbTh3/+ mice.
Mitoxantrone alleviated expanded erythropoiesis in β-thalassemic mice. (A-B) Representative images (A) and weight statistics of spleens (B). Percentages of Ter119+ cells assayed by flow cytometry in bone marrow (C) and spleens (D). Erfe mRNA levels detected by qRT-PCR in bone marrow (E) and spleens (F). The groups mean WT and HbbTh3/+ (Th3/+) mice treated with either Mito or PBS. The bar plot represents the mean ± SD of samples (n = 6-8 mice per group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Mitoxantrone alleviated expanded erythropoiesis in β-thalassemic mice. (A-B) Representative images (A) and weight statistics of spleens (B). Percentages of Ter119+ cells assayed by flow cytometry in bone marrow (C) and spleens (D). Erfe mRNA levels detected by qRT-PCR in bone marrow (E) and spleens (F). The groups mean WT and HbbTh3/+ (Th3/+) mice treated with either Mito or PBS. The bar plot represents the mean ± SD of samples (n = 6-8 mice per group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Mitoxantrone improves ineffective erythropoiesis in β-thalassemic mice
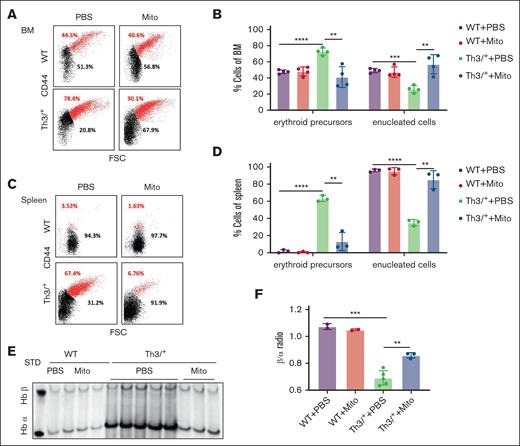
To investigate aberrant erythroid differentiation in hematopoietic organs, we used a well-established approach involving staining with Ter119/CD44 antibodies as well as cell size by flow cytometry to analyze the distribution of nucleated and enucleated erythroid lineage cells in mouse bone marrow and spleen samples.18 We demonstrate decreased percentage of erythroid precursors (nucleated) and increased percentage of enucleated erythroid cells in bone marrow and spleen of mitoxantrone-treated HbbTh3/+ mice (Figure 3A-D). Because the accumulation and precipitate of excessive free α-globin chains is implicated in the shortened survival of circulating β-thalassemic RBCs, we separated RBC membrane proteins using triton-acetic acid-urea gel electrophoresis to assess the effect of mitoxantrone on α-globin chain precipitation. Our data demonstrate significantly reduced α-globin precipitation in mitoxantrone-treated HbbTh3/+ mice (Figure 3E-F). Taken together, we demonstrate mitoxantrone-mediated impact on erythroid lineage cells by reducing α-globin precipitation and proceeded to determine the mechanism of mitoxantrone’s effect.
Mitoxantrone improved ineffective erythropoiesis in β-thalassemic mice. Representative density plots (A) of flow cytometry analysis with CD44 (y-axis)/Ter119/FSC (x-axis) as markers of nucleated (red) and enucleated (black) erythroid cells and statistics (B) in bone marrow Ter119+ cells. Representative density plots (C) of flow cytometry analysis with CD44 (y-axis)/Ter119/FSC (x-axis) as markers of nucleated (red) and enucleated (black) erythroid cells and statistics (D) in spleen Ter119+ cells. Representative image (E) and densitometry quantification (F) of α-globin precipitation on circulation erythrocyte membrane via triton-acetic acid-urea gel analysis. The groups mean WT and HbbTh3/+ (Th3/+) mice treated with either Mito or PBS. The bar plot represents the mean ± SD of samples (n = 6-8 mice per group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. FSC, forward scatter.
Mitoxantrone improved ineffective erythropoiesis in β-thalassemic mice. Representative density plots (A) of flow cytometry analysis with CD44 (y-axis)/Ter119/FSC (x-axis) as markers of nucleated (red) and enucleated (black) erythroid cells and statistics (B) in bone marrow Ter119+ cells. Representative density plots (C) of flow cytometry analysis with CD44 (y-axis)/Ter119/FSC (x-axis) as markers of nucleated (red) and enucleated (black) erythroid cells and statistics (D) in spleen Ter119+ cells. Representative image (E) and densitometry quantification (F) of α-globin precipitation on circulation erythrocyte membrane via triton-acetic acid-urea gel analysis. The groups mean WT and HbbTh3/+ (Th3/+) mice treated with either Mito or PBS. The bar plot represents the mean ± SD of samples (n = 6-8 mice per group). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. FSC, forward scatter.
Mitoxantrone promotes autophagy in reticulocytes of β-thalassemic mice
According to previous research, both the reduction in corpuscular hemoglobin production and autophagy of uncoupled α-globin could decrease α-globin precipitates in β-thalassemia.17,21,26 Because mean corpuscular hemoglobin concentration or mean erythrocyte volume is not decreased in mitoxantrone-treated HbbTh3/+ mice (supplemental Figure 2A-B), we evaluated whether mitoxantrone decreased α-globin precipitation via affecting autophagy.
Autophagy plays a key role in the degradation of excess free globin chains and mitochondrial clearance (mitophagy) to support effective maturation of RBCs.27-30 Using transmission electron microscopy analysis of reticulocytes, we see that mitoxantrone decreases globin precipitates in HbbTh3/+ mice (Figure 4A), consitent with the results of triton-acetic acid-urea gel analysis (Figure 3E-F). Further transmission electron microscopy analysis at higher magnification indicates that mitoxantrone induces the formation of autophagy vesicles of mitochondria in reticulocytes of HbbTh3/+ mice (Figure 4B). We therefore conclude that mitoxantrone may promote autophagy in HbbTh3/+ mouse reticulocytes. It has been reported that mTOR has a central role in this pathway and is activated to block autophagy, leading to cytotoxicity associated with ineffective erythropoiesis in β-thalassemic mice.31,32 Here, we explored the effect of mitoxantrone on the expression of key autophagy proteins, including mTOR, LC3, and P62.33-35 β-thalassemic mice have much higher levels of mTOR, P62, and LC3 protein expression in reticulocytes than WT mice (Figure 4C-D). Mitoxantrone treatment reduces P62 and LC3 accumulation significantly in reticulocytes of HbbTh3/+ mice, correlated with a decrease in mTOR protein concentration (Figure 4C-D), further indicating that mitoxantrone promotes autophagy in reticulocytes from HbbTh3/+ mice. These results suggest that autophagy activation contributes to the therapeutic effects of mitoxantrone in HbbTh3/+ mice.
Mitoxantrone promoted autophagy in reticulocytes of β-thalassemia mice. (A) Globin precipitates (indicated by black arrows) in enriched reticulocytes analyzed by transmission electron microscopy (TEM). Scare bars, 2 μm. (B) Mitochondria in enriched reticulocytes analyzed by TEM. Red arrows indicate damaged mitochondria within double-membrane structures. Orange arrows indicate naked intact mitochondria. Scare bars, 200 nm. (C) Representative images of western blot assay for mTOR, p62, and LC3 protein expression in enriched reticulocytes. β-actin was used as a loading control. (D) Densitometric analysis of protein levels detected above using ImageJ software. The groups (n = 4-6 mice, respectively) mean WT and HbbTh3/+ (Th3/+) mice treated with either PBS or mitoxantrone. The bar plot represents the mean ± SD of samples. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Mitoxantrone promoted autophagy in reticulocytes of β-thalassemia mice. (A) Globin precipitates (indicated by black arrows) in enriched reticulocytes analyzed by transmission electron microscopy (TEM). Scare bars, 2 μm. (B) Mitochondria in enriched reticulocytes analyzed by TEM. Red arrows indicate damaged mitochondria within double-membrane structures. Orange arrows indicate naked intact mitochondria. Scare bars, 200 nm. (C) Representative images of western blot assay for mTOR, p62, and LC3 protein expression in enriched reticulocytes. β-actin was used as a loading control. (D) Densitometric analysis of protein levels detected above using ImageJ software. The groups (n = 4-6 mice, respectively) mean WT and HbbTh3/+ (Th3/+) mice treated with either PBS or mitoxantrone. The bar plot represents the mean ± SD of samples. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001.
Discussion
In this study, we demonstrate that mitoxantrone, an FDA–approved antitumor agent currently in clinical use, not only partially reverses anemia, extramedullary erythropoiesis in the spleen, and erythroblast hyperplasia in the bone marrow but also ameliorates ineffective erythropoiesis, decreases α-globin precipitates in RBCs from the HbbTh3/+ β-thalassemia intermedia mouse model, and promotes autophagy in reticulocytes from HbbTh3/+ mice.
Autophagy is a process that transports cytosolic macromolecules and even whole organelles to the lysosomes for degradation.36,37 A large amount of globin is produced during terminal erythropoiesis, during which autophagy of excess free globin chains is required.38-40 Our results show that mitoxantrone treatment improves ineffective erythropoiesis in HbbTh3/+ mice via autophagy clearance of globin precipitation. In β-thalassemic reticulocytes, autophagy adaptor protein SQSTM1/P62 and nonlipidated LC3 accumulate.17 Furthermore, inhibition of the mTOR signaling pathway was shown to be therapeutic in HbbTh3/+ mice.17,31,32 Coincidently, mitoxantrone was previously found to induce autophagy by suppressing mTOR signaling during apoptosis in tumor cells.41-43 Our findings that mitoxantrone decreases α-globin precipitation and increases the formation of autophagy vesicles along with depression of mTOR, LC3, and P62 in HbbTh3/+ mouse reticulocytes indicate that mitoxantrone clears uncoupled α-globin via increasing autophagic flux in erythroid lineage cells. Our findings provide robust preclinical evidence that mitoxantrone is an interesting novel potential therapeutic approach for patients with β-thalassemia.
Mitoxantrone has been approved by the FDA for the treatment of diseases such as acute myeloid leukemia, malignant lymphoma, and breast cancer.7,9,10 It is thought to be safe and is relatively inexpensive. Our study shows that mitoxantrone treatment has no effect on body weights of all mice, whereas mitoxantrone reduces white blood cells in all mice. Myelosuppressive effects of mitoxantrone on white blood cells are known in patients with cancer. Based on the fact that inducing apoptosis basically explains mitoxantrone’s therapeutic effect on tumor cells, we also analyzed whether mitoxantrone played a role in apoptosis of mouse bone marrow and spleen cells. Our data demonstrate that mitoxantrone does not alter apoptosis in erythroid lineage cells of HbbTh3/+ mice (supplemental Figure 3), indicating that the therapeutic effect of mitoxantrone in HbbTh3/+ mice is independent of apoptosis. The dose of mitoxantrone we used here is relatively low compared with that used for treating tumors.44 Unexpectedly, we also notice that mitoxantrone inhibits bone marrow erythroid cells in WT mice, although there was no significant change in peripheral RBC parameters at present. Considering the possibility that chronic mitoxantrone administration may have renal and gastrointestinal side effects as previously reported,8 future studies are necessary to optimize the mitoxantrone administration regimen based on the specific therapeutic index for β-thalassemia. Nevertheless, our research provides a promising, potentially translatable repurposing use of mitoxantrone as a novel therapy for patients with β-thalassemia.
Acknowledgments
The authors thank all Central South University Laboratory Animal Center animal caretakers for help with the animal experiments.
This work was supported by the grants from Key Research and Development Program of Hunan Province (2022SK2037); Natural Science Foundation of Hunan Province (2021JJ30892); National Key Research and Development Program of China (2018YFA0107800); Major Science and Technology Program of Hainan Province (ZDKJ2021037); Hainan Province Science and Technology Project (LCYX202102, LCYX202203, and LCYX202301); Supported by Specific Research Fund of The Innovation Platform for Academicians of Hainan Province; Project Supported by Hainan Province Clinical Medical Center; US National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK107670, R01DK095112, and R56/R01DK132146 to Y.Z.G.); and Graduate Student Independent Exploration Innovation Fund of the Central South University (2024ZZTS0507 to H.Z.).
Authorship
Contribution: H.Z., R.L., and Z.F. performed research and acquired the data; H.C. and R.L. designed experiments, analyzed the data, and wrote the manuscript; L.N., Y.M., F.S., J.M., Z.S., Y.Z.G., and J.L. provided technical support; and H.C. and Y.Z.G. reviewed and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Huiyong Chen, Molecular Biology Research Center, Central South University, Changsha, N/A 410078, China; email: chenhuiyong@csu.edu.cn.
References
Author notes
H.Z. and R.L. contributed equally to this study.
Data sharing is not applicable because this study did not involve any DNA/RNA sequencing and high-throughput data sets.
The full-text version of this article contains a data supplement.