Key Points
Myosin-1C is used for actomyosin-mediated expulsion of an essential blood clotting factor (VWF).
Myosin-1C links the exocytic actomyosin ring to PIP2 on the plasma membrane, forming anchor points that allow for maximal VWF secretion.
Visual Abstract
Blood endothelial cells control the hemostatic and inflammatory response by secreting von Willebrand factor (VWF) and P-selectin from storage organelles called Weibel-Palade bodies (WPBs). Actin-associated motor proteins regulate this secretory pathway at multiple points. Before fusion, myosin Va forms a complex that anchors WPBs to peripheral actin structures, allowing for the maturation of content. After fusion, an actomyosin ring/coat is recruited and compresses the WPB to forcibly expel the largest VWF multimers. Here, we provide, to our knowledge, the first evidence for the involvement of class I myosins during regulated VWF secretion. We show that the unconventional myosin-1C (Myo1c) is recruited after fusion via its pleckstrin homology domain in an actin-independent process. This provides a link between the actin ring and phosphatidylinositol 4,5-bisphosphate (PIP2) at the membrane of the fused organelle and is necessary to ensure maximal VWF secretion. This is an active process requiring Myo1c ATPase activity because inhibition of class I myosins using the inhibitor pentachloropseudilin or expression of an ATPase-deficient Myo1c rigor mutant perturbs the expulsion of VWF and alters the kinetics of the exocytic actin ring. These data offer a novel insight into the control of an essential physiological process and provide a new way in which it can be regulated.
Introduction
Endothelial cells (ECs) contain rod-shaped storage organelles called Weibel-Palade bodies (WPBs),1 which owe their unique shape to their main cargo: the prohemostatic glycoprotein, von Willebrand factor (VWF).2 VWF dimerizes in the endoplasmic reticulum and concatemerizes as it passes through the trans-Golgi network (TGN), forming long parallel proteinaceous tubules that are packaged into WPBs. Other cargos include the proinflammatory receptor P-selectin,3 cytokines, and agents that control tonicity; thus, exocytosis of WPB is a crucial event important during hemostasis and inflammation.4
Regulated secretion of VWF occurs rapidly in response to stimulation with secretagogues released during injury and inflammation.5,6 Secreted VWF tubules unfurl to form strings (up to 1 mm long) anchored to the EC surface. These serve as a platform for platelet aggregation and thrombus formation.7 This process instigates the primary hemostatic response but is also causally associated with thrombotic diseases such as peripheral vascular disease, myocardial infarction, and stroke.8 Responsible for 1 in 4 deaths,9 thrombosis is a leading cause of death worldwide. Although current therapy options are numerous, they are complicated by the risk of excess bleeding and cerebral hemorrhage.10 As such, there remains a profound medical need for more nuanced treatment strategies.
Circulating levels of VWF are prognostic for cardiovascular disease,11 and control of regulated secretion of VWF is being actively investigated as a therapeutic strategy to reduce the burden of thrombotic diseases. Aptamers and antibodies targeting VWF are currently being tested in the clinic to limit thrombotic pathologies such as thrombotic thrombocytopenic purpura12 and stroke.13 We have previously identified cellular machinery that regulates the expulsion of VWF, and targeting this process represents an exciting therapeutic approach. ECs recruit actin and nonmuscle myosin to sites of WPB exocytosis as rings; these contract in a process aided by septins14 to forcibly extrude the ultra large VWF multimers apically (into the blood vessel lumen).15
Myosins are molecular motor proteins that mediate organelle trafficking and contractile processes during muscle contraction, cytokinesis, and protein secretion.16-18 Conventional class II myosins dimerize and bind to adjacent, oppositely orientated, actin filaments via both head regions to exert force. This is known as the “sliding filament hypothesis.”19 Monomeric class I myosins are referred to as unconventional and lack these abilities. They are localized at cell membranes in ruffles, filopodia, and the leading edge during migration.17 Structurally, class I myosins are composed of an actin and adenosine triphosphate (ATP)–binding head domain, a variable neck region and a tail domain.20 The “neck” (or lever) region contains calmodulin (light chain) binding IQ (isoleucine-glutamine) motif(s), which acts as a regulatory domain, similar to the light chains of class II myosins.21 Lastly, class I myosins possess a pleckstrin homology (PH) domain in the tail region that facilitates binding to phosphoinositides.22 In some settings, class I myosins transport intracellular vesicles along actin filaments.23,24 They have also been shown to tether GLUT4-containing vesicles to actin during exocytosis.25 Whereas lung surfactant secreting alveolar type II (ATII) cells use actin and class I myosins to aid vesicle compression during lamellar body exocytosis.26
A pivotal role of a subset of myosin isoforms in WPB trafficking and VWF secretion has previously been described. WPBs are anchored to actin structures in the cell periphery by a tripartite complex of Rab27a, MyRIP, and myosin Va.27,28 Nonmuscle myosin IIA,29 nonmuscle myosin IIB,15 and myosin Vc30 have been implicated in the actomyosin-mediated expulsion of VWF. However, the role of class I myosins has not been characterized.
We previously used peroxidase proteomics to identify proteins in close proximity to WPBs in unstimulated and stimulated conditions.14 This powerful approach identified differential proximity of actin-binding motor proteins to the WPB surface in resting ECs and in response to stimuli. Here, we describe the function of the class I myosin motor, myosin 1C (Myo1c), and suggest a crucial role in linking the contractile actin ring to the plasma membrane (PM) to augment vesicle compression.
Methods
Cell culture
Human umbilical vein ECs (HUVECs; catalog no. 12203) and Human Dermal Microvascular ECs (HDMECs; catalog no. 12212) were purchased from PromoCell. ECs were cultured as described elsewhere.31 HDMECs were cultured using PromoCell Ready-to-use Growth Medium MV (catalog no. 22020).
Immunofluorescence (IF) and western blotting
This was performed exactly as described elsewhere.15 The commercial suppliers of antibodies used here are provided in supplemental Table 1. Confocal imaging was performed using the Zeiss LSM 800 and Nikon CSU-W1 SoRa spinning disk microscope with 0.1 to 0.2 μm interval Z stacks for fixed sample imaging. When necessary, image brightness and contrast were adjusted for clarity and in alignment with the American Society of Hematology author guidelines.
Live cell imaging
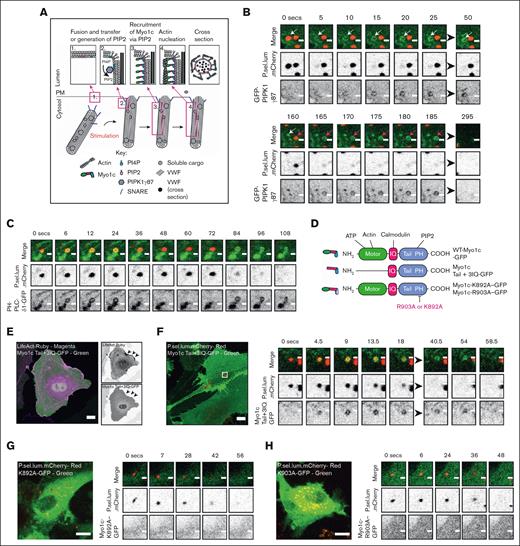
Myo1c–green fluorescent protein (GFP), Myo1c-Tail+3IQ-GFP, Myo1c-K892A–GFP, and Myo1c-R903A–GFP were kind gifts from Michael Ostap.22 PH-PLCδ1-GFP was a gift from Christian Halaszovich.32 GFP-Myo1c was a gift from Martin Bähler (Addgene; plasmid no. 134832).33 GFP-Myo1c (G108R) was generated in our laboratory. GFP-PIPK1 gamma 87 was a gift from Pietro De Camilli (Addgene; plasmid no. 22300).34 GFP-VWF was a gift from J. Voorberg and J.A. Van Mourik (Sanquin Research Laboratory, Amsterdam, The Netherlands).35 P-Selectin lumenal domain mCherry (P.sel.lum.mCherry) was previously cloned in our laboratory.15 LifeAct-GFP was a gift from B. Baum (University College London, London, United Kingdom).36 HUVECs were incubated at 37°C and 5% CO2, and 0.5-1μm interval Z stacks were obtained continuously for 5 to 10 minutes according to experimental objective.
Myo1C mutagenesis
Mutation of glycine 108 to arginine (G108R) changes a conserved residue of the nucleotide binding region in the motor domain and results in a rigor mutant.37,38 The rigor mutant was generated as described by Edelhei et al,39 using the forward primer 5’ gatttctggagagagtcgggcaggcaagaca 3’ and the reverse primer 5’ gtcttgcctgcccgactctctccagaaatc 3’. Mutated residues are shown in bold, isolated clones were sequenced for verification.
Assessment of target protein inhibition on VWF secretion using near-infrared fluorescent dot blot
Small interfering RNA (siRNAs) targeting Myo1c (catalog no. L-015121-00-0005) were purchased as SMARTpools from Dharmacon (Horizon Discovery). Firefly luciferase targeted siRNA was made by Eurofins Genomics (sequence 5' cgu-acg-cgg-aau-acu-ucg 3'). Electroporation of HUVECs, VWF secretion assay, and near-infrared–fluorescent dot blot was performed as described in our previous research.14 Phorbol 12-myristate 13-acetate (PMA; 100 ng/mL), thrombin (1 U/mL), vascular endothelial growth factor (VEGF; 40 ng/mL), histamine (100 μM), adrenaline (10 μM), or 3-isobutyl-1-methyl xanthine (IBMX; 100 μM) were used to stimulate WPB exocytosis. For myosin 1 inhibition, HUVECs were exposed to pentachloropseudilin (PCLP; AOBIOUS, catalog no. AOB33969) for 30 minutes or 16 hours (5-20 μM) before stimulation with secretagogue.
In vivo
Procedures conducted using mice were in alignment with the institutional Animal Welfare Ethical Review Body and UK Home Office guidelines. Eight-week-old, male, C57BL/6 mice (Charles River) were housed under controlled environmental conditions (12-hour light/dark cycles at ambient temperature and humidity) on a standard chow diet. Whole-mount staining and imaging of cremasteric venules were performed as described elsewhere.40
Results
APEX2 proximity proteomics identified differentially enriched myosin isoforms as putative regulators of WPB dynamics
Myosin isoforms have pleotropic functions in secretory vesicle trafficking. They are essential for prefusion and postfusion exocytic processes, including the anchoring of vesicles to peripheral actin, remodeling of cortical actin, stabilization/linking the fusion pore to the PM, and force-driven compression to mediate cargo expulsion.41 To discern which myosin isoforms were of importance in regulated VWF secretion, we consulted our publicly available proximity proteomics data set.14,42
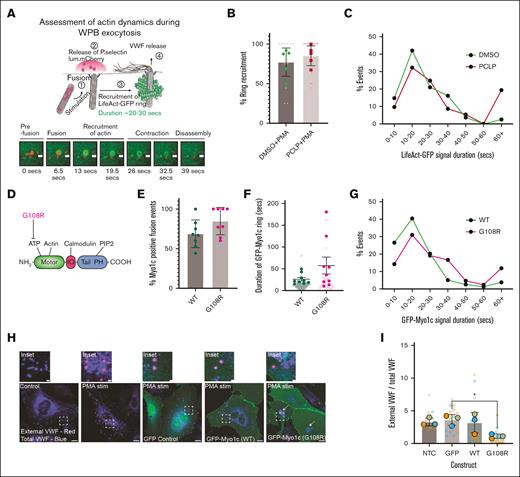
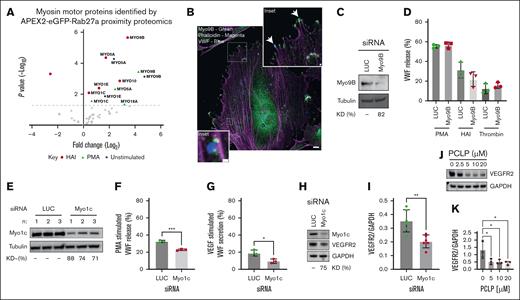
A volcano plot was generated to illustrate which myosin isoforms were most upregulated and most significantly enriched proximal to WPBs (Figure 1A). Myosin Va (MYO5A) forms a tripartite complex with Rab27a and MYRIP to anchor WPBs to actin structures in the cell periphery.43 As expected, myosin Va was significantly enriched in both unstimulated and stimulated (PMA or Histamine/Adrenaline/IBMX [HAI]) Rab27a-proximity proteomic data sets.14 Unexpectedly, the class IX myosin 9B (Myo9B) was the most highly enriched and statistically significant myosin isoform proximal to WPBs in both resting and stimulated cells. This was confirmed using IF, in which some Myo9B (green) could be seen proximal to VWF (blue) near actin structures (magenta) and likely at focal adhesions (Figure 1B). This unusual myosin motor has a Rho GTPase activating protein domain in its tail region.44 Because Rho activation has previously been implicated in VWF secretion45,46 we did not anticipate that Myo9B was a positive regulator of VWF secretion. Indeed, knockdown (KD) (∼82%) of Myo9B by siRNA did not affect VWF secretion after exposure to distinct secretagogues (Figure 1C-D). Subsequently, we chose to investigate the class I myosin family, of which 2 isoforms were significantly enriched in proximity to WPBs (isoforms C and E; Figure 1A: Bold). Of these, only Myo1c was enriched exclusively after secretagogue stimulation (both PMA and HAI), indicating a potential role in regulated exocytosis. To assess the functional role of Myo1c, we assessed the effect of depleting the endogenous pool of Myo1c on regulated VWF secretion. siRNA-mediated depletion resulted in KD efficiencies of 71% to 88% (Figure 1E). In agreement with a role in WPB exocytosis, Myo1c KD reduced VWF secretion in response to PMA (P < .005; Figure 1F). Through the use of independent siRNAs against Myo1c (supplemental Figure 1A-C), we confirmed that the depletion of Myo1c reduced VWF secretion in response to PMA and HAI. Furthermore, Myo1c KD reduced VWF secretion in response to the potent physiological regulators of VWF secretion VEGF165 (P < .05; Figure 1G) and thrombin (P < .01; supplemental Figure 1D). A complicating factor is that Myo1c depletion has been reported to disrupt recycling of lipid rafts47 and the trafficking of VEGFR2 to the PM, resulting in lysosomal degradation.48 We confirmed that endogenous VEGFR2 concentrations were significantly reduced in Myo1c KD cells (P < .01) (Figure 1H-I) as well as in HUVECs treated overnight with the pan class I myosin inhibitor, PCLP (P < .5; Figure 1J-K). To avoid this as a confounding factor in our investigations, we hereafter used PMA as the experimental secretagogue, because this chemical is a cell permeable protein kinase C (PKC) activator bypassing PM-receptor signaling.
WPB proximal myosin motors. (A) Volcano plot of myosin isoforms in close proximity to WPBs, previously identified by Rab27a-targeted APEX2 proximity proteomics. Blue represents significantly enriched in unstimulated cells; green, significantly enriched in PMA stimulated cells; magenta, significantly enriched in HAI stimulated cells; and gray, not statistically significant, compared with mock transfected HUVECs. Paired t test. (B) Unstimulated HUVECs were fixed and subject to IF analysis to localize Myo9B (green) in relation to VWF (blue) and actin (magenta). Myo9B staining was present in the cytoplasm and at the end of actin stress fibers reminiscent of focal adhesions. In some cases, VWF localized proximal to Myo9B puncta. Scale bar, 10 μm. Inset 1 μm (C) western blotting of tubulin and Myo9b in HUVEC lysate after 2 rounds of electroporation of 300 pMoles luciferase (LUC) and Myo9B targeting siRNA. Representative blot. (D) VWF secretion in response to PMA, HAI, and thrombin was assessed by NIR dot blot (n = 3). (E) Western blotting of tubulin and Myo1c in HUVEC lysate after 2 rounds of electroporation of 500 pM LUC and Myo1c targeting siRNA. (F-G) LUC and Myo1c KD HUVEC were exposed to PMA (100 ng/mL) or (G) VEGF165 (40 ng/mL), and VWF secretion was quantified by NIR dot blot (n = 3). t test. ∗∗∗P < .005; ∗∗P < .01. (H-I) Western blotting and (I) densitometry of Myo1c, VEGFR2, and GAPDH in LUC and Myo1c KD HUVEC (n = 6). (J-K) HUVECs were treated with the pan class I myosin inhibitor PCLP for 16 hours and endogenous levels of GAPDH and VEGRF2 determined by western blotting. One-way analysis of variance (ANOVA), ∗P < .05 (n = 3). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KD, knock down; NIR, near infrared.
WPB proximal myosin motors. (A) Volcano plot of myosin isoforms in close proximity to WPBs, previously identified by Rab27a-targeted APEX2 proximity proteomics. Blue represents significantly enriched in unstimulated cells; green, significantly enriched in PMA stimulated cells; magenta, significantly enriched in HAI stimulated cells; and gray, not statistically significant, compared with mock transfected HUVECs. Paired t test. (B) Unstimulated HUVECs were fixed and subject to IF analysis to localize Myo9B (green) in relation to VWF (blue) and actin (magenta). Myo9B staining was present in the cytoplasm and at the end of actin stress fibers reminiscent of focal adhesions. In some cases, VWF localized proximal to Myo9B puncta. Scale bar, 10 μm. Inset 1 μm (C) western blotting of tubulin and Myo9b in HUVEC lysate after 2 rounds of electroporation of 300 pMoles luciferase (LUC) and Myo9B targeting siRNA. Representative blot. (D) VWF secretion in response to PMA, HAI, and thrombin was assessed by NIR dot blot (n = 3). (E) Western blotting of tubulin and Myo1c in HUVEC lysate after 2 rounds of electroporation of 500 pM LUC and Myo1c targeting siRNA. (F-G) LUC and Myo1c KD HUVEC were exposed to PMA (100 ng/mL) or (G) VEGF165 (40 ng/mL), and VWF secretion was quantified by NIR dot blot (n = 3). t test. ∗∗∗P < .005; ∗∗P < .01. (H-I) Western blotting and (I) densitometry of Myo1c, VEGFR2, and GAPDH in LUC and Myo1c KD HUVEC (n = 6). (J-K) HUVECs were treated with the pan class I myosin inhibitor PCLP for 16 hours and endogenous levels of GAPDH and VEGRF2 determined by western blotting. One-way analysis of variance (ANOVA), ∗P < .05 (n = 3). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; KD, knock down; NIR, near infrared.
Myo1c is recruited during exocytosis
Myo1c has proposed roles in membrane fusion of GLUT4-containing vesicles,49 compression of lung surfactant secreting lamellar bodies,26 and for linking actin to the PM during compensatory endocytosis in frog eggs.50 IF and superresolution spinning disk microscopy of HUVECs showed that Myo1c did not colocalize with VWF in unstimulated cells (Figure 2A: box and inset). After secretagogue stimulation, WPBs fuse with the PM and collapse as the pH of the organelle shifts from acidic to neutral,4 and Myo1c was apparent surrounding fused WPB. Endogenous Myo1c was localized as punctae within the actin ring but encapsulating VWF (Figure 2A; supplemental Figure 2A,B). Using the actin polymerization inhibitor cytochalasin E with and without stimulus, we noted that Myo1c recruitment is independent of actin (Figure 2A). As a complementary approach, we used live cell imaging of HUVEC transiently expressing GFP-tagged Myo1c.33 Coexpression with LifeAct-Ruby illustrated its colocalization with actin at the leading edge51 (supplemental Video 1), whereas coexpression with P.sel.lum.mCherry15 (a fusion marker that is stored in WPBs and lost upon fusion with the PM) allowed for the assessment of Myo1c recruitment dynamics during WPB exocytosis. In response to PMA, Myo1c-GFP was clearly recruited to WPBs after fusion and was present on 68.65% ± 6.17 of events (Figure 2B; supplemental Video 2). In agreement with this, addition of an Alexa Fluor conjugated anti-VWF antibody to the culture media during stimulation revealed that the Myo1c ring appeared before expulsion and labeling of VWF (Figure 2C). By imaging HUVEC expressing LifeActRuby and Myo1c-GFP, we noted that the Myo1c signal preceded recruitment of the actin ring (supplemental Figure 2D).
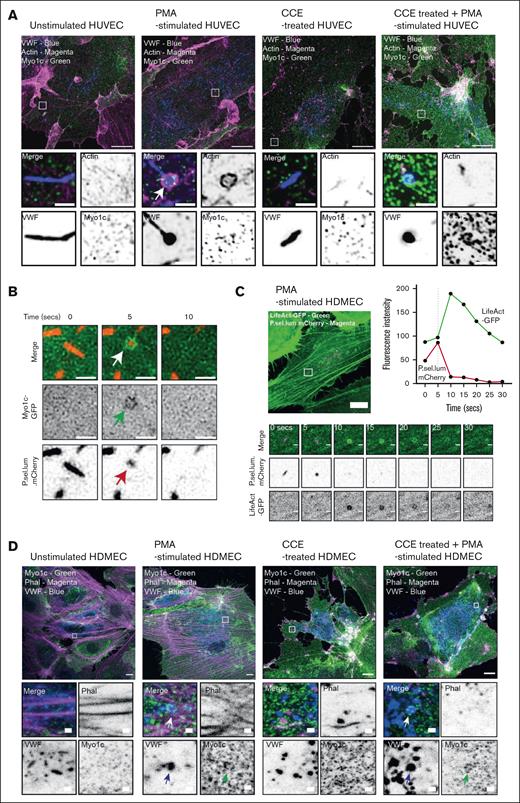
ECs use Myo1c as part of the WPB exocytic machinery. (A) Super-resolution imaging and immunofluorescent localization of endogenous Myo1c (green), actin (magenta), and VWF (blue) in unstimulated or PMA (100 ng/mL) stimulated HUVEC in the presence and absence of 1 μM of the actin polymerization inhibitor cytochalasin E (CCE). Scale bar, 10 μm. Inset, 1 μm. Myo1c is recruited independently of actin but was dependent on stimulation with PMA. (B) Myo1c-GFP encapsulates WPB after fusion as determined by live cell super-resolution spinning disk imaging of PMA stimulated (100 ng/mL) HUVEC coexpressing a Myo1c-GFP and the WPB fusion marker P.sel.lum.mCherry. Scale bar, 1 μm. Arrows indicate point of collapse/fusion of vesicle (C) Live cell imaging of LifeAct-GFP and P.sel.lum.mCherry expressing HDMEC indicated the utility of actin rings to expel VWF after stimulation. Scale bar, 10 μm. Inset, 1 μm. Z stacks of 0.5 μm were acquired continuously for 10 minutes (Zeiss LSM 800). (D) Confocal imaging and IF analyses of endogenous Myo1c in HDMEC that were left untreated or stimulated with PMA, CCE, or CCE and PMA. Arrows illustrate where Myo1c is recruited to fused/collapsed WPB. Scale bar, 10 μm.
ECs use Myo1c as part of the WPB exocytic machinery. (A) Super-resolution imaging and immunofluorescent localization of endogenous Myo1c (green), actin (magenta), and VWF (blue) in unstimulated or PMA (100 ng/mL) stimulated HUVEC in the presence and absence of 1 μM of the actin polymerization inhibitor cytochalasin E (CCE). Scale bar, 10 μm. Inset, 1 μm. Myo1c is recruited independently of actin but was dependent on stimulation with PMA. (B) Myo1c-GFP encapsulates WPB after fusion as determined by live cell super-resolution spinning disk imaging of PMA stimulated (100 ng/mL) HUVEC coexpressing a Myo1c-GFP and the WPB fusion marker P.sel.lum.mCherry. Scale bar, 1 μm. Arrows indicate point of collapse/fusion of vesicle (C) Live cell imaging of LifeAct-GFP and P.sel.lum.mCherry expressing HDMEC indicated the utility of actin rings to expel VWF after stimulation. Scale bar, 10 μm. Inset, 1 μm. Z stacks of 0.5 μm were acquired continuously for 10 minutes (Zeiss LSM 800). (D) Confocal imaging and IF analyses of endogenous Myo1c in HDMEC that were left untreated or stimulated with PMA, CCE, or CCE and PMA. Arrows illustrate where Myo1c is recruited to fused/collapsed WPB. Scale bar, 10 μm.
To confirm our results in an alternative EC type, we assessed whether human dermal microvascular ECs (HDMECs) used actin rings during WPB exocytosis. Live cell imaging of HDMEC expressing LifeAct-GFP and P.sel.lum.mCherry confirmed that this phenomenon is not specific to venous ECs from the umbilical vein (Figure 2C). IF studies demonstrated that HDMECs also recruit Myo1c during VWF secretion (Figure 2D). Once more, this was shown to be independent of actin. Through whole-mount IF imaging of the murine cremaster muscle, we determined that microvascular ECs express Myo1c in vivo (supplemental Figure 3). This demonstrates that HUVECs are a physiologically relevant model for studying Myo1c function, and Myo1c has a postfusion role. For the remainder of the investigations, we used HUVEC as our model system.
PIP2 mediated recruitment of Myo1c
Phosphoinositides control targeted membrane traffic and are differentially distributed between cellular compartments.52 Myo1c has a PIP2 binding PH domain in its tail region.22 Based on research in ATII cells,26 we anticipated that Myo1c was potentially recruited to fusing WPBs via this region (Figure 3A). Lipids on the organelle and PM play a variety of roles in exocytosis in diverse secretory systems.53 Phosphatidic acid and PIP2 are likely important in WPB exocytosis because phospholipase D1 (PLD1) has an established role in VWF secretion.54 PIP2 sensors (PH-PLCδ1-YFP) and enzymes (PIP5Kγ87) have previously been shown to be recruited to site of WPB fusion.55 We independently confirmed that GFP-PIPK1γ87 (Figure 3B) and PH-PLCδ1-GFP (Figure 3C) were present at sites of WPB fusion after collapse of the organelle. Interestingly, we on occasion noted the presence of GFP+ vacuoles in GFP-PIPK1γ87–expressing cells that were coated in Myo1c, actin, and septin 7 (another lipid binding protein associated with WPB exocytosis; supplemental Figure 4). These data indicate that the presence of PIP2 on the PM drives protein recruitment.
The PH domain of Myo1c is required for its recruitment during WPB exocytosis. (A) A schematic of the proposed spatiotemporal dynamics of Myo1c recruitment during WPB exocytosis. (B) Live cell imaging of the GFP-PIPK1γ87 and P.sel.lum.mCherry in secretagogue (HAI) stimulated HUVEC illustrates postfusion recruitment. Two exocytic events are seen here. Scale bars are 1 μm. White and magenta arrows indicate independent fusion events. (C) The PIP2 sensor PH-PLCδ1-GFP is also recruited after fusion. Scale bars are 1 μm. (D) A schematic of the Myo1c structural domains and location of truncation or site directed mutations. (E) HUVEC coexpressing LifeAct-Ruby and Myo1c-Tail+3IQ-GFP indicated the importance of the myosin head domain for interacting with actin. Scale bars are 10 μm. (F) Myo1c-Tail+3IQ-GFP is recruited to WPBs after fusion. (G-H) GFP-tagged Myo1c fusion proteins harboring mutations in their PH domain (K892A/R903A) are not recruited to WPBs during exocytosis. Scale bars are 10 μm (F,G,H). Inset scale bars are 1 μm. For live cell confocal imaging experiments, 0.5 μm Z stacks were acquired continuously for 5 to 10 minutes (Zeiss LSM 800).
The PH domain of Myo1c is required for its recruitment during WPB exocytosis. (A) A schematic of the proposed spatiotemporal dynamics of Myo1c recruitment during WPB exocytosis. (B) Live cell imaging of the GFP-PIPK1γ87 and P.sel.lum.mCherry in secretagogue (HAI) stimulated HUVEC illustrates postfusion recruitment. Two exocytic events are seen here. Scale bars are 1 μm. White and magenta arrows indicate independent fusion events. (C) The PIP2 sensor PH-PLCδ1-GFP is also recruited after fusion. Scale bars are 1 μm. (D) A schematic of the Myo1c structural domains and location of truncation or site directed mutations. (E) HUVEC coexpressing LifeAct-Ruby and Myo1c-Tail+3IQ-GFP indicated the importance of the myosin head domain for interacting with actin. Scale bars are 10 μm. (F) Myo1c-Tail+3IQ-GFP is recruited to WPBs after fusion. (G-H) GFP-tagged Myo1c fusion proteins harboring mutations in their PH domain (K892A/R903A) are not recruited to WPBs during exocytosis. Scale bars are 10 μm (F,G,H). Inset scale bars are 1 μm. For live cell confocal imaging experiments, 0.5 μm Z stacks were acquired continuously for 5 to 10 minutes (Zeiss LSM 800).
To investigate the mechanism of its recruitment, we used truncated and point mutants of Myo1c (Figure 3D). By coexpressing the GFP-tagged neck and tail domain of Myo1c (Myo1c-Tail+3IQ-GFP) together with LifeAct-Ruby, we demonstrate that the N-terminus is necessary for appropriate Myo1c targeting to actin at the leading edge (Figure 3E, inset; supplemental Video 3). Consistent with the hypothesis that Myo1c is recruited to fusing WPB via its tail-resident PH domain; Myo1c-Tail+3IQ-GFP localized to fused WPBs after secretagogue stimulation (Figure 3F). We used a GFP-tagged Myo1c fusion proteins harboring point mutations in the phosphoinositide-binding PH domain (K892A/R903A) to show that the interaction with PIP2 is necessary for recruitment to WPB at fusion (Figure 3G-H). Unlike the wild-type (WT) and Myo1c-Tail+3IQ construct, Myo1c-K892A–GFP and Myo1c-R903A–GFP localized to the cytosol and were not recruited to WPBs after fusion. Taken together, these data indicate that Myo1c is recruited to WPB after fusion via its PH domain.
The effect of type I myosin inhibition on VWF secretion and exocytic actin ring dynamics
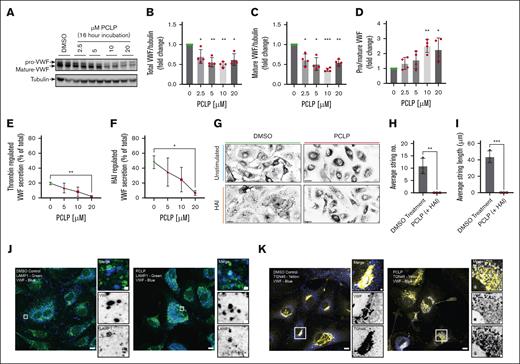
To investigate the role of the motor (head) domain, we used the pan-myosin I inhibitor, PCLP.56 PCLP is a potent allosteric inhibitor of myosin ATPase that shows selectivity for class I myosins at low doses (IC50, ∼1-10 μM). However, at higher doses, other myosin classes (eg, nonmuscle myosin IIB; IC50, ∼90 μM)56 are affected. Here, pre-exposure to 2.5 to 20 μM PCLP for 16 hours resulted in an obvious trafficking defect, whereby the endogenous levels of total and mature VWF were decreased (Figure 4A-C; P < .05). The ratio of pro-VWF to mature VWF was increased in a dose-dependent fashion (Figure 4D; P < .05-.01). Regulated VWF secretion (Figure 4E-F) and string formation (Figure 4G-I) were almost completely abolished in PCLP-treated cells. Moreover, IF of LAMP1 (Figure 4J) and TGN46 (Figure 4K) illustrated the appearance of VWF-positive lysosomes and a gross defect in morphology of the TGN. This demonstrates that class I myosins play a role in VWF trafficking as well as secretion. To determine the specific role of Myo1c in VWF trafficking, we next assessed the effect of 4 different siRNA oligonucleotides targeting Myo1c on VWF levels by western blotting. Quantification of the levels of mature and pro-VWF were unchanged by Myo1c KD indicating that Myo1c is not essential for WPB biogenesis. The broader effect of PCLP likely reflects effects on other class I myosins during WPB biogenesis (Figure 5).
HUVEC exposed to PCLP for 16 hours exhibit a VWF trafficking defect. HUVEC were exposed to dimethyl sulfoxide (DMSO) or a range of concentrations of PCLP and incubated overnight (16 hours). (A) Immunoblotting of the resulting lysates displayed changes in pro-VWF and mature VWF in relation to tubulin. Densitometry indicated a decrease in total VWF (B) and mature-VWF levels (C) alongside a dose-dependent increase in the ratio of pro/mature VWF (D). n = 4 Ratio paired t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005 (E) 16-hour incubation with PCLP resulted in inhibition of regulated secretion of VWF in response to thrombin and (F) HAI. (G) HUVEC preincubated with DMSO or PCLP for 16 hours were stimulated with HAI for 10 minutes before application of 5 dyne/cm2 shear stress. VWF strings were visualized by immunofluorescence and confocal microscopy. (H-I) The number (H) and length (I) of VWF strings secreted under flow in response to HAI in the presence or absence of DMSO or PCLP (n = 3). (J) IF analyses using anti-LAMP1 (green) and anti-VWF (blue) antibodies indicated numerous VWF positive lysosomes in PCLP-treated cells. (K) IF analyses using anti-TGN46 (yellow) and anti-VWF (blue) antibodies indicated a gross defect in TGN morphology (fragmented and swollen) in PCLP-treated cells. Scale bars, 10 μm. Inset scale bars, 1 μm.
HUVEC exposed to PCLP for 16 hours exhibit a VWF trafficking defect. HUVEC were exposed to dimethyl sulfoxide (DMSO) or a range of concentrations of PCLP and incubated overnight (16 hours). (A) Immunoblotting of the resulting lysates displayed changes in pro-VWF and mature VWF in relation to tubulin. Densitometry indicated a decrease in total VWF (B) and mature-VWF levels (C) alongside a dose-dependent increase in the ratio of pro/mature VWF (D). n = 4 Ratio paired t test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .005 (E) 16-hour incubation with PCLP resulted in inhibition of regulated secretion of VWF in response to thrombin and (F) HAI. (G) HUVEC preincubated with DMSO or PCLP for 16 hours were stimulated with HAI for 10 minutes before application of 5 dyne/cm2 shear stress. VWF strings were visualized by immunofluorescence and confocal microscopy. (H-I) The number (H) and length (I) of VWF strings secreted under flow in response to HAI in the presence or absence of DMSO or PCLP (n = 3). (J) IF analyses using anti-LAMP1 (green) and anti-VWF (blue) antibodies indicated numerous VWF positive lysosomes in PCLP-treated cells. (K) IF analyses using anti-TGN46 (yellow) and anti-VWF (blue) antibodies indicated a gross defect in TGN morphology (fragmented and swollen) in PCLP-treated cells. Scale bars, 10 μm. Inset scale bars, 1 μm.
Short term PCLP treatment (30 minutes) allows for postfusion analysis of the role of type I myosins in WPB exocytosis by acutely inhibiting the myosin I ATPase activity without affecting WPB biogenesis (Figure 5A). Accordingly, we first used PCLP to assess the effect on PMA-induced VWF secretion. Preincubation with 10 to 40 μM PCLP significantly reduced VWF secretion (P < .05-.01; Figure 5B). We next imaged HUVEC coexpressing GFP-VWF and P.sel.lum.mCherry to investigate the effect of type I myosin inhibition on VWF expulsion (Figure 5C). PCLP significantly (P < .01) increased the lag time for GFP-VWF to be expelled after the loss of the WPB fusion marker, P.sel.lum.mCherry (mean ± standard error of the mean, 61s ± 9 vs 172s ± 13; Figure 5D-E). To address whether this result was specific to Myo1c or a broader effect on class I myosins, we repeated this loss of function assay using siRNA. We achieved KD efficiencies of ∼68% (Figure 5F). This correlated with a marked increase in the average delay between the loss of P.sel.lum.mCherry and GFP-VWF (42s ± 8.4 vs 99s ± 10.2; Figure 5G). Given the heterogenous nature of siRNA transfection efficiencies across a monolayer of cells, we postulate the phenotype is likely an underestimation, and this indicates Myo1c is the predominant class I myosin influencing WPB exocytosis.
Acute inhibition of class I myosins and Myo1c depletion perturbs the expulsion of VWF. (A) Schematic of Myo1c domains and mechanism of inhibition by PCLP. (B) Pharmacological inhibition of the ATP binding domain with 10 to 40 μM PCLP reduces VWF release. (n = 6) ∗P < .05; ∗∗P < .01, ratio paired t test. (C) Schematic of live cell imaging approach to study WPB fusion dynamics and VWF expulsion. Scale bar, 1 μm. (D) HUVECs were electroporated with the P.sel.lum.mCherry and GFP-VWF constructs and imaged by confocal microscopy. Preincubation with 20 μM PCLP increased the time taken for VWF to be expelled after loss of the fusion marker (P.sel.lum.mCherry). Paired t test, ∗∗P < .01. (n = 3; DMSO: 9 cells, 63 events; PCLP: 9 cells, 38 events; mean ± standard error of the mean [SEM]). (E) A frequency distribution of events. (F) LUC and Myo1c KD HUVEC were used to test whether these effects were specific to Myo1c or a broader effect of class I myosins. Western blotting determined the efficiency of target protein KD. (G) Myo1c siRNA depletion increased the time taken for VWF to be expelled after loss of P.sel.lum.mCherry. ∗P < .05; paired t test.
Acute inhibition of class I myosins and Myo1c depletion perturbs the expulsion of VWF. (A) Schematic of Myo1c domains and mechanism of inhibition by PCLP. (B) Pharmacological inhibition of the ATP binding domain with 10 to 40 μM PCLP reduces VWF release. (n = 6) ∗P < .05; ∗∗P < .01, ratio paired t test. (C) Schematic of live cell imaging approach to study WPB fusion dynamics and VWF expulsion. Scale bar, 1 μm. (D) HUVECs were electroporated with the P.sel.lum.mCherry and GFP-VWF constructs and imaged by confocal microscopy. Preincubation with 20 μM PCLP increased the time taken for VWF to be expelled after loss of the fusion marker (P.sel.lum.mCherry). Paired t test, ∗∗P < .01. (n = 3; DMSO: 9 cells, 63 events; PCLP: 9 cells, 38 events; mean ± standard error of the mean [SEM]). (E) A frequency distribution of events. (F) LUC and Myo1c KD HUVEC were used to test whether these effects were specific to Myo1c or a broader effect of class I myosins. Western blotting determined the efficiency of target protein KD. (G) Myo1c siRNA depletion increased the time taken for VWF to be expelled after loss of P.sel.lum.mCherry. ∗P < .05; paired t test.
Next, we studied actin dynamics during WPB exocytosis using a similar approach substituting GFP-VWF for LifeAct-GFP (Figure 6A). This demonstrated that type I myosin inhibition with PCLP had no effect on the percentage of WPB fusion events that recruited an actin ring (Figure 6B). This indicated that Myo1c is not required for actin polymerization. However, an increased proportion of persisting (>60s) actin coats/rings was noted in PCLP-treated cells (2.4% vs 19.3%; Figure 6C), demonstrating that actin ring contraction required Myo1c ATP hydrolysis (supplemental Videos 4-5). This phenotype was confirmed by generating a dominant negative GFP-Myo1c rigor mutant via the introduction of a point mutation in the ATP-binding site (G108R; Figure 6D). Live cell imaging of GFP-Myo1c WT and GFP-Myo1c (G108R) determined that both were recruited to WPBs during exocytosis, and the percentage of fusion events that were positive for Myo1c-GFP signal was comparable with that of actin rings (WT, 68.7% ± 6.2; vs G108R, 84.79% ± 6.1; Figure 6E). The WT signal persisted for ∼26 seconds, which also matches the dynamics of the actin ring.14,15 However, the rigor mutant persisted for longer (∼57s ± 19), mirroring what we observed when imaging actin dynamics in the presence of PCLP (Figure 6F). This was also reflected in the distribution of frequency of events. We noted a striking similarity to the change in actin ring dynamics in which the percentage GFP-Myo1c rings that lasted >60 seconds was increased after PCLP treatment (3.8% vs 11.9%; Figure 6G). Using an Alexa Fluor-conjugated anti-VWF antibody in the medium, we monitored VWF as it is secreted from the cell (Figure 6H). We compared the amount of VWF secreted by the cell in relation with total VWF while overexpressing GFP, GFP-Myo1c WT, or GFP-Myo1c (G108R). Concordant with our previous data, overexpression of the “rigor” mutant resulted in reduced secretion of VWF (Figure 6I). However, this must be caveated with potential off-target effects during WPB biogenesis at the TGN (Figure 6H arrows; supplemental Figure 6). Taken together, these data indicate a role for Myo1c and its ATPase domain in augmenting compression of the vesicle likely through actin coat organization and linkage.
Inhibition of Myo1 ATPase activity through pharmacological inhibition or point mutation (G108R) affects the actomyosin machinery associated with exocytosis. (A) Schematic of live cell imaging approach to study actin dynamics during WPB exocytosis. Scale bar, 1 μm. (B) PMA stimulated HUVEC coexpressing LifeAct-GFP and P.sel.lum.mCherry in the presence or absence of PCLP. The percentage of WPB fusion events that recruited an actin ring were unchanged in DMSO and PCLP (20 μM) treated cells. (C) The lifetime (seconds) of LifeAct-GFP signal at fusion sites was quantified in DMSO and PCLP-treated HUVEC. The distribution of frequency of events is presented here (n = 5 DMSO: 15 cells, 81 events; PCLP: 18 cells, 93 events; mean ± SEM). (D) Schematic of site-directed mutagenesis for the generation of a Myo1c rigor mutant. (E) HUVEC coexpressing GFP-tagged Myo1c constructs and P.sel.lum.mCherry were stimulated with PMA and the percentage of exocytic events that recruit GFP-Myo1c WT or G108R was quantified (n = 3; WT: 8 cells and 119 events; G108R: 8 cells and 58 events). (F) HUVEC coexpressing GFP-tagged Myo1c constructs and P.sel.lum.mCherry were stimulated with PMA and the duration of GFP signal in a ring shape forming at the site of WPB fusion was quantified. (n = 3; WT: 9 cells and 79 events; PCLP: 9 cells and 42 events). (G) The distribution of frequency closely resembles actin ring dynamics--panel C. For live cell confocal imaging experiments, 0.5 μm Z stacks were acquired continuously for 5 to 10 minutes (Zeiss LSM 800). (H) HUVEC expressing GFP, GFP-Myo1c (WT) or (G108R) were stimulated with PMA (100 ng/mL) for 10 minutes and labeled for external VWF (red) and total VWF (blue). Scale bar, 1 μm. (I) Quantification of the ratio of externalized VWF to total VWF. ∗P < .05, 1-way ANOVA; n = 3 NTC. Arrows indicate swollen intracellular VWF signal in cells expressing the G108R point mutant. NTC, nontransfected control.
Inhibition of Myo1 ATPase activity through pharmacological inhibition or point mutation (G108R) affects the actomyosin machinery associated with exocytosis. (A) Schematic of live cell imaging approach to study actin dynamics during WPB exocytosis. Scale bar, 1 μm. (B) PMA stimulated HUVEC coexpressing LifeAct-GFP and P.sel.lum.mCherry in the presence or absence of PCLP. The percentage of WPB fusion events that recruited an actin ring were unchanged in DMSO and PCLP (20 μM) treated cells. (C) The lifetime (seconds) of LifeAct-GFP signal at fusion sites was quantified in DMSO and PCLP-treated HUVEC. The distribution of frequency of events is presented here (n = 5 DMSO: 15 cells, 81 events; PCLP: 18 cells, 93 events; mean ± SEM). (D) Schematic of site-directed mutagenesis for the generation of a Myo1c rigor mutant. (E) HUVEC coexpressing GFP-tagged Myo1c constructs and P.sel.lum.mCherry were stimulated with PMA and the percentage of exocytic events that recruit GFP-Myo1c WT or G108R was quantified (n = 3; WT: 8 cells and 119 events; G108R: 8 cells and 58 events). (F) HUVEC coexpressing GFP-tagged Myo1c constructs and P.sel.lum.mCherry were stimulated with PMA and the duration of GFP signal in a ring shape forming at the site of WPB fusion was quantified. (n = 3; WT: 9 cells and 79 events; PCLP: 9 cells and 42 events). (G) The distribution of frequency closely resembles actin ring dynamics--panel C. For live cell confocal imaging experiments, 0.5 μm Z stacks were acquired continuously for 5 to 10 minutes (Zeiss LSM 800). (H) HUVEC expressing GFP, GFP-Myo1c (WT) or (G108R) were stimulated with PMA (100 ng/mL) for 10 minutes and labeled for external VWF (red) and total VWF (blue). Scale bar, 1 μm. (I) Quantification of the ratio of externalized VWF to total VWF. ∗P < .05, 1-way ANOVA; n = 3 NTC. Arrows indicate swollen intracellular VWF signal in cells expressing the G108R point mutant. NTC, nontransfected control.
Discussion
We previously described the presence of a contractile actomyosin ring that is recruited to the WPB surface after fusion with the PM and aiding efficient VWF secretion.15 This represents an unexploited therapeutic target for the prevention of thrombotic pathologies. Actomyosin-mediated expulsion of VWF requires upstream protein kinase C α57 and p21 activated kinase 214 signaling, Spire1-mediated actin nucleation,30 zyxin and α-actinin–mediated organization,58 and controlled compression (via septin14 and non-muscle myosin isoforms15,29). However, the mechanism by which the actomyosin ring is attached to the vesicle membrane and how this influences exocytosis are unclear. We demonstrate that Myo1c is recruited to the membrane of fused WPBs by its PH domain, in an actin-independent fashion. Perturbation of Myo1c function through pharmacological inhibition or siRNA-mediated depletion reduced VWF secretion in human ECs, and detailed live cell imaging experiments implicate a role in augmenting WPB exocytosis through providing additional traction points for the actin ring. This represents, to our knowledge, the first description of how class I myosin motors contribute to endothelial secretion of VWF secretion.
As a functional read out, we assessed the effect of siRNA-mediated depletion of Myo1c on VWF secretion in response to PMA and VEGF165. These secretagogues were chosen because they stimulate increases in cytosolic cAMP and activation of PKC, which is thought to be required for ring recruitment.29,57,58 SiRNA depletion of Myo1c modestly reduced VWF secretion in response to PMA, but a greater effect was observed when HUVECs were stimulated with VEGF165. Myo1c is known to regulate VEGFR2 trafficking to the PM, and Myo1c KD leads to VEGFR2 degradation.48 It is plausible that this is not specific to VEGFR2 but also other membrane bound receptors (notably, we also saw a marked effect of Myo1c depletion on thrombin-stimulated VWF release). For this reason, we draw our conclusions from investigations on ECs stimulated with the membrane permeable PKC agonist PMA.
Although other class I myosins such as Myo1e59 have roles in other secretory systems, we have focused on delineating a role for Myo1c. In addition to actin-membrane tethering, Myo1c aids insulin stimulated PM fusion of GLUT4-containing vesicles, where it is recruited before vesicle fusion.23,60 In contrast, we observed that GFP-tagged Myo1c is recruited to the WPB surface after fusion, similar to that reported by Kittelberger et al.26 Therefore, we exclude that Myo1c is acting as an organelle transporter. Instead, we propose a similar role to that seen in surfactant exocytosis by ATII cells26 and cortical granules in Xenopus eggs,50 whereby Myo1c links the actin coat to the vesicle membrane. To fulfill this role, Myo1c requires a tight interaction with both actin and the WPB membrane surface. We show that the PH domain of Myo1c binds PIP2 that is recruited to WPB after fusion.55 Inhibition of the myosin head ATPase domain using PCLP resulted in constrained release of VWF and delayed actin ring contractility, although it did not reduce the proportion of fusion events that recruited actin. We sought to confirm these findings by generating an ATP hydrolysis deficient (rigor) mutant (G108R). In the yeast homologue (Myo5), this mutation inhibits endocytosis and prevents membrane invagination.38 A similar rigor mutant has also been generated by mutating a lysine 111 (K111R) that inhibits lipid raft exocytosis.47 The Myo1c rigor mutant reduced the amount of VWF secreted, and live cell imaging showed that it persisted at the site of fusion longer than the full-length Myo1c control. The timing of WT Myo1c recruitment mirrored the spatiotemporal dynamics of the actin ring, further suggesting a role in aiding this force driven process. The change in ring kinetics after inhibition with PCLP were strikingly similar to those of G108R. Overall, these changes closely resemble the defect in actin ring contraction observed when NMII isoforms are inhibited using blebbistatin,15 consistent with active Myo1c augmenting VWF release by providing anchor points for the actomyosin ring.
Here, an acute PCLP exposure time of 30 minutes was needed to assess actin ring dynamics during WPB exocytosis. Longer incubation times had drastic effects on protein trafficking. Sixteen-hour incubation with PCLP resulted in disruption of the TGN, a near complete abrogation of regulated VWF secretion, ineffective VWF biogenesis, as well as the clear observation of VWF signal in LAMP1+ lysosomes. This may reflect defects at the Golgi61 or inhibition of autophagosome-lysosome fusion,62 with subsequent effects on WPB turnover, lysosomal colocalization, and degradation of VWF. Importantly, we did not observe this phenotype in Myo1c-depleted cells and therefore hypothesize that this trafficking defect is caused by inhibitory action of PCLP on other class I myosins.56 Notably, Myo1b promotes the formation of tubules and carriers at remodeling TGN membranes63 and has a role in secretory granule biogenesis in pancreatic beta cells64 and neuroendocrine cells.65 A specific role in WPB biogenesis is therefore also a possibility.
Finally, as a hypothesis of the spatiotemporal recruitment of these molecules, we present a putative working model (see the visual abstract). Enriched PIP2 concentrations occur at the site of WPB fusion as a result of membrane mixing and/or through PIPK1γ87 mediated production from its precursor PI4P. PIP2 at the WPB surface then leads to the rapid recruitment of Myo1c via its lipid binding PH domain. De novo Spire1 mediated actin nucleation30 follows (or occurs simultaneously) with the Myo1c motor domain binding to the resulting actin coat/ring. We suggest that NMII isoforms are recruited after actin, such as seen in Xenopus eggs,66 lamellar bodies,67 rodent,68 and Drosophila salivary granules.69 Activation of these isoforms then leads to vesicle compression and expulsion of VWF.
Overall, these data provide, to our knowledge, the first evidence of class I myosins participating in VWF secretion from ECs. To our knowledge, this is the first description of a role for Myo1c in the field of thrombosis and hemostasis. As such, these data aid our fundamental understanding of the molecular mechanisms governing primary hemostasis.
Acknowledgments
The authors acknowledge the CMR Advanced Bio-Imaging Facility of Queen Mary University of London for the help and advice with microscopy. The authors thank Chris Stefan (University College London) for his critical appraisal of this manuscript. The authors thank their funders and collaborators.
This work was supported by British Heart Foundation grant PG/22/11208. T.P.M. was funded by Barts Charity project grant MGU05434.
Authorship
Contribution: S.E.-M. and T.D.N. developed the methodology and wrote the original draft of the manuscript; P.M. and M.F. generated and provided essential tools and reagents; S.E.-M., T.D.N., T.P.M., T.A.J.M., and G.M. performed the investigation; T.D.N. supervised the study; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sammy El-Mansi, Centre for Microvascular Research, William Harvey Research Institute, Charterhouse Square, Faculty of Medicine and Dentistry, Queen Mary University of London, London EC1M 6BQ, United Kingdom; email: s.elmansi@qmul.ac.uk; and Thomas D. Nightingale, Centre for Microvascular Research, William Harvey Research Institute, Charterhouse Square, Faculty of Medicine and Dentistry, Queen Mary University of London, London EC1M 6BQ, United Kingdom; email: t.nightingale@qmul.ac.uk.
References
Author notes
Mass spectrometry data are available via the PRIDE40 partner repository (data set identifier PXD036983 and 10.6019/PXD036983).
Other original data and constructs are available upon reasonable request from the corresponding authors, Thomas D. Nightingale (t.nightingale@qmul.ac.uk) and Sammy El-Mansi (s.elmansi@qmul.ac.uk).
The full-text version of this article contains a data supplement.

![Acute inhibition of class I myosins and Myo1c depletion perturbs the expulsion of VWF. (A) Schematic of Myo1c domains and mechanism of inhibition by PCLP. (B) Pharmacological inhibition of the ATP binding domain with 10 to 40 μM PCLP reduces VWF release. (n = 6) ∗P < .05; ∗∗P < .01, ratio paired t test. (C) Schematic of live cell imaging approach to study WPB fusion dynamics and VWF expulsion. Scale bar, 1 μm. (D) HUVECs were electroporated with the P.sel.lum.mCherry and GFP-VWF constructs and imaged by confocal microscopy. Preincubation with 20 μM PCLP increased the time taken for VWF to be expelled after loss of the fusion marker (P.sel.lum.mCherry). Paired t test, ∗∗P < .01. (n = 3; DMSO: 9 cells, 63 events; PCLP: 9 cells, 38 events; mean ± standard error of the mean [SEM]). (E) A frequency distribution of events. (F) LUC and Myo1c KD HUVEC were used to test whether these effects were specific to Myo1c or a broader effect of class I myosins. Western blotting determined the efficiency of target protein KD. (G) Myo1c siRNA depletion increased the time taken for VWF to be expelled after loss of P.sel.lum.mCherry. ∗P < .05; paired t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/17/10.1182_bloodadvances.2024012590/2/m_blooda_adv-2024-012590-gr5.jpeg?Expires=1769089608&Signature=OJA2Vom5RxALz9n2MG1bY4VVsXfieXZeyhVOTEdZYuETRy1sK4eRecELAhBd9f~IycFFhJ0LE5R~wZjiAGjLmb7DnqagJPA6AHZW6y8Q16cT0Dce~HmBPPCjIQA~y0-VL8hhTQCIwfSp60xYyzJdQ8nB1HWAxK28UkyrUS-3VPUmfMWBpP5fFo~dCgKQapHgmY2KpkRJx-MFH3cbIFcNNYB4yXZ~6T4CFKjgtc9nQfOutq7Uk3wJpOf2PcF5Lopgbc~wbz7cpx6UOul-eUNGKL2cMK6SovMhTKc-d1oL6Ku42B~Ecgm3FDN6tf2K2QSusOzis3ljASXj3ShfXuLvfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)