Key Points
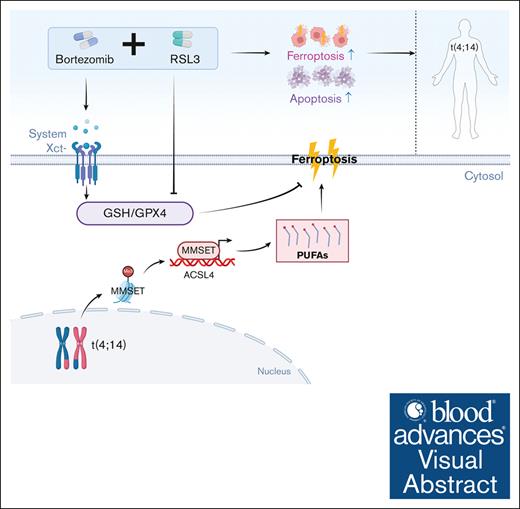
MM with t(4;14) translocation is vulnerable to class II FINs because of the upregulation of the MMSET-ACSL4-PUFAs axis.
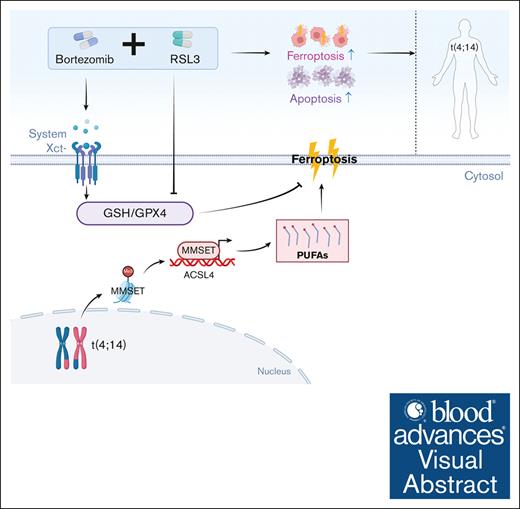
Class II FINs and bortezomib synergize to trigger t(4;14)-positive MM cell death by attenuating glutathione levels.
Visual Abstract
Multiple myeloma (MM) is a clonal plasma cell malignancy that is characterized by genetic heterogeneity. The cytogenetic abnormality t(4;14) strongly predicts poor outcome in patients with MM, even in the era of novel drugs. Ferroptosis is a new approach to antitumor therapy, but the relationship between ferroptosis and MM cytogenetic abnormalities remains largely unclear. In this study, we show that t(4;14)-positive but not t(4;14)-negative MM cells are susceptible to class II ferroptosis inducers (FINs) in a preclinical setting, which is dependent on the significant upregulation of the MM SET domain-containing protein (MMSET). Mechanistically, MMSET upregulates acyl-coenzyme A synthetase long-chain family member 4 transcription by binding to its promoter region, leading to increased polyunsaturated fatty acid (PUFA) levels and enhanced sensitivity of t(4;14)-positive MM cells to ferroptosis. Supplementation with PUFAs efficiently restores the susceptibility of t(4;14)-negative MM cells to ferroptosis. In addition, combining class II FIN treatment with bortezomib in t(4;14)-positive MM cells attenuates cellular glutathione and induces both apoptosis and ferroptosis levels by inhibiting the increase in solute carrier family 7 member 11, demonstrating synergistic antitumor activity in vitro and in a xenograft model. Taken together, our findings suggest that targeting ferroptosis with class II FINs is a novel and promising therapeutic approach to improve the outcome of t(4;14)-positive patients with MM.
Introduction
Multiple myeloma (MM) is a hematologic malignancy that originate from the clonal proliferation of plasma cells.1 Despite recent therapeutic advances, MM remains incurable.2 MM is characterized by cytogenetic complexity and heterogeneity.3 Chromosomal translocation t(4;14), the most common identified cause of high-risk MM (∼10%-15%) among newly diagnosed cases,4 strongly predicts poor outcomes in patients with MM, even in the era of novel drugs.5
It is well-established that t(4;14)(p16;q32) increases the expression of the MM SET domain-containing protein through the immunoglobulin heavy-chain enhancer.6 MMSET is widely recognized as a crucial oncogene that contributes to progression and drug resistance in t(4;14)-positive MM.7,8 As a histone methyltransferase, MMSET facilitates transcription by marking the dimethylation of histone H3 at K36, loosening the chromatin structure, and interacting with other enzymes that modify histones.9,10 Because of alternative splicing, MMSET has multiple transcripts and corresponding isoforms,11 which include MMSET II. This full-length form is considered the main carcinogen.12,13 Researchers have focused on the development of effective inhibitors of MMSET.14 Particularly, KTX-1001, a novel compound that targets the catalytic SET domain, has entered a phase I clinical trial in patients with relapsed and refractory MM. However, structural and preclinical data are still lacking.15 Although combination chemotherapy regimens based on proteasome inhibitors can improve the survival of patients with t(4;14)-positive MM in the short term by inducing apoptosis, they ultimately fail to overcome the relapse outcome because of the anti-apoptotic effect of MMSET. Accordingly, there is an urgent need for new treatment strategies for patients with t(4;14)-positive MM.
Ferroptosis is an iron-dependent, nonapoptotic form of cell death that is characterized by the accumulation of massive lipid peroxidation.16 There are primarily 2 classes of ferroptosis inducers (FINs) that are distinguished based on different mechanisms.17 Erastin, a drug classified as a class I FIN, directly inhibits the cystine/glutamate transporter solute carrier family 7 member 11 and indirectly inhibits glutathione peroxidase 4 (GPX4) to induce ferroptosis.18 Class II FINs, such as RAS-selective lethal 3 (RSL3), directly inhibit GPX4 activity, which leads to the induction of ferroptosis.19 Recently, accumulating evidence suggests that inducing ferroptosis enhances the effectiveness of chemotherapy, targeted therapy, immunotherapy, and radiotherapy20,21; even drug-resistant or tolerant tumor cells have a distinct susceptibility to ferroptosis.22,23 These findings indicate that FINs possess a strong antitumor activity, and they may achieve greater efficacy in combination therapy settings.24 However, predicting the sensitivity to ferroptosis in cancer remains a topic of debate that depends on the specific integrated genetic and metabolic network.25-27 In our past research, the gene for acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) was abnormally upregulated and determined to be a crucial forecaster of ferroptosis in MM.28 However, the mechanism of how ACSL4 regulates MM ferroptosis susceptibility needs to be examined further.
In this study, we demonstrated that t(4;14)-positive MM cells are susceptible to class II FINs because of the upregulation of the MMSET/ACSL4/polyunsaturated fatty acids (PUFAs) axis. Coupling class II FINs with bortezomib (BTZ) exhibited synergistic antitumor activity that led to both apoptosis and ferroptosis by inhibiting the increase in xCT and attenuating cellular glutathione levels in t(4;14)-positive MM. These findings represent a promising treatment strategy for t(4;14)-positive MM.
Materials and methods
Patient samples
A total of 16 healthy donors and 8 patients with MM were recruited from the Union Hospital (Wuhan, China) between September 2020 and March 2022. Patients with MM were diagnosed according to the International Myeloma Working Group criteria and had not received any previous treatment. Additional clinical information pertaining to the healthy donors and patients with MM has been provided in supplemental Table 1. Primary MM cells were isolated from bone marrow aspirates of patients with MM using human CD138 microbeads (No.130-051-301, Miltenyi) in accordance with the suggested protocols. Bone marrow mononuclear cells and peripheral blood mononuclear cells were separated from bone marrow aspirates or peripheral blood from healthy donors using Ficoll–Hipaque density sedimentation. Informed consent was obtained from all participants and was authorized by the institutional review board of the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2022-0477-01).
Reagents
Details of the reagents are presented in supplemental Table 2.
Lentiviral transduction
Lentivirus particles were obtained from Genechem Biotechnology (Shanghai, China). NCI-H929 and OPM-2 cells were transduced with lentivirus that expressed short hairpin RNA (shRNA) against human MMSET II (shMMSET) and human ACSL4 (shACSL4), lentivirus that overexpressed ACSL4, or a negative control (shNC) at a multiplicity of infection of 100 under optimal conditions. Lentivirus particles that overexpressed MMSET were transduced with shNC and shMMSET and used for transduction of OPM-2 cells to generate the add-back strain of MMSET expression. Cells were then cultured for 48 hours, and the RPMI-1640 medium with 10% fetal bovine serum was used to replace the supernatant containing lentivirus. Three days after transduction, cells were chosen utilizing puromycin (Biosharp, 1.0 μg/mL for NCI-H929 and 1.5 μg/mL for OPM-2) for a duration of 4 to 7 days. The short hairpin sequences pertaining to the genes employed in this study can be found in supplemental Table 3.
Real-time quantitative reverse transcription PCR
The total RNA was extracted using TRIzol from Takara (Otsu, Japan). Real-time quantitative reverse transcription PCR (RT-qPCR) was conducted in triplicate using the ChamQ SYBR qPCR Master Mix from Vazyme (Nanjing, China). The 2–ΔΔCT method was used to analyze the results. β-actin was employed as the internal reference. All primers were synthesized by Tsingke Biotechnology (Beijing, China), and the sequences are provided in supplemental Table 4.
ChIP-qPCR assay
Chromatin immunoprecipitation (ChIP) was performed using the Magna ChIP A/G ChIP Kit (no. 17-10058, Merck Millipore, MA) according to the manufacturer's protocol. The antibodies used for ChIPs were MMSET (no. 75359, Abcam) and normal mouse immunoglobulin G (sc-3877, Santa Cruz Biotechnology). Input controls consisted of 2% chromatin before immunoprecipitation. Purified DNA from cross-linked cells was used for qPCR with a subset of primers that targeted the ACSL4 promoter. The sequences of primers are shown in supplemental Table 5.
Animal models and treatments
Female nonobese, diabetic or severe combined immunodeficient (NOD SCID) mice (6 weeks, GemPharmatech, China) were subcutaneously injected with 1 × 107 NCI-H929 cells with or without MMSET knockdown in 200 μL serum-free RPMI-1640 medium to establish a human MM xenograft model. After ∼2 weeks, when the average volume of established tumors reached ∼50 to 80 mm3, the mice were randomized to receive either solvent control (vehicle) or RSL3 (10 mg/kg, intraperitoneally [IP]), every 2 days) treatment for 3 weeks. For combination therapy, female NOD SCID mice were subcutaneously injected with 1 × 107 NCI-H929 cells in 200 μL serum-free RPMI-1640 medium to establish a human MM xenograft model. Once the average volume of established tumors reached ∼50 to 80 mm3, the mice were randomized to either receive solvent control, BTZ (0.5 mg/kg, IP, biweekly), RSL3 (5 mg/kg, IP, every 2 days), or BTZ plus RSL3 treatment for 3 weeks. Body weights and tumor volumes of mice were recorded every 3 days. Tumor volumes were measured using calipers and calculated as , where is the longest diameter and is the shortest diameter. The in vivo combination effect was evaluated by using the coefficient of drug interaction (CDI) formula, , , , and , and a CDI < 0.7 was considered as synergistic effect.29 Mice were euthanized after completing the medication or when the tumor volume reached 2000 mm3. Tumors were dissected, weighed, and subjected to immunohistochemical analysis. Details of the primary and secondary antibodies used for western blotting and immunohischemistry are shown in the supplemental Table 6.
Statistical analysis
All experimental data and illustrative images stem from at least 3 independent experiments. The results are presented as mean ± standard deviation. The half-maximal inhibitory concentration of the treatment was calculated using GraphPad Prism 8.0. Correlation analysis was performed using Spearman correlation analysis in IBM SPSS Statistics 26.0. Statistical analysis was conducted using GraphPad Prism 8.0, employing Student’s t test, one-way analysis of variance with multiple comparison tests, and two-way analysis of variance with multiple comparison tests when necessary, and statistical significance was set at P < .05.
Additional detailed materials and methods are listed in the supplemental Methods.
The study was approved by the Institutional Review Board at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2022-0477-01). All patients provided written informed consent. All animal experiments were performed according to the National Guidelines for Animal Usage in Research (China) and were approved by the Experimental Animal Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology (No.2020-S091).
Results
t(4;14)-positive MM is vulnerable to class II FINs
We have previously shown that ACSL4 affects ferroptosis sensitivity in MM.29 When investigating the mechanism, we observed a dichotomized phenomenon in that there was a significant difference in ACSL4 expression levels between t(4;14)-positive and -negative MM cell lines from the Cancer Cell Line Encyclopedia database (Figure 1A), which prompted us to explore the association between t(4;14) translocation status and ferroptosis in MM. We then screened publicly available drug susceptibility data from the Cancer Therapeutics Response Portal version 2 database using PharmacoDB.30 In Cancer Therapeutics Response Portal version 2, data were available for erastin (class I FINs) and RSL3 (class II FINs). We extracted drug sensitivity data as the percentage of area above the drug dose response curve for all MM cell lines that were evaluated (13 for erastin and 21 for RSL3). When stratified by t(4;14) translocation status, t(4;14)-positive MM cells had higher area above the drug dose-response curve % values for RSL3 but not for erastin when compared with t(4;14)-negative cells, indicating greater drug sensitivity for class II FINs (Figure 1B; supplemental Figure 1A). In addition, we performed gene set enrichment analysis for the differentially expressed genes of MMSET knockout cells using publicly available data sets. The ferroptosis and PUFA synthesis pathways exhibited enrichment in t(4;14)-positive KMS-11 cells in both the GSE24746 and GSE29148 data sets listed in the Gene Expression Omnibus (supplemental Figure 1B; supplemental Table 7).
t(4;14)-positive MM is vulnerable to class II FINs. (A) Relative expression levels of ACSL4 in t(4;14)-positive and t(4;14)-negative MM cell lines from the cancer cell line encyclopedia data set. (B) Sensitivity to RSL3 in t(4;14)-positive or -negative MM cell lines mined from the cancer therapeutics response portal version 2 database. Higher area above the drug dose-response curve % values indicate greater drug sensitivity (unpaired, 2-tailed Wilcoxon rank-sum test comparing t(4;14)-positive and -negative MM cell lines). (C) Relative cell viability of t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with RSL3 at the indicated concentrations for 24 hours. The half-maximal inhibitory concentration (IC50) of the MM cells was calculated using GraphPad 8.0 software and is shown in the right of figure. (D) Violin plots of the relative cell viability in t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with 1 μM RSL3 for 24 hours. (E) Relative cell viability of primary cells from patients with t(4;14)-positive MM (n = 4) and t(4;14)-negative MM (n = 4) treated with 0.5 μM RSL3. (F) Relative cell viability of t(4;14)-positive NCI-H929 and OPM-2 cells pretreated with the apoptosis inhibitor 10 μM Z-VAD-FMK (Z-VAD), the necrosis inhibitor 1 μM necrosulfonamide (NAS), the autophagy inhibitor 500 μM 3-methyladenine (3-MA), or ferroptosis inhibitors including 0.1 μM liproxstatin-1 (Lip-1), 1 μM ferrostatin-1 (Fer-1), and 10 μM deferoxamine mesylate (DFOM) for 2 hours and then treated with 0.2 μM RSL3 for 24 hours. (G-H) Lipid ROS levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (dimethylsulfoxide [DMSO]), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 6 hours. (I) Relative PTGS2 mRNA levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO) and 0.5 μM RSL3 (RSL3) for 24 hours. (J) The relative malondialdehyde (MDA) concentrations of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 24 hours. (K) Electron microscopy images of t(4;14)-positive NCI-H929 and t(4;14)-negative U-266 cells treated with or without 0.5 μM RSL3 for 24 hours. The black arrowhead indicates mitochondria, which show membrane rupture and reduced cristae in t(4;14)-positive MM and relatively intact mitochondrial structure in t(4;14)-negative MM following RSL3 treatment. The levels of shrunken or ruptured mitochondria were analyzed using ImageJ software. The left scale bar: 1 μm. The right scale bar: 0.5 μm. ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
t(4;14)-positive MM is vulnerable to class II FINs. (A) Relative expression levels of ACSL4 in t(4;14)-positive and t(4;14)-negative MM cell lines from the cancer cell line encyclopedia data set. (B) Sensitivity to RSL3 in t(4;14)-positive or -negative MM cell lines mined from the cancer therapeutics response portal version 2 database. Higher area above the drug dose-response curve % values indicate greater drug sensitivity (unpaired, 2-tailed Wilcoxon rank-sum test comparing t(4;14)-positive and -negative MM cell lines). (C) Relative cell viability of t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with RSL3 at the indicated concentrations for 24 hours. The half-maximal inhibitory concentration (IC50) of the MM cells was calculated using GraphPad 8.0 software and is shown in the right of figure. (D) Violin plots of the relative cell viability in t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with 1 μM RSL3 for 24 hours. (E) Relative cell viability of primary cells from patients with t(4;14)-positive MM (n = 4) and t(4;14)-negative MM (n = 4) treated with 0.5 μM RSL3. (F) Relative cell viability of t(4;14)-positive NCI-H929 and OPM-2 cells pretreated with the apoptosis inhibitor 10 μM Z-VAD-FMK (Z-VAD), the necrosis inhibitor 1 μM necrosulfonamide (NAS), the autophagy inhibitor 500 μM 3-methyladenine (3-MA), or ferroptosis inhibitors including 0.1 μM liproxstatin-1 (Lip-1), 1 μM ferrostatin-1 (Fer-1), and 10 μM deferoxamine mesylate (DFOM) for 2 hours and then treated with 0.2 μM RSL3 for 24 hours. (G-H) Lipid ROS levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (dimethylsulfoxide [DMSO]), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 6 hours. (I) Relative PTGS2 mRNA levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO) and 0.5 μM RSL3 (RSL3) for 24 hours. (J) The relative malondialdehyde (MDA) concentrations of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 24 hours. (K) Electron microscopy images of t(4;14)-positive NCI-H929 and t(4;14)-negative U-266 cells treated with or without 0.5 μM RSL3 for 24 hours. The black arrowhead indicates mitochondria, which show membrane rupture and reduced cristae in t(4;14)-positive MM and relatively intact mitochondrial structure in t(4;14)-negative MM following RSL3 treatment. The levels of shrunken or ruptured mitochondria were analyzed using ImageJ software. The left scale bar: 1 μm. The right scale bar: 0.5 μm. ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
To test our hypothesis, we assessed the efficacy of erastin and RSL3 on t(4;14)-positive and -negative MM cells in vitro. Our results showed an almost 6-fold difference in the mean half-maximal inhibitory concentration of RSL3 between the 2 types of MM cells (0.54 μM for the t(4;14)-positive group and 3.21 μM for the t(4;14)-negative group) (Figure 1C). When treating with 1 μM RSL3, there was a significant difference in the cell activity based on the t(4;14) status (Figure 1D). However, erastin had no detectable toxicity in MM cells, even when used in high concentrations, irrespective of the t(4;14) status (supplemental Figure 1C). To validate the impact of RSL3, we administered another class II FIN, namely ML210, and observed comparable results (supplemental Figure 1D). In addition, we conducted a preclinical evaluation of the therapeutic capacity of RSL3 in primary cells taken from patients with t(4;14)-positive and -negative MM. In agreement with the preceding results, significantly enhanced sensitivity was found in primary MM cells derived from patients with t(4;14)-positive MM (Figure 1E). In addition, bone marrow mononuclear cells and peripheral blood mononuclear cells obtained from healthy donors were examined for their sensitivity to RSL3. There was minimal toxicity in the presence of RSL3 in normal cells (supplemental Figure 2A-B).
To confirm the types of cell death, we subsequently treated t(4;14)-positive MM cells with various inhibitors of cell death. Only pretreatment with the ferroptosis inhibitors liproxstatin-1 and ferrostatin-1 or the iron chelator deferoxamine mesylate could partially rescue MM cells from RSL3- or ML210-induced ferroptosis. However, pretreatment with the apoptosis inhibitor Z-VAD-FMK, necrosis inhibitor necrosulfonamide, or autophagy inhibitor 3-methyladenine did not have the same effect (Figure 1F; supplemental Figure 3A-B). The cellular total reactive oxygen species (ROS) levels increased significantly in t(4;14)-positive MM cells when treated with class II FINs (supplemental Figure 4A). This increase was primarily caused by the elevation in cellular lipid ROS levels (Figure 1G-H; supplemental Figure 4B). Furthermore, the expression of the ferroptosis marker gene PTGS2 messenger RNA (mRNA) (Figure 1I; supplemental Figure 5A) and the accumulation of the lipid peroxidation product malondialdehyde (Figure 1J; supplemental Figure 5B) were significantly higher in class II FIN-treated t(4;14)-positive MM cells. Electron microscopy revealed morphologic changes specific to ferroptosis, such as decreased or absent mitochondrial cristae and ruptured outer mitochondrial membrane,30,31 in t(4;14)-positive MM cells (Figure 1K). Mitochondrial depolarization, assessed using JC-1 staining, was also detected in t(4;14)-positive MM cells (supplemental Figure 5C). However, none of these changes were observed in t(4;14)-negative MM cells at the same concentration of class II FINs (Figure 1G-K; supplemental Figures 4-5). These findings suggest that MM cells that are chromosomal t(4;14) positive, but not those that are t(4;14) negative, are susceptible to class II FINs.
Knockdown of MMSET in t(4;14)-positive MM cells produces resistance to ferroptosis
To determine whether the overexpressed MMSET was critical for ferroptosis sensitivity in t(4;14)-positive MM cells, we established stable NCI-H929 and OPM-2 cell lines that expressed (shRNA) that targeted MMSET or control scramble shRNA as a negative control (Figure 2A). Although a decrease of 10% to 20% in cell activity was observed in MMSET knockdowns when compared with the control, knockdown of MMSET led to a significant resistance to class II FINs in t(4;14)-positive MM cells (Figure 2B-C; supplemental Figure 6A-B), and propidium iodide staining analysis confirmed the results (supplemental Figure 6C-D). This finding was further supported by pretreatment with multiple cell death inhibitors (Figure 2C; supplemental Figure 6B). The PTGS2 mRNA (Figure 2D; supplemental Figure 7A) and malondialdehyde (Figure 2E; supplemental Figure 7B) levels were consistently markedly lower in the MMSET-knockdown group than in the control after class II FINs treatment. There was a substantial reduction in lipid ROS levels in MMSET knockdowns with class II FIN treatment (Figure 2F; supplemental Figure 7C). Electron microscopy showed a reduction in the mitochondrial alterations, such as crista disappearance and outer membrane rupture,16,31 which was accompanied by a decrease in mitochondrial depolarization levels (Figure 2G; supplemental Figure 7D-G), signifying a decrease in ferroptosis levels in the MMSET-knockdown group when compared with the control group. In addition, we constructed MMSET knockdowns and conducted add-back experiments in t(4;14)-positive OPM-2 cells to validate the role of MMSET on ferroptosis sensitization (supplemental Figure 8A). We observed that MM cells after MMSET knockdown were resistant to ferroptosis, whereas the sensitivity to class II FINs was significantly elevated when MMSET knockdown was reversed (supplemental Figure 8B-C).
Knockdown of MMSET in t(4;14)-positive MM causes resistance to ferroptosis. (A) Knockdown efficiency was analyzed by western blotting in t(4;14)-positive NCI-H929 and OPM-2 cells transfected with shRNAs against MMSET (shMMSET) and scramble shRNA as the negative control (shNC) for 48 hours. GAPDH was used as the endogenous control. (B) Showing the relative cell viability of NCI-H929 and OPM-2 cells with or without MMSET knockdown treated with RSL3 at indicated concentrations for 24 hours. The IC50 analysis was conducted using GraphPad software 8.0. (C) Relative cell death analysis of NCI-H929 and OPM-2 cells with or without MMSET knockdown that were pretreated with the indicated cell death inhibitors for 2 hours and then treated by RSL3 (0.5 μM for NCI-H929 and 0.2 μM for OPM-2) for 24 hour. (D-E) The relative PTGS2 mRNA levels (D) or MDA concentrations (E) of NCI-H929 and OPM-2 cells with or without MMSET knockdown with RSL3 treatment (0.5 μM for NCI-H929 and 0.2 μM for OPM-2) for 24 hours. (F) Lipid ROS levels of NCI-H929 and OPM-2 cells with or without MMSET knockdown, treated with RSL3 (0.5 μM for NCI-H929 and 0.2 μM for OPM-2) for 6 hours in the presence or absence of Fer-1. (G) Electron microscopy images of of NCI-H929 cells with or without MMSET knockdown treated with 0.5 μM RSL3 or DMSO. The black arrowhead indicates mitochondria with membrane rupture and reduced cristae in the group of shNC and relatively intact mitochondrial structures in the shMMSET group with RSL3 treatment. The levels of shrunken mitochondria were analyzed using ImageJ software. The left scale bar: 1 μm. The right scale bar: 0.5 μm. (H-I) Relative cell viability of NCI-H929 and OPM-2 cells with MMSET knockdown (H) or t(4;14)-negative U-266 cells (I) treated with RSL3 at the indicated concentrations for 24 hours in the presence of PUFAs (5 μM arachidonic acid or adrenic acid) or MUFAs (oleic acid) and followed by treatment with the ferroptosis inhibitor Fer-1 or not. ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
Knockdown of MMSET in t(4;14)-positive MM causes resistance to ferroptosis. (A) Knockdown efficiency was analyzed by western blotting in t(4;14)-positive NCI-H929 and OPM-2 cells transfected with shRNAs against MMSET (shMMSET) and scramble shRNA as the negative control (shNC) for 48 hours. GAPDH was used as the endogenous control. (B) Showing the relative cell viability of NCI-H929 and OPM-2 cells with or without MMSET knockdown treated with RSL3 at indicated concentrations for 24 hours. The IC50 analysis was conducted using GraphPad software 8.0. (C) Relative cell death analysis of NCI-H929 and OPM-2 cells with or without MMSET knockdown that were pretreated with the indicated cell death inhibitors for 2 hours and then treated by RSL3 (0.5 μM for NCI-H929 and 0.2 μM for OPM-2) for 24 hour. (D-E) The relative PTGS2 mRNA levels (D) or MDA concentrations (E) of NCI-H929 and OPM-2 cells with or without MMSET knockdown with RSL3 treatment (0.5 μM for NCI-H929 and 0.2 μM for OPM-2) for 24 hours. (F) Lipid ROS levels of NCI-H929 and OPM-2 cells with or without MMSET knockdown, treated with RSL3 (0.5 μM for NCI-H929 and 0.2 μM for OPM-2) for 6 hours in the presence or absence of Fer-1. (G) Electron microscopy images of of NCI-H929 cells with or without MMSET knockdown treated with 0.5 μM RSL3 or DMSO. The black arrowhead indicates mitochondria with membrane rupture and reduced cristae in the group of shNC and relatively intact mitochondrial structures in the shMMSET group with RSL3 treatment. The levels of shrunken mitochondria were analyzed using ImageJ software. The left scale bar: 1 μm. The right scale bar: 0.5 μm. (H-I) Relative cell viability of NCI-H929 and OPM-2 cells with MMSET knockdown (H) or t(4;14)-negative U-266 cells (I) treated with RSL3 at the indicated concentrations for 24 hours in the presence of PUFAs (5 μM arachidonic acid or adrenic acid) or MUFAs (oleic acid) and followed by treatment with the ferroptosis inhibitor Fer-1 or not. ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
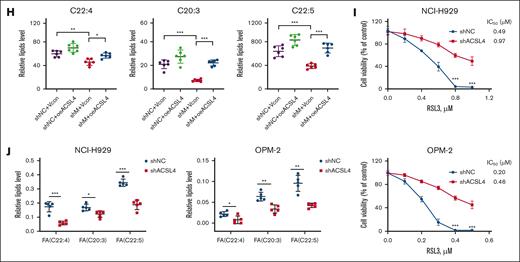
Because it is likely that MMSET is involved in PUFAs synthesis (supplemental Figure 1B), we investigated whether PUFAs have an impact on MMSET-dependent ferroptosis sensitivity. The results indicated that adding low concentrations of PUFAs, such as arachidonic acid and adrenic acid, significantly increased the sensitivity of MMSET-knockdown t(4;14)-positive (Figure 2H; supplemental Figure 8D) or t(4;14)-negative MM cells to ferroptosis (Figure 2I; supplemental Figure 8E-F). In contrast, monounsaturated fatty acids, like oleic acid, did not have a notable effect. In addition, treatment with the Fer-1 ferroptosis inhibitor significantly rescued cell death induced by PUFA treatment (Figure 2H-I; supplemental Figure 8D-F). To verify the influence of MMSET on lipid composition, we performed an untargeted metabolomic analysis of the constructed MMSET knockdown and add-back OPM-2 cells. There were several PUFAs, including C22:4, C20:3, and C22:5, that decreased in MMSET knockdown, whereas the PUFA levels were evidently upregulated when MMSET expression was restored in the add-back cells (supplemental Figure 8G). Collectively, our results identified that the MMSET gene was crucial for promoting MM ferroptosis sensitivity and demonstrated the important role of PUFAs in regulating MMSET-induced sensitivity to ferroptosis.
MMSET enhances ferroptosis susceptibility by regulating ACSL4
There are 3 primary processes that impact ferroptosis, specifically ferritin metabolism, lipid metabolism, and glutathione (GSH) metabolism (supplemental Figure 9A). To uncover the potential mechanism of MMSET-related ferroptosis sensitivity, we selected genes that are involved in ferroptosis as candidates. The Cancer Cell Line Encyclopedia database was used to study the correlation between ACSL4, solute carrier family 7 member 11, dihydroorotate dehydrogenase, ferroptosis suppressor protein 1 (FSP1), GPX4, and MMSET. Among these genes, only the ACSL4 mRNA levels were found to be higher in t(4;14)-positive than in t(4;14)-negative MM cell lines (Figure 1A). ACSL4 mRNA levels were also positively correlated with MMSET mRNA levels (r = 0.592; P = .006) (n = 20) (supplemental Figure 9B). In the public data set GSE57863, the expression levels of ACSL4 were significantly decreased in MMSET knockout t(4;14)-positive KMS-11 cells (supplemental Figure 9C). Furthermore, the expression levels of MMSET were found to be correlated with ACSL4 expression levels in patients with MM in the public data set GSE6691 (r = 0.594; P = .042) (n = 12) (supplemental Figure 9D). Similarly, comparable results were observed in samples taken from patients with MM at our hospital (Figure 3A-B).
Notably, the expression of ACSL4 significantly decreased at both the mRNA (supplemental Figure 9E) and protein levels (Figure 3C) in NCI-H929 and OPM-2 cells after MMSET knockdown when compared with the control. In addition, ACSL4 expression levels were decreased by MMSET knockdown and reversed after MMSET was added back in OPM-2 cells (supplemental Figure 9F). Because MMSET is a histone methylase that regulates the opening of the genome transcriptionally, our investigation focused on whether it had transcriptional regulation of ACSL4 expression. We conducted a luciferase reporter assay, which indicated that ACSL4 promoter activity was significantly decreased following MMSET knockdown in NCI-H929 and OPM-2 cells (Figure 3D). The subsequent ChIP-qPCR assay results confirmed that MMSET was enriched at the promoter regions of ACSL4, and ACSL4 levels were reduced by MMSET knockdown in OPM-2 cells (Figure 3E). Therefore, our results suggest that MMSET modulates ACSL4 transcriptionally by binding to its promoter region.
To investigate whether MMSET enhances the sensitivity of MM cells to ferroptosis through ACSL4, we conducted experiments in NCI-H929 and OPM-2 cells with MMSET downregulation and ACSL4 overexpression (supplemental Figure 9G). The results suggest that MM cells with MMSET knockdown developed resistance to ferroptosis, whereas the upregulation of ACSL4 re-sensitized MM cells to ferroptosis (Figure 3F; supplemental Figure 9H). Consistent results were also observed in the lipid ROS assay (Figure 3G; supplemental Figure 9I). To investigate the effect of the MMSET/ACSL4 axis on fatty acid levels, we performed targeted metabolomic analysis in a co-transfected system of OPM-2 cells, which showed a significant decrease in several PUFAs, including C22:4, C20:3, and C22:5, following MMSET knockdown. In contrast, ACSL4 overexpression led to a substantial increase in these PUFAs (Figure 3H).
In addition, ACSL4-knockdown NCI-H929 and OPM-2 cells were constructed (supplemental Figure 10A). Further results indicated a significant reduction in ferroptosis sensitivity as a consequence of ACSL4 knockdown in both NCI-H929 and OPM-2 cells (Figure 3I; supplemental Figure 10B). Pretreatment with the ACSL4 inhibitor rosiglitazone also significantly shielded MM cells from ferroptosis triggered by class II FINs (supplemental Figure 10C-D). Subsequently, we performed a lipidomic analysis on NCI-H929 and OPM-2 cells with or without ACSL4 knockdown and discovered that multiple PUFAs (C22:4, C20:3, C22:5), primarily adrenic acid (C22:5), were considerably reduced by ACSL4 knockdown (Figure 3J). Conversely, the sensitivity of MM cells to ferroptosis following ACSL4 knockdown was significantly restored by supplementing them with low doses of PUFAs (supplemental Figure 10E-F). Furthermore, we observed that PUFA levels did not influence the GPX4 protein levels in t(4;14)-positive MM cells (supplemental Figure 10G-H). Collectively, our findings demonstrate that the MMSET/ACSL4/PUFAs axis has a crucial role in promoting t(4;14)-positive MM cell sensitivity to ferroptosis.
MMSET promotes t(4;14)-positive MM ferroptosis sensitivity via ACSL4 in vivo
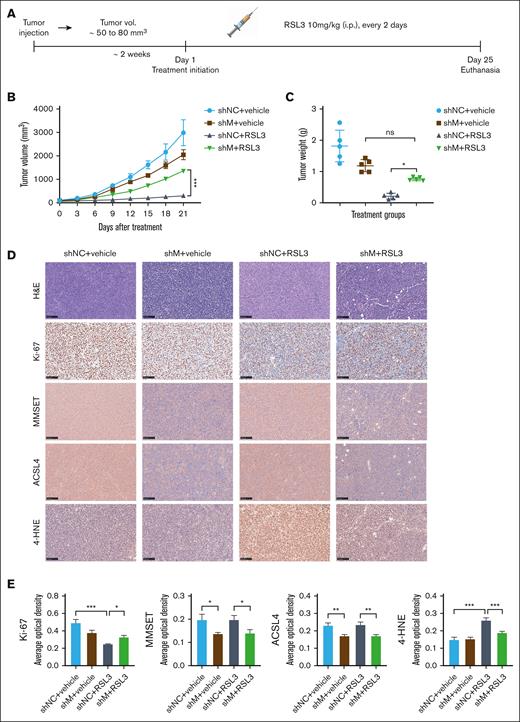
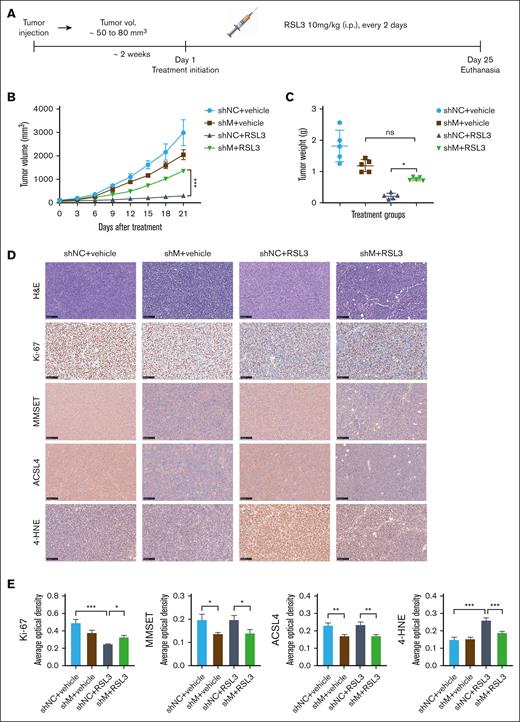
We examined the impact of the MMSET and ACSL4 axis on the sensitivity of t(4;14)-positive MM to ferroptosis in vivo. To establish a xenograft model, we injected NCI-H929 cells, with or without MMSET knockdown, subcutaneously into NOD SCID mice. Two weeks after xenotransplantation, the mice were randomized and treated with either solvent control or RSL3 (10 mg/kg) IP for 3 weeks (Figure 4A). According to our in vitro data, treatment with RSL3 led to significant growth inhibition of NCI-H929 xenograft tumors, whereas MMSET knockdown seemed to confer resistance to RSL3-induced ferroptosis (Figure 4B-C; supplemental Figure 11A). The expression levels of the proliferation marker ki-67, the oxidative stress indicator 4-hydroxynonenal (4-HNE), MMSET, and ACSL4 in xenograft tumors were quantitatively analyzed using Image J, which provided additional evidence that the MMSET and ACSL4 axis promoted sensitivity to ferroptosis in t(4;14)-positive MM (Figure 4D-E). Moreover, during the treatment period, the mice did not experience any significant adverse effects, such as sudden mortality or notable weight loss (supplemental Figure 11B), indicating that RSL3 was well tolerated in vivo.
MMSET promotes t(4;14)-positive MM ferroptosis sensitivity via ACSL4 in vivo. (A) A schematic diagram of the xenograft model. NCI-H929 cells with MMSET knockdown (shM) or without knockdown (shNC) were injected subcutaneously into NOD SCID mice to establish a xenograft model. When tumor volumes (vol.) reached ∼50 to 80 mm3, the mice were randomized to receive solvent control (vehicle) or RSL3 (10 mg/kg) IP for 3 weeks (n = 5). (B) Growth curves of the xenograft tumors. Tumor volumes of each group were calculated every 3 days. (C) Weights of the xenograft tumors. Tumor weights of each group were analyzed on the day of mice euthanasia. (D) Immunohistochemical analysis of ki-67, MMSET, ACSL4, and 4-HNE protein levels in tumor tissues from the 4 groups of mice with the indicated treatment. Scale bar: 100 μm. (E) Expression levels of targeted proteins in mice of the treatment groups were quantitatively analyzed using Image J. ns, not significant; P > .05, ∗ P < .05, ∗∗ P < .01, and ∗∗∗ P < .001.
MMSET promotes t(4;14)-positive MM ferroptosis sensitivity via ACSL4 in vivo. (A) A schematic diagram of the xenograft model. NCI-H929 cells with MMSET knockdown (shM) or without knockdown (shNC) were injected subcutaneously into NOD SCID mice to establish a xenograft model. When tumor volumes (vol.) reached ∼50 to 80 mm3, the mice were randomized to receive solvent control (vehicle) or RSL3 (10 mg/kg) IP for 3 weeks (n = 5). (B) Growth curves of the xenograft tumors. Tumor volumes of each group were calculated every 3 days. (C) Weights of the xenograft tumors. Tumor weights of each group were analyzed on the day of mice euthanasia. (D) Immunohistochemical analysis of ki-67, MMSET, ACSL4, and 4-HNE protein levels in tumor tissues from the 4 groups of mice with the indicated treatment. Scale bar: 100 μm. (E) Expression levels of targeted proteins in mice of the treatment groups were quantitatively analyzed using Image J. ns, not significant; P > .05, ∗ P < .05, ∗∗ P < .01, and ∗∗∗ P < .001.
RSL3 and BTZ synergistically induce t(4;14)-positive MM cell death by inhibiting xCT expression and attenuating cellular GSH levels
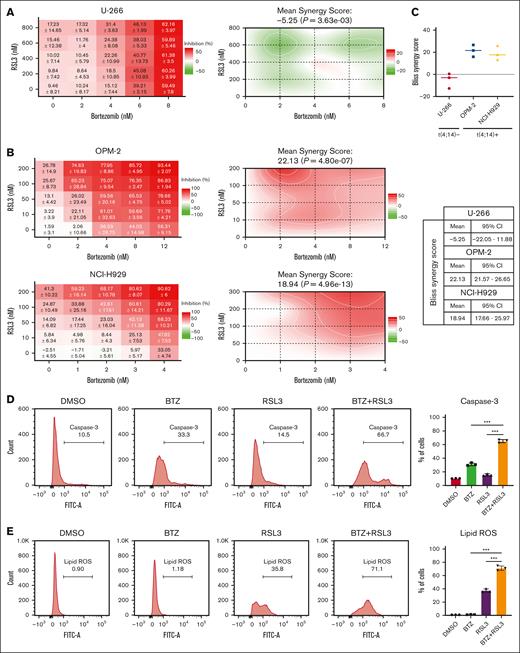
BTZ has become a cornerstone drug in the treatment of MM since its clinical approval. However, BTZ-based combination chemotherapy regimens show limited effect in patients with t(4;14)-positive MM.32,33 Based on our findings, we hypothesized that combining FINs could improve the sensitivity of MM cells to BTZ. Combining dose-response data and Bliss synergy scores analyzed by SynergyFinder 2.0, we evaluated the effect of the combination.34 A strong, significant synergistic effect was observed in t(4;14)-positive NCI-H929 and OPM2 cells, whereas only an additive effect was observed in t(4;14)-negative U-266 cells (Figure 5A-C). Furthermore, we tested the combination of RSL3 and BTZ in MMSET knockdown and add-back OPM-2 cells. There was only an additive effect observed in MMSET knockdown cells, whereas an apparent synergy effect was observed in MMSET add-back cells (supplemental Figure 12A-B), indicating that MMSET primed the synergistic effect by regulating the sensitivity of ferroptosis. To elucidate the cell death pathways involved in this synergistic effect, we conducted assays for activated caspase-3 and lipid ROS levels. The results indicated that the combination therapy did not only enhance apoptosis but also enhanced ferroptosis in t(4;14)-positive MM cells in contrast with the single agent, revealing the presence of mixed cell death pathways (Figure 5D-E; supplemental Figure 12C-D).
RSL3 and BTZ synergistically induce t(4;14)-positive but not t(4;14)-negative MM cell death. (A-B) Dose-response matrices of t(4;14)-negative U-266 (A) and t(4;14)-positive NCI-H929 and OPM-2 cells (B) treated with a range concentration of BTZ and/or RSL3 for 24 hours. (C) Bliss synergy scores of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells were plotted for 3 independent experiments. The mean Bliss synergy score and 95% confidence interval (CI) for each cell line are shown in the right table. (D-E) Caspase-3 (D) or lipid ROS (E) levels of t(4;14)-positive NCI-H929 cells treated with 1 nM BTZ and/or 0.2 μM RSL3 for 24 hours analyzed by flow cytometry. ∗∗∗ P < .001.
RSL3 and BTZ synergistically induce t(4;14)-positive but not t(4;14)-negative MM cell death. (A-B) Dose-response matrices of t(4;14)-negative U-266 (A) and t(4;14)-positive NCI-H929 and OPM-2 cells (B) treated with a range concentration of BTZ and/or RSL3 for 24 hours. (C) Bliss synergy scores of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells were plotted for 3 independent experiments. The mean Bliss synergy score and 95% confidence interval (CI) for each cell line are shown in the right table. (D-E) Caspase-3 (D) or lipid ROS (E) levels of t(4;14)-positive NCI-H929 cells treated with 1 nM BTZ and/or 0.2 μM RSL3 for 24 hours analyzed by flow cytometry. ∗∗∗ P < .001.
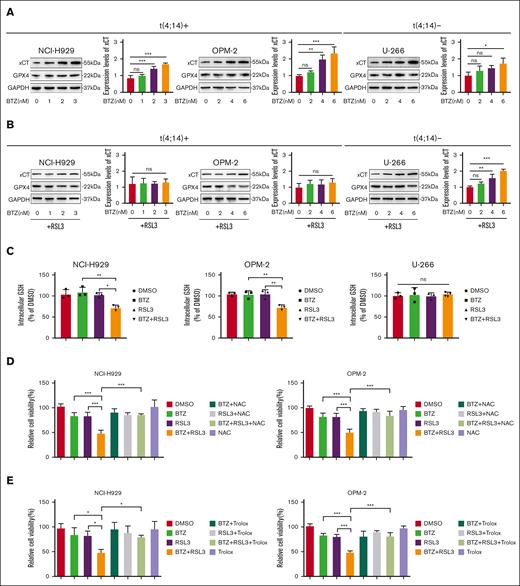
We then investigated the molecular basis that underlie the synergistic effect of the combination therapy by examining the expression levels of ferroptosis-related proteins. Our findings demonstrated that BTZ treatment led to a gradual upregulation in the expression of the ferroptosis-associated protein xCT in MM, independent of t(4;14) status, whereas there was no change in the expression of GPX4 (Figure 6A). Intriguingly, the concurrent administration of RSL3 eliminated the increase in xCT induced by BTZ in t(4;14)-positive MM cells, but not in t(4;14)-negative MM cells (Figure 6B). Because xCT regulates the transportation of cysteine to promote GSH synthesis, we investigated the levels of cellular reduced GSH in MM that was treated with BTZ or RSL3 or both. Our results suggested that t(4;14)-positive MM cells showed lower levels of reduced GSH with combination treatment, although no significant changes were noted in t(4;14)-negative MM cells (Figure 6C). Further research revealed that the combination therapy–induced cell death of t(4;14)-positive MM was prevented by N-acetylcysteine (a precursor of GSH) or trolox (a scavenger of lipid ROS) (Figure 6D-E). These findings were supported by flow cytometry data on the intracellular lipid ROS and activated caspase 3 levels (supplemental Figure 13A-D). Overall, our results suggest that RSL3 hinders the upregulation of xCT caused by BTZ monotherapy in t(4;14)-positive MM and causes a reduction in GSH levels, thereby leading to a synergistic antitumor effect.
RSL3 potentiates BTZ activity by abrogating xCT upregulation upon BTZ treatment and attenuating GSH levels in t(4;14)-positive MM. (A-B) Western blotting analysis of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells with BTZ single treatment (A) or RSL3 combination treatment (B) for 48 hours. GAPDH was used as the internal control, and the intensities were measured using ImageJ. (C) Cellular reduced glutathione levels were detected in t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with BTZ or RSL3 alone or together for 24 hours. (D-E) Relative cell viability were detected in t(4;14)-positive NCI-H929 and OPM-2 cells treated with BTZ or RSL3 alone or together for 24 hours in the presence of NAC (a GSH precursor) (D) or trolox (a lipid ROS scavenger) (E). ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
RSL3 potentiates BTZ activity by abrogating xCT upregulation upon BTZ treatment and attenuating GSH levels in t(4;14)-positive MM. (A-B) Western blotting analysis of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells with BTZ single treatment (A) or RSL3 combination treatment (B) for 48 hours. GAPDH was used as the internal control, and the intensities were measured using ImageJ. (C) Cellular reduced glutathione levels were detected in t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with BTZ or RSL3 alone or together for 24 hours. (D-E) Relative cell viability were detected in t(4;14)-positive NCI-H929 and OPM-2 cells treated with BTZ or RSL3 alone or together for 24 hours in the presence of NAC (a GSH precursor) (D) or trolox (a lipid ROS scavenger) (E). ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
RSL3 and BTZ synergistically inhibit t(4;14)-positive tumor growth in vivo
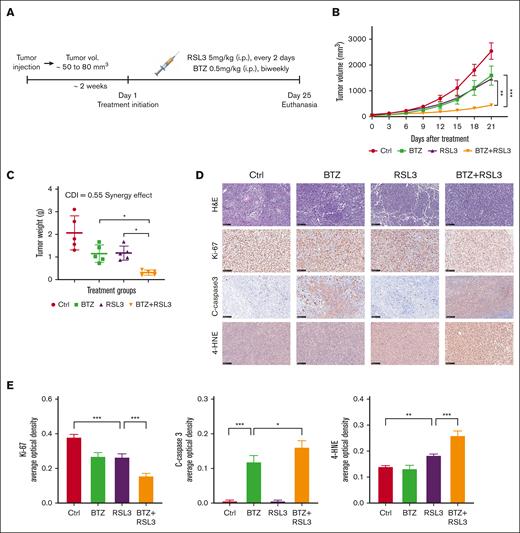
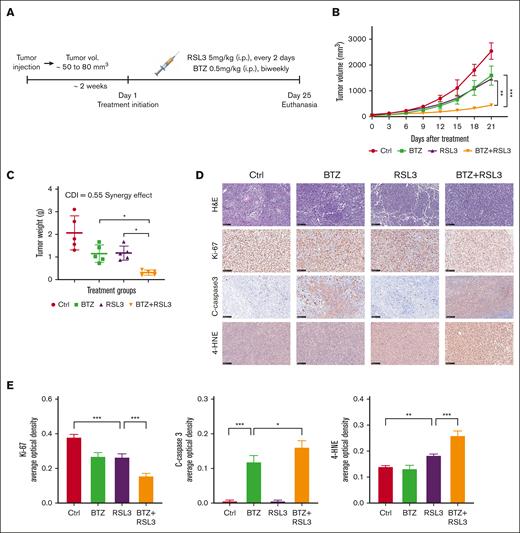
To investigate the therapeutic impact of combining RSL3 and BTZ in vivo, we developed a t(4;14)-positive MM xenograft model using NOD SCID mice. The mice were randomly assigned to treatment groups and received either the solvent control, RSL3 (5 mg/kg, every 2 days), BTZ (0.5 mg/kg, biweekly), or RSL3 in combination with BTZ through IP injections for 3 weeks (Figure 7A). Based on our in vitro findings, treatment with BTZ or RSL3 alone led to a partial delay in tumor growth of NCI-H929 xenografts, whereas the combined treatment of RSL3 and BTZ significantly inhibited tumor growth and induced mixed cell death pathways, including apoptosis and ferroptosis (Figure 7B-D; supplemental Figure 14A). The calculated CDI demonstrated a significant synergistic effect of 0.55 in vivo based on mice tumor weights (Figure 7C). The combination therapy induced increased apoptosis and ferroptosis levels, which was confirmed by quantitative analysis of immunohistochemistry (Figure 7D-E). In addition, there were no significant unfavorable outcomes during treatment (supplemental Figure 14B), suggesting that there was no significant additional toxicity with combination therapy.
RSL3 and BTZ synergistically inhibit t(4;14)-positive tumor growth in vivo. (A) Schematic diagram of the xenograft model. NCI-H929 cells were injected subcutaneously into NOD SCID mice to establish a xenograft model. When tumor (vol.) reached ∼50 to 80 mm3, the mice were randomized to receive eith solvent control (vehicle), BTZ (0.5 mg/kg, biweekly), RSL3 (5 mg/kg, every 2 days), or BTZ plus RSL3 treatment IP for 3 weeks (n = 5). (B) Growth curves of the xenograft tumors. Tumor volumes of each group were assessed every 3 days. (C) Weights of xenograft tumors. Tumor weights of each group were evaluated on the day of mice euthanasia. The CDI was calculated to test for synergy using mean tumor weight measurements. The CDI value of 0.55 indicates synergy (defined as CDI < 1, with CDI < 0.7 indicating a significant synergistic effect). (D) Immunohistochemical analysis of ki-67, cleaved-caspase3, and 4-HNE protein levels in tumor tissues from NCI-H929-xenografted mice treated with solvent control (Ctrl), BTZ, RSL3, or BTZ plus RSL3. Scale bar: 100 μm. (E) Expression levels of targeted proteins in mice of treatment groups were quantitatively analyzed using Image J. ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
RSL3 and BTZ synergistically inhibit t(4;14)-positive tumor growth in vivo. (A) Schematic diagram of the xenograft model. NCI-H929 cells were injected subcutaneously into NOD SCID mice to establish a xenograft model. When tumor (vol.) reached ∼50 to 80 mm3, the mice were randomized to receive eith solvent control (vehicle), BTZ (0.5 mg/kg, biweekly), RSL3 (5 mg/kg, every 2 days), or BTZ plus RSL3 treatment IP for 3 weeks (n = 5). (B) Growth curves of the xenograft tumors. Tumor volumes of each group were assessed every 3 days. (C) Weights of xenograft tumors. Tumor weights of each group were evaluated on the day of mice euthanasia. The CDI was calculated to test for synergy using mean tumor weight measurements. The CDI value of 0.55 indicates synergy (defined as CDI < 1, with CDI < 0.7 indicating a significant synergistic effect). (D) Immunohistochemical analysis of ki-67, cleaved-caspase3, and 4-HNE protein levels in tumor tissues from NCI-H929-xenografted mice treated with solvent control (Ctrl), BTZ, RSL3, or BTZ plus RSL3. Scale bar: 100 μm. (E) Expression levels of targeted proteins in mice of treatment groups were quantitatively analyzed using Image J. ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.
Discussion
Bioinformatics research based on publicly available gene expression databases has highlighted the connection between ferroptosis-related genes and cytogenetic abnormalities in MM,35 however, the fundamental correlation requires further elucidation. This study demonstrated that the susceptibility of t(4;14)-positive MM to ferroptosis and showed that class II FINs may be a promising new approach to treating patients with t(4;14)-positive MM, especially in combination with BTZ. Importantly, our findings offer a fresh therapeutic approach. Although some tumor cells may be highly aggressive or resistant to drugs, targeting their metabolic vulnerabilities presents potential avenues for effective treatment.36 Indeed, FINs are among the potential agents that target metabolic vulnerability by inducing ferroptosis.37 It seems paradoxical that MMSET promotes the malignant progression of t(4;14)-positive MM and sensitizes it to ferroptosis. Notably, the robust reducing system in MM effectively balances the heightened levels of ROS caused by abnormal immunoglobulin secretion,38 making it difficult for surviving MM cells, even with MMSET overexpression, to undergo ferroptosis spontaneously. Our findings show that only class II FINs are required to disturb the redox equilibrium, and MMSET enhances the vulnerability of t(4;14)-positive MM to ferroptosis under these conditions. The antiferroptosis pathway, in parallel with the GPX4 antioxidant pathway, also includes ferroptosis suppressor protein 1 (FSP1)-Coenzyme Q10. However, in our previous study, low expression of FSP1 in t(4;14)-positive MM was observed.28 These results reflect the dependence of the antioxidant pathway of t(4;14)-positive cells on GPX4 activity, but not on its basic expression levels, and therefore the susceptibility to the inhibition of GPX4 activity.
ACSL4 was identified as the target gene of MMSET that enhances sensitivity to ferroptosis in t(4;14)-positive MM. Our study is consistent with the recent report that ACSL4 plays a pivotal role in ferroptosis triggered by class II but not class I FINs.25 Nevertheless, our findings suggest a notable influence of the MMSET/ACSL4 axis on PUFA biosynthesis, which renders MM cells vulnerable to ferroptosis and deviates from the previous study that suggested that ACSL4-mediated phospholipid synthesis promotes ferroptosis.26 The recent analysis provides evidence that activated ACSL4 catalyzes the biosynthesis of lipids containing PUFAs, which leads to the accumulation of lipid peroxidation products.27 This signifies the multifaceted role of ACSL4 in promoting ferroptosis. In our previous study, ACSL4 functioned as an oncogene that influenced lipid metabolism in MM,28 breast cancer, and hepatocellular carcinoma.39,40 The augmented expression of fatty acid transporters in MM and cancer-associated adipocytes in the bone marrow turns on a malignant state in plasma cells and activates the cellular lipolysis pathway while enhancing the susceptibility to ferroptosis through the synthesis of PUFAs.41,42 PUFAs are unstable fatty acids that are known to induce ferroptosis when present at high concentrations and increase the effectiveness of BTZ in MM.43 Therefore, the regulation of fatty acids by the MMSET/ACSL4 axis seems to have a dual function, contingent on the level of fatty acids. Further investigation is required to comprehend the involvement of the MMSET/ACSL4 axis in fatty acid metabolism and its potential role in the pathogenesis of MM.
Our findings demonstrate that the combined use of RSL3 and BTZ induces mixed cell death, including both apoptosis and ferroptosis, in t(4;14)-positive MM. It should be noted that kinase profiling of the anticancer agent reveals significant overlap with the ferroptosis and apoptosis kinome activities in MM,44 precisely elucidating the features of co-existing ferroptotic and apoptotic cell death. The upregulation of ferroptosis-associated xCT may be a compensatory detoxification mechanism that antagonizes the cellular ROS levels induced by BTZ independent of chromosomal t(4;14) status in MM. In addition, RSL3 enhances BTZ activity by attenuating cellular GSH levels in t(4;14)-positive MM and abrogating xCT upregulation caused by BTZ treatment. In our experiments, we observed a synergistic effect between BTZ and RSL3 in t(4;14)-negative U-266 cells when the concentration of RSL3 was increased, but we decided to exclude this result because of the potential toxicity of high drug concentrations. The decrease of xCT-based GSH levels can enhance the potency of both BTZ-induced apoptosis45 and FIN-triggered ferroptosis, leading to a synergistic effect in t(4;14)-positive MM. However, further exploration is required to determine why RSL3 does not decrease GSH levels when administered alone but instead weakens them when combined with BTZ. It should be noted that class II FINs could enhance the efficacy of lenalidomide in MM.46 In addition, in our expanded drug combination experiments, RSL3 enhanced the sensitivity of other antimyeloma drugs, such as melphalan and doxorubicin (supplemental Figure 15A-D), which indicates that combination therapy based on FINs shows great application prospects. By comparing the synergistic Bliss scores, the combination of BTZ with RSL3 showed the most significant synergistic effect, primarily because of the significant inhibition of increased xCT induced by BTZ treatment. While treatment of MM cells with the class I FIN, erastin, only minimally affected the cells, indicating a strong compensatory response within the MM cells against the inhibitory effects of cystine-glutamate transporter blockade, supporting the complex mechanisms involved in this biological process. Overexpression of xCT on the cell membrane is another potential mechanism for erastin resistance.47 Despite its insensitivity, erastin and doxorubicin demonstrate synergetic antitumor effects in MM.48 The combination of BTZ and sulfasalazine, another class I FIN, also proved to be highly effective in blocking exosome-based communication between the bone marrow stromal cells and MM cells.49
Our research indicates the need for further exploration of the impact of class II FINs in treating MM in a clinical environment. The in vivo application of RSL3 has been under scrutiny by the scientific community because of its suboptimal pharmacokinetics. Despite this, we maintain a positive outlook on the potential use of FINs. One promising development is the rapid progress in nano-delivery systems that could facilitate FIN delivery.50 In addition, several US Food and Drug Administration–approved agents, including fingolimod ,51 artemisinin,52 and sulfasalazine,20 have shown the ability to induce MM ferroptosis and the potential to overcome BTZ resistance.49 Interestingly, statins is reported to be an actionable treatment for t(4;14)-positive MM by inhibiting the mevalonate pathway.53 It might be possible to test whether ferroptosis inhibitors can rescue cell death in t(4;14)-positive MM cells by statins. Collectively, preclinical models are necessary to further evaluate the efficacy of these agents as t(4;14)-positive MM therapies. These results may have immediate clinical trial applications if successful.
Acknowledgments
The authors thank BIOTREE Biotech Co Ltd. (Shanghai, China) for the lipidomic analysis. The ChIP assay and qPCR were performed with help from Wuhan IGENEBOOK Biotechnology Co., Ltd. (Wuhan, China).
This work was supported by the National Natural Science Foundation of China (grant numbers 81974007 and 82270214 to C.S. and grant No. 82070228 to B.Z.).
Authorship
Contribution: J.Z. and F.F. designed the research; J.Z. and Y.L. performed the research and collected data; L.Z., C.M., and Q.L. provided technical support; H.Y., F.Z., and J.L. analyzed and interpreted the data; A.X. and J.X. performed statistical analyses; J.Z. and B.Z. wrote the manuscript; Y.H. and C.S. revised the manuscript and funded the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Chunyan Sun, Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Dadao, Wuhan 430022, China; email: suncy0618@163.com; Yu Hu, Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Dadao, Wuhan 430022, China; email: dr_huyu@126.com; and Bo Zhang, Department of Hematology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277 Jiefang Dadao, Wuhan 430022, China; email: zhangbo19871987@126.com.
References
Author notes
J.Z. and Y.L. contributed equally to this work.
Public data sets related to this article are available in the Gene Expression Omnibus (accession numbers GSE24746, GSE29148, GSE57863, and GSE6691) (https://www.ncbi.nlm.nih.gov/geo/). Our metabolomics data have been deposited in MetaboLights database (accession numbers MTBLS10427 and MTBLS10428) (https://www.ebi.ac.uk/metabolights/).
The full-text version of this article contains a data supplement.

![t(4;14)-positive MM is vulnerable to class II FINs. (A) Relative expression levels of ACSL4 in t(4;14)-positive and t(4;14)-negative MM cell lines from the cancer cell line encyclopedia data set. (B) Sensitivity to RSL3 in t(4;14)-positive or -negative MM cell lines mined from the cancer therapeutics response portal version 2 database. Higher area above the drug dose-response curve % values indicate greater drug sensitivity (unpaired, 2-tailed Wilcoxon rank-sum test comparing t(4;14)-positive and -negative MM cell lines). (C) Relative cell viability of t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with RSL3 at the indicated concentrations for 24 hours. The half-maximal inhibitory concentration (IC50) of the MM cells was calculated using GraphPad 8.0 software and is shown in the right of figure. (D) Violin plots of the relative cell viability in t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with 1 μM RSL3 for 24 hours. (E) Relative cell viability of primary cells from patients with t(4;14)-positive MM (n = 4) and t(4;14)-negative MM (n = 4) treated with 0.5 μM RSL3. (F) Relative cell viability of t(4;14)-positive NCI-H929 and OPM-2 cells pretreated with the apoptosis inhibitor 10 μM Z-VAD-FMK (Z-VAD), the necrosis inhibitor 1 μM necrosulfonamide (NAS), the autophagy inhibitor 500 μM 3-methyladenine (3-MA), or ferroptosis inhibitors including 0.1 μM liproxstatin-1 (Lip-1), 1 μM ferrostatin-1 (Fer-1), and 10 μM deferoxamine mesylate (DFOM) for 2 hours and then treated with 0.2 μM RSL3 for 24 hours. (G-H) Lipid ROS levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (dimethylsulfoxide [DMSO]), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 6 hours. (I) Relative PTGS2 mRNA levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO) and 0.5 μM RSL3 (RSL3) for 24 hours. (J) The relative malondialdehyde (MDA) concentrations of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 24 hours. (K) Electron microscopy images of t(4;14)-positive NCI-H929 and t(4;14)-negative U-266 cells treated with or without 0.5 μM RSL3 for 24 hours. The black arrowhead indicates mitochondria, which show membrane rupture and reduced cristae in t(4;14)-positive MM and relatively intact mitochondrial structure in t(4;14)-negative MM following RSL3 treatment. The levels of shrunken or ruptured mitochondria were analyzed using ImageJ software. The left scale bar: 1 μm. The right scale bar: 0.5 μm. ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/19/10.1182_bloodadvances.2023010335/2/m_blooda_adv-2023-010335-gr1.jpeg?Expires=1768113402&Signature=ltLDrEqcbJX~NZUR7UyebveStPfi589vLMjYXZHv7ZIXstmsW-QSa0cwd4eLxjiDjmaRhwiyaGbKersyizNv73xa~PhuhqzvjGIok8~80q~wsWxC8XXiNrG4dkUgpSCk7W0MglctcftBa2POqEXG1BipqIywuz-tS2XYvqSMM-fz28AEoIra2ODP8c7g8mjRFntZxmXq3D5yCMLyRVz3R~gj7RIstsOec~WESWZwhncIS56tNJ2HrLCeMQN4ZoOtGxA4Wprze2aT1-8oZZh6bOcpAmGJW1ef1GcEMpkYuXaFR3hTVo1wVUKS~TuKIOGDrvwtq9xzSrext03xx~GObQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![t(4;14)-positive MM is vulnerable to class II FINs. (A) Relative expression levels of ACSL4 in t(4;14)-positive and t(4;14)-negative MM cell lines from the cancer cell line encyclopedia data set. (B) Sensitivity to RSL3 in t(4;14)-positive or -negative MM cell lines mined from the cancer therapeutics response portal version 2 database. Higher area above the drug dose-response curve % values indicate greater drug sensitivity (unpaired, 2-tailed Wilcoxon rank-sum test comparing t(4;14)-positive and -negative MM cell lines). (C) Relative cell viability of t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with RSL3 at the indicated concentrations for 24 hours. The half-maximal inhibitory concentration (IC50) of the MM cells was calculated using GraphPad 8.0 software and is shown in the right of figure. (D) Violin plots of the relative cell viability in t(4;14)-positive NCI-H929, OPM-2, and KMS-11 cell lines and t(4;14)-negative U-266, MM.1S, and RPMI8226 cell lines treated with 1 μM RSL3 for 24 hours. (E) Relative cell viability of primary cells from patients with t(4;14)-positive MM (n = 4) and t(4;14)-negative MM (n = 4) treated with 0.5 μM RSL3. (F) Relative cell viability of t(4;14)-positive NCI-H929 and OPM-2 cells pretreated with the apoptosis inhibitor 10 μM Z-VAD-FMK (Z-VAD), the necrosis inhibitor 1 μM necrosulfonamide (NAS), the autophagy inhibitor 500 μM 3-methyladenine (3-MA), or ferroptosis inhibitors including 0.1 μM liproxstatin-1 (Lip-1), 1 μM ferrostatin-1 (Fer-1), and 10 μM deferoxamine mesylate (DFOM) for 2 hours and then treated with 0.2 μM RSL3 for 24 hours. (G-H) Lipid ROS levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (dimethylsulfoxide [DMSO]), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 6 hours. (I) Relative PTGS2 mRNA levels of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO) and 0.5 μM RSL3 (RSL3) for 24 hours. (J) The relative malondialdehyde (MDA) concentrations of t(4;14)-positive NCI-H929 and OPM-2 cells and t(4;14)-negative U-266 cells treated with solvent (DMSO), 0.2 μM RSL3 (D0.2), or 0.5 μM RSL3 (D0.5) for 24 hours. (K) Electron microscopy images of t(4;14)-positive NCI-H929 and t(4;14)-negative U-266 cells treated with or without 0.5 μM RSL3 for 24 hours. The black arrowhead indicates mitochondria, which show membrane rupture and reduced cristae in t(4;14)-positive MM and relatively intact mitochondrial structure in t(4;14)-negative MM following RSL3 treatment. The levels of shrunken or ruptured mitochondria were analyzed using ImageJ software. The left scale bar: 1 μm. The right scale bar: 0.5 μm. ns, not significant; P > .05, ∗ P <0 .05, ∗∗ P < .01, and ∗∗∗ P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/19/10.1182_bloodadvances.2023010335/2/m_blooda_adv-2023-010335-gr1.jpeg?Expires=1768607626&Signature=ymhypl7578YpNE6qSn-tJ-mGWG9ecFpRgGC1OtTGOBpj8~19ugAZ30g2jqaraJZmrVs2XlBKX~S0ridZZtR-IyqdU7wOTfCEUZLDEoXqYYcL1UPEe8V6-bl-RIo0~BqSR-wRvl6matKxFfZOHBrYbOjx7X5ion7dHcJ7bWIxeR5GOuQuA9W~MhFuYyiG~RKewLedmcCZhGRHcvTJEn0WFGl51BaeC0RT4TaJaqvjDHqw91ok7v2na3SYcgeXNbJpCkeaO5K8yy0UUzC9vLw8W20UDIfOHqGYJuATfEUhRNSLzTDVqLpv4x2T9MiwVx7P0etzWDNPP~mwYw~f9VCvLQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)