Visual Abstract
Gene therapy for severe hemophilia A uses an adeno-associated virus (AAV) vector and liver-specific promoters that depend on healthy hepatocyte function to achieve safe and long-lasting increases in factor VIII (FVIII) activity. Thus, hepatocyte health is an essential aspect of safe and successful gene therapy. Many people living with hemophilia A have current or past chronic hepatitis C virus infection, metabolic dysfunction–associated steatosis or steatohepatitis, or other conditions that may compromise the efficacy and safety of AAV-mediated gene therapy. In addition, gene therapy may induce an immune response to transduced hepatocytes, leading to liver inflammation and reduced FVIII activity. The immune response can be treated with immunosuppression, but close monitoring of liver function tests and factor levels is necessary. The long-term risk of hepatocellular carcinoma associated with gene therapy is unknown. Routine screening by imaging for hepatocellular carcinoma, preferable every 6 months, is essential in patients at high risk and recommended in all recipients of hemophilia A gene therapy. This paper describes our current understanding of the biologic underpinnings of how liver health affects hemophilia A gene therapy, and provides practical clinical guidance for assessing, monitoring, and managing liver health both before and after gene therapy.
Introduction
The availability of gene therapy to treat severe hemophilia A (HA) has advanced the potential for unprecedented reduction in bleeds and factor use, and improved quality of life through a single intravenous infusion.1-3 With this therapy (AAV5-hFVIII-SQ, valoctocogene roxaparvovec), an engineered human factor VIII (hFVIII) complementary DNA is delivered to hepatocytes by an adeno-associated virus (AAV) vector. The complementary DNA codes for hFVIII in which the B domain is replaced by a 14-amino-acid linker (hFVIII-SQ).4 Transduced hepatocytes then produce hFVIII-SQ and secrete it into the circulation. In individuals with severe HA (FVIII activity of <1%), treatment with valoctocogene roxaparvovec increased circulating FVIII activity to 11.9% to 62.3% (interquartile range) at 49 to 52 weeks, reduced bleeds and factor use by >90%, and improved quality of life.1-3 Although HA gene therapy costs up to $2 million to $3 million, it has been shown to be cost-effective when compared with the lifetime costs of factor replacement therapy, hospitalization, morbidity, and disability.5,6
Two major clinical concerns regarding liver-targeted gene therapy for HA are (1) how to optimize long-term hFVIII-SQ expression, and (2) how to minimize the risks of gene therapy to liver health. The phase 3 trial of valoctocogene roxaparvovec included many design elements centered on these 2 concerns, specifically, exclusion of individuals with active hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, advanced fibrosis or cirrhosis (stage F3 or F4 on METAVIR score), as well as close monitoring of gene therapy recipients for signs of liver inflammation or hepatocellular carcinoma (HCC).1 These concerns and their clinical implications were the focus of a scientific roundtable held on 23 June 2023, sponsored by BioMarin Pharmaceutical, Inc, manufacturer of valoctocogene roxaparvovec. The roundtable brought together an international group of experts with diverse backgrounds in HA, hematology, gene therapy, and hepatology, whose roundtable presentations, expert opinions, and recommendations were the basis for this paper. This paper explores the current understanding of biologic mechanisms underpinning the potential impact of chronic liver disease on the safety and efficacy of gene therapy in individuals with HA, and how gene therapy affects subsequent liver health, concluding with a description of clinical strategies to address these issues based on currently available knowledge. Because liver-specific issues related to gene therapy are less common with FIX gene therapy,7 the roundtable and this paper focused on FVIII gene therapy.
Liver-targeted gene therapy for HA
Systemic delivery of AAV vectors carrying therapeutic genes results in the transduction of different tissues, depending on the AAV serotype. HA gene therapy uses serotypes such as AAV5, which preferentially delivers therapeutic genes to hepatocytes. Once delivered to the hepatocyte, the AAV vector is uncoated, releasing the hF8-SQ transgene cassette into the nucleus. The transgene primarily remains episomal (independent of the genome) with a minor proportion being integrated into the host genome.8-10 This fate of the transgene underscores the advantage of targeting hepatocytes; they are usually quiescent and do not undergo frequent cell turnover, which could result in loss of the transgene from daughter cells.11 In theory, therefore, transduction of quiescent hepatocytes can provide long-term expression of the therapeutic gene. To further enhance liver specificity of hFVIII-SQ expression, the hF8-SQ transgene of valoctocogene roxaparvovec uses a liver-selective promoter.12
Despite effective targeting of transgene expression to hepatocytes, the durability of hFVIII-SQ expression after valoctocogene roxaparvovec therapy is limited in some patients. Based on the chromogenic substrate assay, the median FVIII activity among participants in the phase 3 trial was 23.9% at 1 year after gene therapy, 11.8% at 2 years, 8.3% at 3 years, 6.7% at 4 years, and projected by quantitative pharmacokinetic modeling to be 5.7% at 5 years.2,13 The limited durability of hFVIII-SQ expression after gene therapy is not well understood, but several mechanisms have been proposed.14 One proposed mechanism is endoplasmic reticulum (ER) stress due to high levels of transgene-directed protein synthesis.15 A second proposed mechanism relates to the fact that liver sinusoidal cells, not hepatocytes, are the natural source of endogenous FVIII production,16-18 and hepatocytes may be less efficient at FVIII secretion than liver sinusoidal endothelial cells. A third proposed mechanism is the immune response to AAV-transduced hepatocytes and the ensuing hepatocyte inflammation, or oxidative stress due to dose-dependent levels of intracellular capsid with unknown degradation kinetics. Finally, the majority of adults with HA have preexisting liver disease, such as chronic HCV contracted through factor replacement therapy or metabolic dysfunction–associated steatotic liver disease (MASLD; formerly known as nonalcoholic fatty liver disease).19 Preexisting liver disease may contribute to the decline in hFVIII-SQ expression, although the role of this explanation remains unknown because patients with significant liver dysfunction were excluded from gene therapy trials.1 Individuals with chronic liver disease are at risk of developing fibrosis; advanced fibrosis or cirrhosis puts them at risk for end-stage liver disease and HCC.20,21 The long-term implications of hepatocyte-targeted gene therapy in these settings is unknown. Thus, the presence of preexisting liver disease is a major factor influencing clinical decision-making in individuals seeking HA gene therapy. Each of these issues will be addressed in this paper.
Liver disease and its impact on HA gene therapy
Hepatocytes have enormous proliferation potential and can repopulate a damaged liver. If there is liver damage from ongoing liver disease, hepatocyte proliferation can help to maintain liver function even in advanced stages of disease. In the setting of gene therapy, proliferation of hepatocytes may be detrimental to long-term expression of the transgene. For example, in mouse models of partial hepatectomy after gene therapy, most AAV-derived transgenes are lost during hepatocyte cell division, resulting in reduced expression of the therapeutic gene.11,22 Accelerated hepatocyte turnover associated with ongoing liver disease could, therefore, result in the reduction or elimination of hFVIII-SQ–expressing hepatocytes and reduced FVIII activity. Furthermore, there is evidence from rodent models that gene therapy administered during ongoing liver inflammation may increase the risk of subsequent HCC.23 However, the vector used in those studies targeted genomic integration into a site known to be associated with HCC, so it is unlikely that those studies are relevant to humans receiving valoctocogene roxaparvovec.
Liver disease is common in people with hemophilia and a leading cause of death,19,24 especially in those with severe hemophilia. For example, data from 6 US Hemophilia Treatment Centers between 2009 and 2013 reported that 48% of young participants with hemophilia (ages 18-34 years) had liver disease, including HCV.25 Because that study preceded the availability of direct-acting antiviral agents for treatment of HCV, most of those with HCV probably had active infection, although viral load data were not reported. Furthermore, 48% were overweight or had obesity, placing them at increased risk for MASLD.
History of chronic HCV
Before nonfactor and gene therapies became available for severe HA, clotting factor replacement therapy was the primary bleed prevention strategy. As a result, many males with severe HA were infected with HCV before viral screening and inactivation technologies were implemented in the mid-1980s.26,27 Among men with hemophilia born before 1983, 84% to 92% have current or past chronic HCV; among those born after 1983 the corresponding value is ∼33%.28 Thus, current or past HCV is a major cause of liver disease, cirrhosis, end-stage liver disease, and HCC in men with hemophilia. Because most were infected with HCV during the first year of life because of treatment with contaminated clotting factor concentrates, men with hemophilia have longer duration of HCV infection than HCV-infected cohorts of a similar age without hemophilia.29,30 As such, ∼25% of adult men with hemophilia and chronic HCV infection have advanced fibrosis (METAVIR ≥ F3).29 In those with HIV-HCV coinfection, there is more rapid progression of HCV-associated liver disease.29 Such patients also have a greater frequency and severity of hepatotoxicity from liver-metabolized drugs than other groups.31 In the setting of gene therapy, such susceptibility to hepatocyte damage could lead to reduction or loss of transgene expression.32
Active HCV infection, defined by a detectable HCV viral load, is a contraindication for HA gene therapy. With the availability of direct-acting antiviral drugs, however, HCV infection can be eradicated in virtually all patients with minimal to no side effects and is considered the standard of care for individuals with hemophilia.33 The impact of prior HCV infection, including type of treatment, time to treatment, and residual disease after treatment, on the response to gene therapy has not been studied and remains unclear.
Advanced fibrosis (F3) and cirrhosis (F4) are also contraindications for HA gene therapy. However, early cirrhosis can regress after HCV eradication in as many as one-third to one-half of patients.34 To what extent livers with regressed cirrhosis are comparable to livers that never developed advanced fibrosis remains poorly understood, and, to date, individuals with regressed cirrhosis remain under HCC screening. Although such individuals may become eligible for gene therapy, per the authors’ expert opinion, they may require more intensive long-term monitoring.
MASLD
MASLD is increasing in global prevalence, and is now present in approximately one-third of the population,35 although the reported prevalence depends on detection methods.19,30 It has also become a leading cause of liver fibrosis and cirrhosis in both the general population and the hemophilia population.19,21,36 A subset of patients with MASLD develop steatohepatitis (metabolic dysfunction–associated steatohepatitis, formerly nonalcoholic steatohepatitis), which is characterized by inflammation and hepatocyte proliferation, and is a major risk factor for HCC in the general population and people with hemophilia.20 Unfortunately, little is known about MASLD in people with hemophilia, in part because of the high bleeding risk of biopsy, and in part because of confounding from comorbidities, such as long-duration HCV infection and associated fibrosis. Furthermore, little is known about how MASLD affects HA gene therapy. Based on the authors’ experience, it is expected that individuals with MASLD may have ongoing hepatocyte damage and proliferation, which could be detrimental to long-term factor expression after HA gene therapy.
Effects of HA gene therapy on liver health
The immune response to HA gene therapy
Although valoctocogene roxaparvovec was generally well tolerated in the phase 3 trial, 86% of recipients developed alanine aminotransferase (ALT) elevations that were treated with corticosteroids or other immunosuppressants.1 In previous studies of systemically administered AAV gene therapies, the most common adverse event was an inflammatory response directed against transduced hepatocytes and which was associated with ALT elevation.37 In some cases, ALT elevations preceded the decline in factor levels, and there is concern that the immune response could adversely affect long-term factor expression.
Mechanistically, much has been learned from earlier AAV gene therapy studies for hemophilia B (HB). The prevailing hypothesis formulated from these early studies to explain this inflammatory response was that vector-induced CD8+ cytotoxic T lymphocytes (CTLs) mount an immune response targeting hepatocytes that present AAV capsid–derived antigens. This mechanism was demonstrated in early HB gene therapy trials, in which a rise in ALT coincided with a decline in transgene expression and detection of capsid-specific CTLs by interferon-gamma enzyme-linked immunospot assays.38,39 However, more recent trials using similar assays, including trials in HA gene therapy, have not fully recapitulated these findings. Specifically, they have not demonstrated a close temporal relationship between ALT elevation and the capsid-specific CTL response.40 Thus, capsid-specific CTL responses may not fully explain the inflammatory response to gene therapy in the liver. Other potential explanations for the inflammatory response could involve innate immune responses to the vector,41 cellular stress, or other mechanisms, but none have been investigated thoroughly.14 Ongoing research is exploring ways to (1) improve vector design to reduce immune responses, and (2) develop more targeted immunomodulators for treating liver inflammation after gene therapy.14
Aside from early liver inflammation (within 6 months of treatment), immune responses in valoctocogene roxaparvovec recipients did not appear to cause significant safety or efficacy concerns,40 although results from long-term follow-up studies are needed to determine HCC risk or other long-term consequences. Trial participants were required to be inhibitor negative at the time of enrollment. Some participants in the valoctocogene roxaparvovec trial developed transient, low-titer binding antibody responses to hFVIII-SQ, but none developed inhibitors.1 Such low-titer antibody responses are known to occur in healthy individuals as well as in persons with HA who received FVIII replacement therapy.42
No patient characteristics or biomarkers have yet been identified that predict anticapsid CTL responses or ALT flares after AAV vector gene therapy. Recent observation of an acute hepatitis outbreak in children, possibly associated with AAV2 infection, showed enrichment for certain HLA class II alleles previously associated with autoimmune hepatitis.43,44 Whether HLA or other risk alleles can predict immune responses to AAV vectors remains unknown.
Hepatocellular stress: the ER and the UPR
Because the full-length FVIII gene cannot be packaged within the AAV capsid, HA gene therapy uses a B-domain–deleted FVIII transgene (hFVIII-SQ), in which the B-domain sequence is replaced by a sequence coding for a 14-amino acid linker.45,46 The B domain is not essential for FVIII activity, and recombinant B-domain–deleted FVIII has been used as factor replacement therapy for several decades. Both full-length FVIII and hFVIII-SQ may induce the unfolded protein response (UPR) associated with accumulation of protein in the ER lumen, oxidative stress, amyloid-like protein formation, and apoptosis in cultured cells including human hepatocytes and in wild-type mice.15,47,48 Thus, it has been hypothesized that hepatocellular ER stress may be due to the size of FVIII, making it difficult to fold and secrete.49-52 Indeed, there is no evidence that use of a B-domain–deleted FVIII gene induces any more cellular stress or hepatotoxicity than a full-length gene.
One approach to minimize induction of chronic cellular stress resulting from an UPR is to use a relatively weak liver-specific promoter that produces expression that is modest but sufficient to drive therapeutic levels of hFVIII-SQ production.46,51,53 Valoctocogene roxaparvovec, for example, uses a weak promoter and spreads the transgene expression load to more cells, as evidenced by unbiased distribution of the hFVIII-SQ gene in human liver biopsy samples.53 In preclinical studies, valoctocogene roxaparvovec therapy does not elicit an UPR in mouse hepatocytes or show evidence of hepatotoxicity even during expression of supraphysiological levels of hFVIII-SQ46,51 (note that there are species differences, with mice expressing higher RNA levels per transgene than primates, resulting in a higher burden on protein translation and folding machinery).53 In addition, human liver biopsies taken from clinical trial participants 2.6 to 4.1 years after valoctocogene roxaparvovec treatment showed no evidence of ER stress in hepatocytes expressing hFVIII-SQ.53 These studies cannot rule out transient ER stress occurring earlier after gene therapy, but histopathologic analyses have revealed no dysplasia, architectural distortion, fibrosis, or chronic inflammation, or necrosis. Furthermore, analyses by confocal microscopy revealed no induction of the ER stress marker GRP78 in hepatocytes expressing hFVIII-SQ.53 Taken together, these studies suggest that careful selection of the type and strength of the promoter may minimize cellular stress after gene therapy. The degree to which ER stress causes ALT increases and liver inflammation after HA gene therapy remains unknown.
Insertional oncogenesis
After intravenous administration of AAV-based gene therapy and hepatocyte transduction, the transgene remains primarily episomal, which is associated with a favorable safety profile. However, the transgene does integrate into the target cell genome at a low frequency, estimated in some studies to be 0.5 to 16 integration sites per 1000 cells.54-58 In liver biopsy samples from patients with HA obtained 2.6 to 4.1 years after valoctocogene roxaparvovec treatment at the approved dose, ∼50% of hepatocyte nuclei were found to contain the hFVIII-SQ therapeutic gene.53 On average, the human liver contains ∼100 billion hepatocytes.58 These estimates suggest that there are likely millions of genomic integration events per patient after HA gene therapy. The degree of transduction and genome integration in nonliver tissues is also unknown. Although insertional oncogenesis due to recombinant AAV genomic integration has been observed in some mouse models after AAV gene therapy,58 it has not been observed in large animal models or humans to date.59 Likewise, there have been no reported cases to date of insertional oncogenesis leading to HCC in recipients of hemophilia gene therapy.60 However, naturally acquired AAV2 can integrate at genomic loci that have previously been implicated in HCC oncogenesis.61 Because malignancy may take decades to develop in humans, screening for development of HCC is an essential part of long-term follow-up after gene therapy.
In recent years, rates of HCC have increased among people with hemophilia, but these increases are consistent with the high rates of HCV infection and MASLD in this population, and are occurring despite the availability of direct-acting antiviral agents against HCV.20 Inevitably, cases of HCC will be identified in people who have had HA gene therapy, especially among older patients or those with a history of chronic HCV. In such cases, it will be important to alert the gene therapy manufacturer and arrange for molecular analysis of the tumor tissue as part of a manufacturer-directed research protocol to ascertain whether gene therapy contributed to the development of HCC. HCC has been reported in a patient who received AAV gene therapy for HB and who had been exposed to HBV and HCV and had ongoing MASLD, but analyses conducted to date have indicated that genomic integration of the therapeutic gene was not responsible for the cancer.60,62 Clinical studies of AAV-based gene therapy have reported 3 additional cancers after hemophilia gene therapy (squamous cell tonsilar carcinoma, acinic cell carcinoma of the salivary gland, and B-cell acute lymphoblastic leukemia) and an epithelioid cancer after gene therapy for spinal muscle atrophy.63 In each of these cases, analyses concluded that the cancer was not related to an AAV integration event.62,63
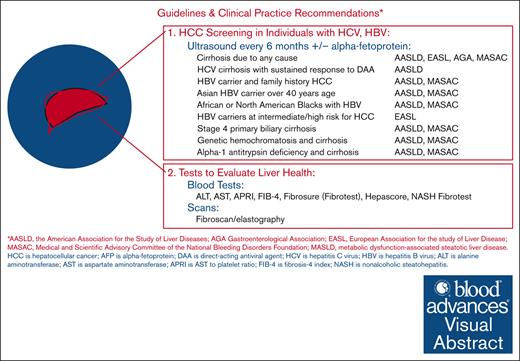
Evaluating and monitoring liver health
Existing guidelines address screening for HCC in people with advanced fibrosis or cirrhosis from any liver disease, including HCV, MASLD, or active HBV infection (Table 1).64 Until recently, few guidelines have distinguished between people with or without bleeding disorders or addressed issues related to liver health in the context of gene therapy for HA.7,59,67,68 The guidance provided here for care of individuals with HA who are considering or who undergo HA gene therapy is summarized in Table 2 and is based on the authors’ expert opinions.
Pretreatment assessment of liver health
Individuals with advanced liver disease (F3, F4, or cirrhosis), active HCV or HBV infection, viral load–positive HIV infection, or radiological liver abnormalities are currently ineligible for HA gene therapy, and such patients should consult with a hepatologist or infectious disease specialist. Avoidance of alcohol and hepatotoxic drugs is also a required prerequisite for HA gene therapy.
Current strategies to assess liver health include measuring liver aminotransferase levels (ALT and aspartate aminotransferase [AST]), using calculations based on standard laboratory values (eg, AST to platelet ratio [APRI] or fibrosis-4 index [FIB-4]), using specialized proprietary liver fibrosis tests (eg, Fibrosure, Fibroscore, or Hepascore), and elastography using either ultrasound or magnetic resonance imaging. Each of these categories of tests has its strengths and weaknesses, as discussed hereafter, and summarized in Table 3.
Aminotransferase levels are best used to assess liver inflammation and the potential ongoing loss of hepatocytes. Accurately determining baseline levels before gene therapy is important, because the need for immunomodulation after gene transfer is based on changes in AST or ALT level. Because AST is more abundant in muscle than ALT, AST levels are less specific for liver damage than ALT levels. Note that many laboratories report normal ALT reference ranges that are higher than what should be considered normal, which should be 29 to 33 IU/L for males and 19 to 25 IU/L for females.69
Many prescribed medications can affect AST or ALT levels, and the best practice is to minimize exposure to known hepatotoxins (eg, isotretinoin, efavirenz, and others) for 4 to 6 weeks before proposed gene therapy as well as after gene therapy. Alcohol is a well-known hepatotoxic agent and should be avoided for 6 to 8 weeks before baseline assessment to allow an accurate baseline aminotransferase level to be determined. Complicating the use of AST and ALT (especially ALT) as markers of liver inflammation after gene therapy is the presence of MASLD. Accurate measurement of baseline ALT level before gene therapy is especially important in patients with fatty liver, who often have elevated transaminase levels that can confound interpretation of how gene therapy affects those levels.
Liver fibrosis is the second important parameter to assess before HA gene therapy. This can be achieved by blood tests and/or elastography. A practical approach is to use 1 of 2 fibrosis prediction calculations based on simple blood tests: the APRI or the FIB-4 index.70 These calculated values have been validated with the level of fibrosis, usually using the METAVIR score of fibrosis as a gold standard. Advantages of these combination tests include ease of calculation and wide availability, as well as disease nonspecificity; that is, they work for fibrosis due to viral hepatitis, chronic alcohol consumption, and MASLD. A disadvantage of the FIB-4 score is that diagnostic cutoffs may need to be adjusted for age, because older people have higher scores.71,72 A disadvantage of the APRI score is that it is relatively insensitive for fibrosis and early cirrhosis. Calculators for both are available at.
Several proprietary blood tests for liver fibrosis have also been developed. These tests use combinations of laboratory values, but some also include patient demographic factors. Some of these proprietary tests are specific to the underlying cause of fibrosis, such as the nonalcoholic steatohepatitis-Fibrotest, which is specific to fibrosis caused by MASLD.
Elastography is a more recent technology to determine the degree of liver fibrosis. There are 2 widely used methods for elastography assessment, vibration-controlled transient elastography (VCTE) and magnetic resonance elastography (MRE), in addition to other transient elastography technologies. VCTE uses a sound beam similar to ultrasound to assess the stiffness of the liver. Liver stiffness has been correlated with the stage of fibrosis for most major liver diseases, which have subtly different correlations. An optional measurement that can be made using VCTE is the continued attenuation parameter. This value, measured in decibels per meter (dB/m), correlates with the percentage of liver fat. MRE provides information about liver stiffness and is able to quantify the amount of liver fat. MRE has a slightly higher diagnostic accuracy than VCTE73 but is usually more expensive.
For individuals with discordant results, such as low FIB-4 score and high elastography scores, consultation with a hepatologist is recommended. Consultation with a hepatologist is also recommended for patients with radiological liver abnormalities, liver function test abnormalities (>1.25× upper limit of normal), or international normalized ratio of ≥1.4.
Posttreatment monitoring of liver health
ALT and FVIII activity
After HA gene therapy, patients should have weekly monitoring of ALT levels for at least 26 weeks (Table 2). Currently, corticosteroids are recommended for treatment of elevated ALT levels associated with gene therapy. Corticosteroids should be continued until ALT levels return to baseline, during which time ALT and FVIII activity levels should be monitored weekly.
HCC screening
The rationale for HCC screening in individuals with chronic liver disease is to detect tumors at an early stage when curative treatments are feasible. Based on annual incidences of HCC in various liver conditions, professional societies have developed HCC screening guidelines. Table 1 presents a summary of recommendations from major professional societies regarding screening for HCC in patients with established risk factors (hepatitis B, hepatitis C, or MASLD). The potential risk of HCC after AAV-mediated gene therapy was addressed by the Expert Working Group on AAV Integration convened by the American Society of Gene and Cell Therapy.59 The Working Group recommended routine follow-up using liver ultrasound or magnetic resonance imaging, and serum α-fetoprotein. Based on expert opinion and the unknown HCC risk posed by AAV vector administration, we concur with the Working Group’s recommendation for routine HCC screening after HA gene therapy. Because the tumor volume doubling time of HCC has been estimated to be 4 to 5 months,74 we favor a screening interval of 6 months, in accordance with most guidelines for HCC screening in chronic liver diseases (Table 1). A screening interval of 6 months is superior to 12 months with respect to survival of patients with HCC.75 Screening methods and recommendations may change in the future as more information and new methods become available. The Working Group recommended liver biopsy only for the purpose of investigating liver tumors detected after gene therapy to determine whether gene therapy was a contributing factor.59 Such investigations should be performed after informing the gene therapy manufacturer and as part of a manufacturer-directed research protocol. More research is needed to better understand how current and future gene therapies affect liver histology and function, underscoring the importance of collaboration among hepatologists, molecular biologists, and immunologists.
Treatment of gene therapy–induced liver inflammation
Elevations in liver enzymes, especially ALT, are presumed to indicate an immune-mediated response targeting transduced hepatocytes and can be associated with reductions in FVIII activity levels. In the phase 3 trial of valoctocogene roxaparvovec, 86% of patients experienced ALT elevations, and most occurred within the first year.1 The median time to first ALT elevation, defined as ≥1.5× baseline or above upper limit of normal, was 7 weeks (range, 0.4-159). The median duration of ALT elevation was 4 weeks (range, 0.1-135). Currently, there are no histological data characterizing the etiology of ALT flares after AAV vector administration, and more research is needed on this issue. Until more research results are available, routine liver biopsies to monitor AAV gene therapy or investigate ALT flares are not recommended in the absence of an appropriate indication for biopsy or a research protocol.
At this time, corticosteroid treatment is recommended in patients exhibiting elevated liver enzymes because of concerns for preserving hFVIII-SQ expression and for liver health. However, there is ongoing debate regarding the role and value of corticosteroids in this setting. In the phase 3 trial, the median duration of corticosteroid use was 35 weeks (range, 3-120). Among 110 patients in the valoctocogene roxaparvovec phase 3 trial who received corticosteroid therapy, a significant number, 79 (72%) had an adverse event related to corticosteroid use, including 1 patient who developed new-onset diabetes mellitus, a common adverse effect of corticosteroid use. ALT levels and FVIII levels should be monitored weekly in patients receiving corticosteroids until ALT levels return to baseline. An alternative immunosuppressant was used in some patients for a median of 26 weeks (range, 6-188).
Conclusion
Liver health should be carefully evaluated in persons with severe HA who are considering gene therapy. Such evaluations should be performed in collaboration with a hepatologist, and they should assess the presence of contraindications to gene therapy as well as conditions that could adversely affect the efficacy and safety of HA gene therapy. After gene therapy, patients should be closely monitored for evidence of liver inflammation, which occurs in most treated patients. Immunosuppressive treatment of liver enzyme elevation, usually involving corticosteroids, is currently the standard approach and is thought to be important for preserving gene therapy efficacy. Long-term monitoring for adverse effects of gene therapy, including screening for HCC and other malignancies, is important. Detailed molecular analysis of malignancies that arise is recommended as part of manufacturer-directed research protocols. A multidisciplinary team, including a hepatologist, hematologist, and hemophilia expert, should be involved in evaluating and monitoring liver health before and after HA gene therapy. Other specialists might include geneticists, psychosocial professionals, and lived-experience experts.
Acknowledgments
Medical writing support was provided by Ken Scholz, affiliated with Innovative BioPharma, LLC, and was funded by BioMarin Pharmaceutical, Inc.
Funding for the roundtable was provided by BioMarin Pharmaceutical Inc.
Authorship
Contribution: M.V.R., and H.M. conceptualized the research question and proposed this publication; M.V.R., Y.P.d.J., B.A.L., D.E.S., S.F., C.E.W., and R.K. gave presentations and participated in discussion at the roundtable; A.D.L., D.J.N., and A.v.D. contributed to the roundtable discussion; and all authors wrote initial drafts of manuscript sections, and reviewed and revised subsequent drafts and approved of the final submission.
Conflict-of-interest disclosure: M.V.R. receives research grants to her institution from BioMarin, Sanofi, Spark Therapeutics, and Takeda; and consulting fees from Be Biopharma, BioMarin, HEMAB, Sanofi, Spark Therapeutics, and Takeda. H.M. is an employee and shareholder of BioMarin. Y.P.d.J. served on scientific advisory boards for BioMarin and Pfizer; received consulting fees from Pfizer; and received research funding from Spark. R.K. serves on scientific advisory boards of BioMarin and Pfizer; and received research funding from Bayer. A.D.L. has received honoraria for participating in advisory board meetings for Pfizer, BioMarin, and CSL Behring; and has received institutional research support from Pfizer, BioMarin, and Spark Therapeutics. B.L. is an employee and shareholder of BioMarin. D.J.N. serves on advisory boards for BioMarin and Hemab. D.E.S. is a consultant for BioMarin and Poseida Therapeutics; and receives licensing royalties from Spark Therapeutics. S.F. is an employee and stockholder of BioMarin. A.v.D. has received honoraria for participating in scientific advisory board panels, consulting, and speaking engagements for BioMarin, Regeneron, Pfizer, Bioverativ/Sanofi, CSL-Behring, Novo Nordisk, Precision Medicine, Spark Therapeutics, Regeneron, Genentech, and UniQure; and is a cofounder and member of the board of directors of Hematherix LLC, a biotech company that is developing superFVa therapy for bleeding complications. C.E.W. is a medical consultant for Genentech, CSL Bering, BioMarin, Sanofi, and Novo Nordisk. B.A.L. serves on the data safety monitoring board for BioMarin, Spark Therapeutics, CSL Behring, and Pfizer.
Correspondence: Margaret V. Ragni, Division Hematology/Oncology, Department of Medicine, University of Pittsburgh Medical Center, Hemophilia Center of Western Pennsylvania, Lothrop at DeSoto Streets, Pittsburgh, PA 15261; email: ragni@pitt.edu.
References
Author notes
Patient data have not been presented. However, laboratory data presented herein are available on request from the corresponding author, Margaret Ragni (ragni@pitt.edu)