Key Points
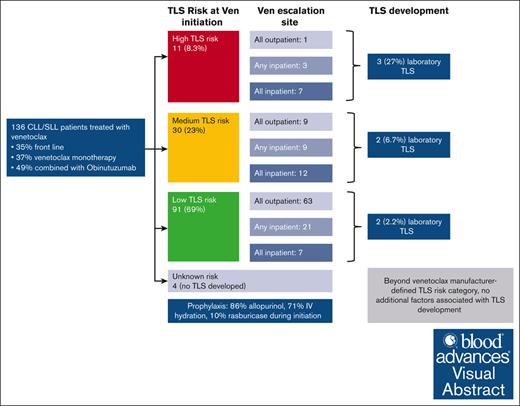
In a real-world series of CLL patients treated with venetoclax, laboratory TLS was rare (5.1% overall; 2.7% among outpatient escalations).
Beyond TLS risk based on ALC and lymph node size, no additional factors were associated with lab TLS onset, and no clinical TLS was seen.
Visual Abstract
Venetoclax is a B-cell lymphoma 2 inhibitor used in chronic lymphocytic leukemia (CLL), which can cause tumor lysis syndrome (TLS). We aimed to determine the incidence of, and risk factors for, TLS among patients with CLL/small lymphocytic lymphoma who received treatment with venetoclax at our institution from 1 January 2016 to 31 December 2020. We included 616 venetoclax escalations among 136 patients with CLL. Overall, 74 patients (54%) underwent escalation exclusively outpatient, 35 (26%) had at least 1 planned hospitalization, and 27 (20%) were escalated exclusively inpatient. During venetoclax initiation, 86% of patients received allopurinol, 71% intravenous hydration, 18% phosphate binders, and 10% prophylactic rasburicase. Among the entire cohort, 7 patients (5.1%) developed laboratory TLS by modified Cairo Bishop criteria and none developed clinical TLS. Incidence of laboratory TLS was 15% for those escalated exclusively inpatient, 2.9% for those with any prophylactic hospitalization, and 2.7% for those escalated exclusively outpatient. Those who developed TLS were more likely to have higher TLS risk, and no additional risk factors were identified. In this single institution retrospective cohort study, laboratory TLS was observed, although clinical TLS was not. Prophylactic measures, including use of IV hydration, may have contributed to low rates of observed TLS in the outpatient setting.
Introduction
Venetoclax is a BCL2 inhibitor approved for the treatment of chronic lymphocytic leukemia (CLL). In the early phase clinical trials of venetoclax monotherapy in relapsed and refractory CLL before institution of dose escalation strategies, tumor lysis syndrome (TLS) was observed and led to deaths and serious adverse events.1 TLS is a life-threatening oncologic emergency caused by rapid death of malignant cells and release of electrolytes and nucleic acids into the bloodstream. Left untreated, TLS can lead to renal failure, arrhythmias, and death.2 These adverse events led to the development of an extended dose ramp-up schedule, over the course of 5 weeks, as well as the establishment of TLS prophylaxis and monitoring strategies. The approved prescribing information for venetoclax provides definitions for TLS risk categories based on CLL burden (absolute lymphocyte count [ALC] and maximum lymph node [LN] size) and baseline renal function, and corresponding recommendations for TLS prophylaxis.3 Current National Comprehensive Cancer Netrwork guidelines and prescribing information recommend that patients with CLL with high tumor burden should be observed in the inpatient setting during at least the first 2 stages of venetoclax escalation, whereas those with low or medium tumor burden may be observed in the outpatient setting with appropriate prophylaxis and monitoring.4
In later phase clinical trials using the 5-week dose ramp, the observed frequency of TLS when treating CLL with venetoclax-based therapies has been low. In the phase 3 MURANO trial of venetoclax and rituximab in the treatment of relapsed or refractory CLL, 6 patients (3.1%) treated with this combination developed grade ≥3 TLS and 1 (0.5%) patient developed clinical TLS. This led to trials that used debulking of CLL before venetoclax: in the CLL14 phase 3, open-label trial among previously untreated patients with CLL, patients were randomized to receive either venetoclax and the anti-CD20 monoclonal antibody obinutuzumab or chlorambucil and obinutuzumab. In the arm receiving venetoclax and obinutuzumab, 3 of 212 (1.4%) patients who received treatment developed laboratory TLS, and no patients developed clinical TLS, although all 3 of those events occurred during obinutuzumab administration, before initiation of venetoclax.5,6
Less is known about the incidence of TLS in patients treated with venetoclax outside of clinical trials. In 1 report from an international retrospective cohort of 297 patients, 96% of whom had relapsed or refractory CLL, 8.4% developed TLS (5.7% laboratory TLS and 2.7% clinical TLS).7 Patients in this cohort had had a median of 3 prior lines of therapy and 80% of patients were treated with venetoclax monotherapy without a debulking agent given before venetoclax. In other real-world studies, incidence of TLS has been reported to be as high as 22% in a French cohort of patients with CLL treated with venetoclax monotherapy,8 and as low as 0% in a retrospective single-center cohort study.9 The incidence of TLS across clinical trials and real-world studies has been reviewed by Sharman et al in 2022.10
In this study, we report on the real-world incidence, prevention, and management of TLS among 136 patients with CLL treated in a single institution outside clinical trials. Our study includes a substantial patient cohort receiving venetoclax as first-line therapy in combination with other agents, thus addressing a gap in the existing literature. Additionally, we specifically report on inpatient vs outpatient escalations of venetoclax and their safety.
Methods
Patients
We conducted a retrospective cohort study among patients aged ≥18 years who were diagnosed with CLL or small lymphocytic lymphoma and received treatment with venetoclax (alone or in combination with other agents) at Memorial Sloan Kettering Cancer Center (MSK) from 1 January 2016 to 31 December 2020. Patients treated on a prospective therapeutic clinical trial were excluded. We included all venetoclax escalations with known dates of escalation and available laboratory results at the time of venetoclax dosing escalation. The primary objective of the study was to assess the association between TLS prevention strategies and incidence of laboratory and clinical TLS in aggregate and as stratified by pretreatment TLS risk. The secondary objectives were to describe strategies used for prevention of TLS in the outpatient and inpatient setting, and to determine the incidence of rasburicase administration for the treatment of isolated hyperuricemia of TLS. This retrospective research protocol was approved by the institutional review board at MSK, and all research was conducted according to the Declaration of Helsinki. Data were extracted from the electronic medical record and stored in a REDCap database for analysis.
Definitions
A dose escalation event was defined as the administration of a new dose of venetoclax at least once. Laboratory and clinical TLS were defined using modified Cairo Bishop criteria.2 TLS risk was calculated based on the size of the largest LN on imaging or physical examination and the ALC as defined by venetoclax prescribing information.3,4 The size of LNs used was the largest known LN by physical examination or imaging, whichever was closest to the day of venetoclax initiation. If measurements were done on the same day, measurement by imaging was prioritized. The ALC was measured on the day of venetoclax initiation. When venetoclax was given as part of combination regimen, paired medications were defined as follows: use of an anti-CD20 monoclonal antibody within 30 days of venetoclax initiation was considered paired medication use. When obinutuzumab was used in combination with venetoclax, dosing usually followed that of the CLL14 trial,5 with venetoclax starting on day 22 of cycle 1 of obinutuzumab. Use of brief overlap of a Bruton tyrosine kinase inhibitor with venetoclax in the first steps of escalation was not considered paired medication use. Creatinine clearance was estimated based on the estimated glomerular filtration rate calculated based on the Chronic Kidney Disease Epidemiology Collaboration model for each patient. This calculation incorporated the patient’s race (as listed in the MSK electronic medical record) until 2021,11 at which point the calculation was changed and no longer incorporated race.12
Interventions
Prophylactic strategies for TLS were extracted from the medication administration record for inpatients and clinic administrations, and from clinical notes and prescription records for outpatients.
Statistical methods
Categorical patient characteristics were summarized by frequency, and continuous characteristics were summarized by the median and interquartile range (IQR). Fisher's exact test or Wilcoxon signed-rank tests were used to determine the significance of the association between occurrence of TLS and discrete or continuous demographic and clinical characteristics. To account for TLS risk score in these associations, exact Cochran-Mantel-Haenszel test was used. Statistical analyses were performed in R version 4.3.0.
Results
During the study period, 136 patients with CLL were treated with venetoclax at our institution outside of clinical trials (Table 1). Among 136 patients, data were available for 616 venetoclax initiation and dose escalation events. Median age was 70 years (IQR, 61.2-76.9) and 86% were of White race. Venetoclax (with or without other agents) was the first line of treatment for 48 patients (35%). Among those treated with venetoclax, 50 patients (37%) received it as monotherapy, 66 (49%) received venetoclax paired with obinutuzumab (started before venetoclax), 16 (12%) with rituximab, and 4 (3%) with ibrutinib. Among the 16 treated with rituximab, 8 (50%) started rituximab before ibrutinib. Among the 4 treated with paired ibrutinib, 2 (50%) started ibrutinib before venetoclax. At venetoclax initiation, 69% had low TLS risk, 23% had medium TLS risk, and 8.3% had high TLS risk as defined by the package insert. Baseline creatinine clearance (per mL/min per 1.73 m2) was >60 for 79% of patients, between 30 and 60 for 19% of patients, and <30 for 1.5% of patients.
Seventy-four (54%) patients underwent venetoclax escalation exclusively in the outpatient setting, 35 (26%) had at least 1 planned hospitalization for venetoclax initiation/escalation, and 27 (20%) were hospitalized for all dose escalations for prevention of TLS. Those admitted for venetoclax initiation/escalation were commonly admitted the evening before initiation/escalation and discharged after day 2 of the initial or escalated venetoclax dose, in the absence of TLS. Table 1 describes the distribution of inpatient vs outpatient escalations based on tumor burden at time of initiation. The use of venetoclax as monotherapy was more common among those escalated fully or partly in the inpatient setting (67% and 40%, respectively) than those escalated fully in the outpatient setting (20%; P < .001). Those escalated fully in the outpatient setting had a smaller size of largest LN before venetoclax initiation (P = .025) and a lower median ALC (P < .001). As a result, 86% of patients escalated fully in the outpatient setting had low TLS risk, compared with 64% of those escalated partly inpatient and 27% of those escalated fully inpatient. Conversely, 1.4% of patients escalated in the outpatient setting had high TLS risk, compared with 27% of those escalated fully inpatient. Overall, 87% (92/106) of patients followed the standard 5-week dose escalation recommended in the US prescribing information for venetoclax.
The strategies used to prevent TLS are shown in Table 2. During their first escalation (ie, initiation), 86% of patients were given allopurinol, 4% febuxostat, 71% IV hydration, and 18% phosphate binders. Prophylactic rasburicase was given to 9.6% of patients for their first escalation, and 5.3% for their second. Table 3 notes prophylactic strategies used in patients who ultimately developed TLS during ≥1 venetoclax initiation/escalation events. During venetoclax initiation, the use of allopurinol and IV hydration did not differ significantly between those who were escalated in the inpatient vs outpatient setting (P = .6 for both), whereas IV diuretic use, rasburicase, and phosphate binders were used more frequently for those escalated fully in the inpatient setting (P < .01 for all 3 comparisons).
Among the entire cohort, 7 patients (5.1%) developed laboratory TLS and no patient developed clinical TLS (Table 1). Three patients had high TLS risk, 2 had intermediate risk, and 2 had low risk, at the time of venetoclax initiation; 5 of those patients were being treated for relapsed/refractory disease, whereas 2 were being treated in the first-line setting. Of 66 patients who received obinutuzumab before venetoclax, 3 (4.5%) developed TLS. Among the 48 patients who were receiving venetoclax in the firs- line setting, the rate of TLS was 4.2%. Among the 43 patients who received venetoclax in combination with obinutuzumab in the first line setting, the rate of TLS was 4.6%. Of 7 patients, 4 first developed TLS during an inpatient escalation and 4 during an outpatient escalation. Figure 1 graphically depicts all venetoclax escalations ordered by baseline TLS risk and highlights inpatient vs outpatient escalations and TLS development. Table 4 describes the treatments given after TLS was identified. The majority of patients received allopurinol, half received IV hydration, and 1 (10%) received potassium binding agents for management of hyperkalemia. No patients required hemodialysis. supplemental Table 1 provides brief case descriptions of the 7 patients who developed TLS. As shown, 5 of 7 patients developed TLS at the third dose level or later; those patients had electrolyte derangements in earlier escalations but only met TLS criteria at later dose levels. One patient was hospitalized for TLS management and the rest were managed as outpatient. Among the 616 dose escalations, there were 10 instances of TLS (1.6%), because 1 patient had TLS during 2 escalations and 1 patient during 3 escalations.
Swimmer plot depicting TLS events and location of dose escalation across all venetoclax initiation and escalation events.
Swimmer plot depicting TLS events and location of dose escalation across all venetoclax initiation and escalation events.
Table 5 describes characteristics among those who did and did not develop TLS. Beyond TLS risk, no other variables (including race, Eastern Cooperative Oncology Group performance status, venetoclax monotherapy vs paired with another agent, creatinine clearance at initiation or at any point during escalation, and site of venetoclax escalation) were found to be significantly associated with TLS. Although baseline creatinine clearance of <60 vs ≥60 mL/min per 1.73 m2 was associated with higher TLS incidence in univariate analysis, this variable was not predictive of TLS incidence upon adjustment for TLS risk classification in a multivariable analysis, indicating that TLS risk classification is an important confounder in other possible associations.
Fifteen patients developed isolated hyperuricemia (ie, in absence of TLS) at least once during their venetoclax escalation (2 patients developed isolated hyperuricemia twice); 4 were treated with IV hydration, and 4 received rasburicase. Of 15 patients, 4 developed laboratory TLS during a subsequent venetoclax escalation.
Among 62 patients who were hospitalized for at least 1 escalation, the median length of hospital stay was 2.0 days per escalation (IQR, 1.53-2.93; range, 1-26). Patients at high baseline risk of TLS had longer median lengths of hospitalization (2.95 days per escalation) than those at low or intermediate risk (2.0 days per escalation). The total number of inpatient hospitalization days for the 62 patients was 604.
Discussion
In this real-world cohort of 136 patients treated with venetoclax for CLL in a single institution, we describe an overall low incidence of laboratory TLS of 5.1% with no observed clinical TLS and no patients requiring hemodialysis. This is consistent with prior reports of low, but not negligible, incidence of laboratory TLS in this population.6,9 Compared to other reports, our study included a higher number of patients treated with venetoclax as their first-line therapy and a higher number of patients who received paired anti-CD20 therapy (obinutuzumab or rituximab) with venetoclax. Consistent with prior reports, patients who developed TLS were more likely to have high TLS risk at baseline, although TLS was observed across TLS risk groups. Of note, most of the TLS instances occurred at the third dose level or beyond. All of the patients with late TLS events (at the third dose escalation of venetoclax or later) exhibited electrolyte derangements (isolated hyperuricemia or hyperphosphatemia) after treatment with lower doses of venetoclax. Thus, clinicians should consider more frequent laboratory monitoring beyond the second escalation when the uric acid or phosphorus become abnormal during early dose escalations, even if patients do not meet formal criteria for laboratory TLS.
Notably, more than half of the patients in our cohort (54%) were not hospitalized for any of their escalations. The incidence of TLS among outpatient escalations remained very low (2.7%) with only 1 patient required hospitalization for TLS management, with all other cases successfully managed in the outpatient setting. Our report thus provides evidence that outpatient escalation of venetoclax is safe for most patients, which often includes upfront debulking of disease with anti-CD20 therapy and robust prophylactic strategies against TLS such as allopurinol, IV hydration, and rasburicase in patients at higher risk of TLS.
Although the potency of venetoclax as a single agent or in combination with other therapy has transformed the therapeutic landscape in CLL, it has also introduced the challenge of mitigating TLS risk while limiting patients’ and providers’ logistical barriers to effective therapy. Expert consensus guidelines have now incorporated venetoclax into guidelines for risk assessment and prevention of TLS in patients with CLL and recognize a group of patients at higher risk of TLS, although do not address the role of debulking therapy before venetoclax and rely on limited real-world data.13 Overall, our findings are consistent with current guideline recommendations: patients with low or medium TLS risk had low incidence of TLS and can likely be treated fully in the outpatient setting, provided the health system can monitor serial electrolyte levels, provide IV hydration, prevent/control hyperuricemia, and deliver appropriate supportive care.4,13 In the investigated cohort, 20% of the patients with fully outpatient escalations had creatinine clearance of <60 mL/min per 1.73 m2 at least once during their escalation. Although renal impairment was not independently associated with TLS development, we would advocate that baseline TLS risk (including ALC, LN size, and estimated creatinine clearance) be used to determine the safety of inpatient vs outpatient escalation, as noted in current prescribing information. The small minority of patients at high baseline TLS risk (8.3% in our cohort) are most likely to benefit from prophylactic hospitalization, given their high likelihood of TLS development (27% in our cohort). Given the increasing costs of inpatient care, this has important economic implications for cancer centers and practices using venetoclax-based therapy in the treatment of CLL. For example, Moore et al estimated an average cost of $2773 per inpatient night for Medicare patients: based on this figure, the total cost of inpatient hospitalization would be $1.7 million for our cohort of 62 patients who were hospitalized at least once and $27 000 per patient on average.
Unlike those escalated exclusively in the outpatient setting, patients who were hospitalized for all their escalations had a relatively high TLS rate of 15%. This likely reflects the baseline characteristics of these patients, whose clinicians decided that they required inpatient admissions for all their venetoclax escalations. This is demonstrated by their higher risk disease burden (only 27% of these patients were low-risk and 27% were high risk) and possibly unmeasured clinical features.
Our study has several limitations: first, as this is a retrospective study, unmeasured covariates could have influenced the decisions around CLL treatment, TLS prophylaxis, and hospitalization that might lead to confounding of our results. Second, because of the low total number of TLS events, we were unable to build multivariable models to incorporate all possible risk factors for TLS development with sufficient power. Third, not all patients had cross-sectional imaging to provide robust characterization of baseline LN size, which may have underestimated disease burden and TLS risk. Of the 4 patients with low or medium baseline TLS risk who developed laboratory TLS, 2 patients had LN size assessed only by physical examination.
In summary, our study supports the safety of venetoclax in CLL treatment and shows that outpatient escalations are safe for most patients with modern debulking and TLS prophylaxis approaches. Ongoing studies are investigating the efficacy of obinutuzumab in decreasing TLS risk before venetoclax initiation as part of a multifaceted strategy to conduct venetoclax dose escalations outpatient whenever feasible.14 This could result in significant cost reductions and simplify the administration of this efficacious medication for both patients with CLL and providers.
Acknowledgment
The research described here was funded through a grant by Sanofi.
Authorship
Contribution: Y.K.V. collected patient data, designed the analysis, and drafted the manuscript; D.N. and A.D. conducted the statistical analysis and designed the tables and figures; S.S., C.K., R.O., M.T., and L.E.R. provided feedback on the manuscript and analysis; M.B.G. supervised the entire work and provided feedback on the manuscript and analysis; and all authors approved the submitted version of the manuscript.
Conflict-of-interest disclosure: Y.K.V. received a 1-time consulting fee from EastRx. L.E.R. has served as a consultant for AbbVie, Ascentage, AstraZeneca, BeiGene, Janssen, Loxo Oncology, Pharmacyclics, Pfizer, and TG Therapeutics; is a member of a data safety monitoring committee for Ascentage; served as a continuing medical education speaker for Dava Oncology, Curio, Medscape, and PeerView; holds minority ownership interest in Abbott Laboratories; received travel support from Loxo Oncology; and has received research funding (paid to the institution) from Adaptive Biotechnologies, AstraZeneca, Genentech, AbbVie, Pfizer, Loxo Oncology, Aptose Biosciences, Dren Bio, and Qilu Puget Sound Biotherapeutics. M.T. consulted or was on advisory boards for AbbVie, AstraZeneca, BeiGene, Janssen, and Loxo Oncology; received travel support from Genmab, Nurix Therapeutics, and Dava Oncology; received honoraria from PeerView Medical Institute, Dava Oncology, Philips Group Oncology Communications, MJH Life Sciences, Intellisphere LLC, and Clinical Care Options; and received research funding (to the institution) from AbbVie, AstraZeneca, BeiGene, GenMab, Nurix Therapeutics, and Genentech. M.B.G. received research support from Sanofi, Amgen, Actinium Pharmaceuticals, and Tigen, and served on advisory boards for Sanofi, Takeda, and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Yannis K. Valtis, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; email: kalogii@mskcc.org.
References
Author notes
The data sets generated and/or analyzed during this study are available on reasonable request (on condition that patient confidentiality is maintained) from the corresponding author, Yannis K. Valtis (kalogii@mskcc.org).
Presented, in part, at the 2024 conference of the American Society for Clinical Oncology (abstract no. 7045), Chicago, IL, 3 June 2024.
The full-text version of this article contains a data supplement.