TO THE EDITOR:
Hematopoietic stem and progenitor cells (HSPCs) are required for hematopoietic cell transplantation (HCT) and HSPC-based gene therapies.1,2 Current practices for harvesting HSPCs with granulocyte colony-stimulating factor (G-CSF) involve a multiday procedure with suboptimal HSPC yields in up to 30% of patients and are associated with some morbidity, including significant bone pain and, on rare occasions, splenic rupture, myocardial infarction, cerebral ischemia, as well as vaso-occlusive episodes in patients with sickle cell disease.3-8 These inherent disadvantages of G-CSF have driven efforts to identify alternative HSPC mobilization strategies.9,10
We and others previously reported that BIO5192, a small molecule inhibitor of the integrin α4β1 (VLA-4), or BOP, a dual α4β1/α9β1 inhibitor, induced rapid and reversible mobilization of murine HSPCs and are synergistic when combined with G-CSF and/or the CXCR4 antagonist plerixafor.11,12 More recently, studies combining a truncated version of the CXCR2 agonist growth-related oncogene protein-β (tGro-β) with plerixafor or a VLA-4 inhibitor demonstrated significant HSPC mobilization within minutes in mice.13-15 However, the transient nature of HSPC mobilization induced by these dual combinations of a CXCR2 agonist, CXCR4 antagonist, or a VLA-4 inhibitor represents an obstacle for their continued development because they may provide insufficient time for adequate collection of HSPCs from donor peripheral blood. Here, we tested the efficacy of murine HSPC mobilization after simultaneous administration of tGro-β with VLA-4 and CXCR4 inhibitors.
We first assessed the HSPC mobilization efficiency of CWHM-823 (a VLA-4 inhibitor14; Figure 1A), plerixafor, and tGro-β alone and in combination. Simultaneous administration of all 3 drugs significantly and synergistically increased the numbers of circulating colony forming units (CFUs; measure of HSPC mobilization) compared with each agent alone and the dual combinations (Figure 1B). However, although the numbers of CFUs remained significantly elevated at 4 hours after administration of the triple combination, the amount of mobilized CFUs had decreased 3.5-fold at 4 hours compared with the mobilization peak at 30 minutes (Figure 1B).
SLU-2609 is a potent inhibitor of VLA4 in vitro and mobilizes CFUs to peripheral blood in mice. (A) Structures of various canonical and novel VLA4 inhibitors. (B) BALB/c mice were injected subcutaneously (SC) with every combination of tGro-β (2.5 mg/kg), CXCR4 inhibitor plerixafor (5 mg/kg), and/or VLA4 inhibitor CWHM-823 (3 mg/kg). tGro-β was always administered as a separate SC injection. Mice treated with the triple combination of a VLA-4 inhibitor, plerixafor and tGro-β were first injected SC with a mixture of the VLA-4 inhibitor plus plerixafor followed immediately thereafter by a SC injection of tGro-β. Peripheral blood samples were collected 30, 120, and 240 minutes after injection. Samples were cultured for 6 to 8 days in mouse methylcellulose complete media and CFUs were quantified. Data are mean ± SD of 2 independent experiments; n = 8 to 10 mice per cohort. (C) G2 acute lymphoblastic leukemia cells expressing VLA-4 were preincubated with the VLA4 inhibitors shown in panel A for 15 minutes at RT. Recombinant soluble VCAM-1/Fc chimera protein was added, and mixtures were cultured for an additional 30 minutes at RT. Binding of VCAM-1 was detected by flow cytometry using a phycoerythrin-conjugated donkey anti-human Fc antibody and compared with a phycoerythrin-conjugated donkey IgG isotype control. Data are mean ± SEM of 3 independent experiments, in which samples were analyzed in duplicate in each experiment. (D) DBA/2J mice were left untreated or injected with 3 mg/kg of the indicated VLA4 inhibitors and peripheral blood samples were collected 30, 120, and 240 minutes after injection. Samples were cultured for 6 to 8 days in mouse methylcellulose complete media, and CFUs were quantified. Data are mean ± SD of 3 independent experiments; n = 15 mice per cohort. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. IgG, immunoglobulin G; RT, room temperature; SD, standard deviation; SEM, standard error of the mean.
SLU-2609 is a potent inhibitor of VLA4 in vitro and mobilizes CFUs to peripheral blood in mice. (A) Structures of various canonical and novel VLA4 inhibitors. (B) BALB/c mice were injected subcutaneously (SC) with every combination of tGro-β (2.5 mg/kg), CXCR4 inhibitor plerixafor (5 mg/kg), and/or VLA4 inhibitor CWHM-823 (3 mg/kg). tGro-β was always administered as a separate SC injection. Mice treated with the triple combination of a VLA-4 inhibitor, plerixafor and tGro-β were first injected SC with a mixture of the VLA-4 inhibitor plus plerixafor followed immediately thereafter by a SC injection of tGro-β. Peripheral blood samples were collected 30, 120, and 240 minutes after injection. Samples were cultured for 6 to 8 days in mouse methylcellulose complete media and CFUs were quantified. Data are mean ± SD of 2 independent experiments; n = 8 to 10 mice per cohort. (C) G2 acute lymphoblastic leukemia cells expressing VLA-4 were preincubated with the VLA4 inhibitors shown in panel A for 15 minutes at RT. Recombinant soluble VCAM-1/Fc chimera protein was added, and mixtures were cultured for an additional 30 minutes at RT. Binding of VCAM-1 was detected by flow cytometry using a phycoerythrin-conjugated donkey anti-human Fc antibody and compared with a phycoerythrin-conjugated donkey IgG isotype control. Data are mean ± SEM of 3 independent experiments, in which samples were analyzed in duplicate in each experiment. (D) DBA/2J mice were left untreated or injected with 3 mg/kg of the indicated VLA4 inhibitors and peripheral blood samples were collected 30, 120, and 240 minutes after injection. Samples were cultured for 6 to 8 days in mouse methylcellulose complete media, and CFUs were quantified. Data are mean ± SD of 3 independent experiments; n = 15 mice per cohort. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. IgG, immunoglobulin G; RT, room temperature; SD, standard deviation; SEM, standard error of the mean.
We next sought to develop novel VLA-4 antagonists that exhibited increased solubility and extended HSPC mobilization in vivo. To do this, we used BIO5192, as well as our previously described VLA-4 inhibitors CWHM-823 and CWHM-824,14 as scaffolds and synthesized variations based on these structures (Figure 1A). We generated SLU-2609 (Figure 1A; supplemental Information Methods), a novel VLA-4 inhibitor, which contains a polyethylene glycol (PEG) chain (16-28 units) to prolong its half-life and solubility. A non-PEGylated compound, SLU-2615, was generated as a control. Using a soluble VCAM-1 binding assay,12,14 we observed that SLU-2609 maintained a half maximal 50% inhibitory concentration in the low nanomolar range (similar to BIO5192, CWHM-823, and CWHM-824), suggesting efficient inhibition of VLA-4 (Figure 1C). Furthermore, SLU-2609 alone induced murine HSPC mobilization to similar levels as BIO5192, with significantly increased numbers of circulating HSPCs at 4 hours compared with SLU-2615, the non-PEGylated control (Figure 1D).
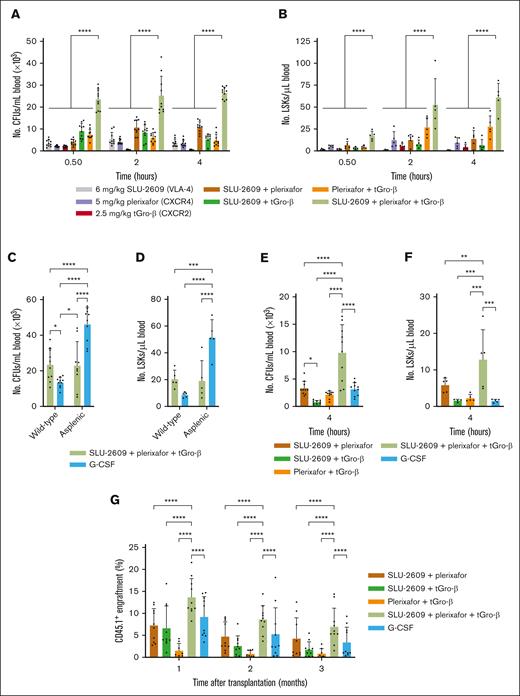
We next assessed the efficacy of murine HSPC mobilization by SLU-2609, plerixafor, and tGro-β alone and in combination. Similar to CWHM-823 (Figure 1B), simultaneous administration of SLU-2609 with plerixafor and tGro-β significantly increased the number of circulating CFUs (Figure 2A) and Lin–Sca-1+c-Kit– (LSK) stem cells (Figure 2B) compared with each agent alone and the dual combinations. However, in contrast to CWHM-823, which displayed reduced HSPC mobilization at 4 hours after treatment (Figure 1B), the triple combination regimen with SLU-2609 induced peak CFU and LSK mobilization after 4 hours. Similar to before, we observed a significant synergistic effect when targeting all 3 receptors simultaneously. Specifically, the addition of the third compound to any 2-compound combination resulted in a significant synergistic increase in mobilized CFUs and LSKs across all time points (P < .0001).
Enhanced mobilization and competitive repopulation with triple combination of SLU-2609, plerixafor, and tGro-β. (A-B) DBA/2J mice were injected with every combination of tGro-β (2.5 mg/kg), CXCR4 inhibitor plerixafor (5 mg/kg), and/or VLA4 inhibitor SLU-2609 (6 mg/kg). tGro-β was always administered as a separate SC injection. Mice treated with the triple combination of a VLA-4 inhibitor, plerixafor, and tGro-β were first injected SC with a mixture of the VLA-4 inhibitor plus plerixafor followed immediately thereafter by a SC injection of tGro-β. Peripheral blood samples were collected 30, 120, and 240 minutes after injection. Numbers of circulating CFUs (A; n = 10) and LSK cells (B; n = 5) were analyzed. Data are mean ± SD. (C-D) Wild-type and splenectomized DBA/2J mice were treated with G-CSF (9 doses every 12 hours; 125 μg/kg per dose) or the triple combination of tGro-β (2.5 mg/kg), plerixafor (5 mg/kg), and SLU-2609 (9 mg/kg). Treatment in splenectomized mice began 7 days after splenectomy. Numbers of circulating CFUs (A) and LSK cells (B) were analyzed from peripheral blood taken after completion of the 5-day G-CSF regimen or 4 hours after injection of the triple combination. Data are mean ± SD of 2 independent experiments; n = 10 mice per cohort. (E-F) C57BL/6J mice were injected with G-CSF (9 doses every 12 hours; 125 μg/kg per dose), tGro-β (2.5 mg/kg), plerixafor (5 mg/kg), and/or SLU-2609 (9 mg/kg). Numbers of circulating CFUs (E; n = 9-10) and LSK cells (F; n = 4-5) were analyzed from peripheral blood taken at 4 hours after injection. Data are mean ± SD. (G) Competitive repopulation assay. Blood (10 μL) from CD45.1+ donors (BALB/c; n = 3 per cohort) mobilized with G-CSF (9 doses every 12 hours; 125 μg/kg per dose), tGro-β (2.5 mg/kg), plerixafor (5 mg/kg), and/or SLU-2609 (9 mg/kg) was mixed with CD45.2+ competitor BM cells (BALB/cJ; n = 2 donors; 2.5 × 105 cells per recipient) and transplanted into lethally irradiated primary CD45.2+ hosts (BALB/cJ; n = 8-10 recipients). The contribution of mobilized cell populations to hematopoiesis was determined by flow cytometry for CD45.1+ donor cells within the CD45+CD3– compartment monthly after transplantation. Data are mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. BM, bone marrow; SD, standard deviation.
Enhanced mobilization and competitive repopulation with triple combination of SLU-2609, plerixafor, and tGro-β. (A-B) DBA/2J mice were injected with every combination of tGro-β (2.5 mg/kg), CXCR4 inhibitor plerixafor (5 mg/kg), and/or VLA4 inhibitor SLU-2609 (6 mg/kg). tGro-β was always administered as a separate SC injection. Mice treated with the triple combination of a VLA-4 inhibitor, plerixafor, and tGro-β were first injected SC with a mixture of the VLA-4 inhibitor plus plerixafor followed immediately thereafter by a SC injection of tGro-β. Peripheral blood samples were collected 30, 120, and 240 minutes after injection. Numbers of circulating CFUs (A; n = 10) and LSK cells (B; n = 5) were analyzed. Data are mean ± SD. (C-D) Wild-type and splenectomized DBA/2J mice were treated with G-CSF (9 doses every 12 hours; 125 μg/kg per dose) or the triple combination of tGro-β (2.5 mg/kg), plerixafor (5 mg/kg), and SLU-2609 (9 mg/kg). Treatment in splenectomized mice began 7 days after splenectomy. Numbers of circulating CFUs (A) and LSK cells (B) were analyzed from peripheral blood taken after completion of the 5-day G-CSF regimen or 4 hours after injection of the triple combination. Data are mean ± SD of 2 independent experiments; n = 10 mice per cohort. (E-F) C57BL/6J mice were injected with G-CSF (9 doses every 12 hours; 125 μg/kg per dose), tGro-β (2.5 mg/kg), plerixafor (5 mg/kg), and/or SLU-2609 (9 mg/kg). Numbers of circulating CFUs (E; n = 9-10) and LSK cells (F; n = 4-5) were analyzed from peripheral blood taken at 4 hours after injection. Data are mean ± SD. (G) Competitive repopulation assay. Blood (10 μL) from CD45.1+ donors (BALB/c; n = 3 per cohort) mobilized with G-CSF (9 doses every 12 hours; 125 μg/kg per dose), tGro-β (2.5 mg/kg), plerixafor (5 mg/kg), and/or SLU-2609 (9 mg/kg) was mixed with CD45.2+ competitor BM cells (BALB/cJ; n = 2 donors; 2.5 × 105 cells per recipient) and transplanted into lethally irradiated primary CD45.2+ hosts (BALB/cJ; n = 8-10 recipients). The contribution of mobilized cell populations to hematopoiesis was determined by flow cytometry for CD45.1+ donor cells within the CD45+CD3– compartment monthly after transplantation. Data are mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. BM, bone marrow; SD, standard deviation.
In addition to increasing the number of circulating HSPCs ∼50-fold (Figure 2A-D), the triple combination also induced rapid pan-hematopoietic cell mobilization (supplemental Figure 1A), with fold change in the magnitude of subset mobilization relative to baseline blood values varying as follows (fold change indicated in parenthesis; supplemental Figure 1B): plasmacytoid dendritic cells (10×) > neutrophils (9.4×) > nonclassical monocytes (6.6×) > B cells (3.8×) > classical monocytes (2.9×) > natural killer cells (2.9×) > eosinophil (2.7×) > conventional dendritic cells (2.1×) > CD8 T cells (1.5×) > CD4 T cells (1.1×). Among T cells, naïve CD8 (twofold) and central memory CD4 (2.2-fold) demonstrated greater mobilization than other effector and memory T-cell subsets (supplemental Figure 3).
To evaluate the role of the spleen in HSPC mobilization, we administered the triple combination regimen to both splenectomized and nonsplenectomized mice. Splenectomies had no effect on the levels of CFU (Figure 2C) or LSK (Figure 2D) mobilization in mice treated with the triple combination of SLU-2609, plerixafor, and tGro-β (P = .918), suggesting that the HSPCs mobilized via this regimen are primarily bone marrow derived. In contrast, splenectomized mice mobilized with a 5-day regimen of G-CSF showed significantly increased levels of circulating CFUs and LSKs compared with wild type mice receiving the same regimen (P < .0001), consistent with previous data suggesting sequestration of HSPCs in the spleen during G-CSF treatment.12 Notably, the triple regimen mobilized significantly higher levels of CFUs and LSKs than G-CSF in wild type DBA/2 mice (Figure 2C-D). A slightly higher percentage of circulating LSK cells were in G0 phase of the cell cycle in mice mobilized with the triple combination than that of mice treated with G-CSF for 5 days (16.2% vs 10.4% in G0 in triple combination vs G-CSF, respectively; P = .04; supplemental Figure 3).
We also compared the triple combination regimen with the standard of care G-CSF in a historically poor mobilizing strain of mouse, C57BL/6J.16 Blood collected 4 hours after treatment of C57BL/6J mice with SLU-2609, plerixafor, and tGro-β showed significantly higher levels of CFUs (Figure 2E) and LSKs (Figure 2F) than blood from mice receiving 5 days of G-CSF injections.
Finally, we assessed whether cells mobilized with the SLU-2609, plerixafor, and tGro-β regimen could provide long-term trilineage engraftment in lethally irradiated mice using a competitive HSCT assay.14 Mice that received transplant with cells mobilized via the triple combination regimen showed significantly higher repopulation of CD45.1+ cells obtained from the mobilized donors than did G-CSF–mobilized grafts at months 1, 2, and 3 (P = .0074, .043, and .0327 respectively), suggesting a higher frequency of engrafting cells within the grafts mobilized via the triple combination. (Figure 2G).
In summary, we designed a novel PEGylated VLA4 inhibitor, SLU-2609, that effectively inhibits interaction of the receptor with VCAM-1 in vitro and mobilizes high levels of HSPCs in combination with plerixafor and tGro-β. This triple combination regimen was well tolerated (no observed toxicities) and outperformed G-CSF in in vivo mobilization assays, as well as in the competitive transplant setting. Thus, not only did this combination mobilize more HSPCs than G-CSF, but it did so in a drastically reduced time frame of hours compared with days required for G-CSF mobilization.
Pepinsky et al17 previously demonstrated that targeted PEGylation of BIO5192 did not significantly reduce its VLA-4 specificity or inhibitory capacity while significantly improving its pharmacokinetic and pharmacodynamic properties. Although HSPC mobilization was not evaluated, a single injection of a 20 kDa PEGylated BIO5192 produced a sustained lymphocytosis in rats that lasted for 6 days and significantly delayed paralysis associated with an experimental autoimmune encephalomyelitis model.17 Similar lymphocytosis was observed in rats after treatment with a 40 kDa branched PEG containing 3 arms of a novel VLA-4 small molecule inhibitor.18 The PEG attached to SLU-2609 in our studies contained a mixture of PEG molecules ranging from 16 to 28 units (0.7-1.2 kDa). Attachment of longer PEG units holds promise to generate VLA-4 targeting compounds with even more favorable pharmacokinetic properties with preserved in vitro and in vivo potency.
The applications of a kinetically favorable, effective mobilizing regimen are not limited to HSPC collection for HCT. Recently, Li et al15 used an in vivo gene therapy strategy for hemoglobinopathies after HSPC mobilization with tGro-β and plerixafor. This strategy, which depends on timely and efficient mobilization followed by in vivo gene targeting, successfully ameliorated the thalassemia phenotype in diseased mice.15 Similarly, because G-CSF is contraindicated in patients with sickle cell disease,6,19 and plerixafor does not reliably yield optimal HSPC numbers for gene-correction therapy applications,20,21 novel HSPC mobilization regimens that are less toxic, more rapid, and more potent than G-CSF and plerixafor are needed. Our triple combination regimen may improve these gene therapy strategies and allow for collection of more HSPCs for HCT.
Acknowledgments: The authors thank the Siteman Flow Cytometry Core for assistance with flow cytometry and the Division of Comparative Medicine at Washington University for their excellent animal care. This work was supported by grants from the NIH/NCI: NCI Research Specialist Award (R50 CA211466; M.P.R.) and an NCI Outstanding Investigator Award (R35 CA197561; J.F.D.). Funding was also provided by the Alvin J. Siteman Cancer Center, Siteman Investment Program with contributions from the National Cancer Institute, Cancer Center Support Grant (P30 CA091842), Barnard Trust, and The Foundation for Barnes-Jewish Hospital, Cancer Frontier Fund (M.P.R.).
Contribution: D.C. and M.P.R. designed and performed experiments, analyzed data, and wrote the manuscript; H.T., M.S., M.J.M., and P.G.R. designed and synthesized the novel VLA-4 inhibitors; S.C., E.C., D.K., L.G., J.K.R., and M.P. designed and performed experiments; F.G. performed statistical analyses; and J.F.D supervised the project, designed the research, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: J.F.D. receives research funding from NeoImmuneTech, MacroGenics, Incyte, and Bioline Rx; has equity ownership in Magenta Therapeutics, Wugen; and is a board member for hC Bioscience Inc, RiverVest Venture Partners. The remaining authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8007, St. Louis, MO 63110; email: jdipersi@wustl.edu.
References
Author notes
D.C. and M.P.R contributed equally to this work.
Data are available upon request from the corresponding author, John F. DiPersio (jdipersi@wustl.edu).
The full-text version of this article contains a data supplement.