Key Points
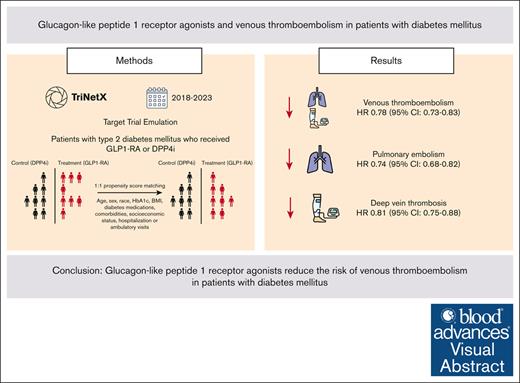
The use of GLP1-RA is associated with a lower risk of 1-year VTE compared with DPP4i in patients with T2DM.
Similar reduction in VTE risk was observed in patients with T2DM regardless of obesity status.
Visual Abstract
Glucagon-like peptide 1 receptor agonists (GLP1-RA) are antidiabetic agents recently approved for weight loss. Obesity is an established risk factor for venous thromboembolism (VTE). Moreover, preclinical studies have shown that GLP1-RA may attenuate thromboxane-induced platelet activation. Therefore, we hypothesized that GLP1-RA use may reduce the risk of VTE. We performed a target trial emulation (TTE) using a population-based database of electronic health records to evaluate whether GLP1-RA use is associated with a reduction in VTE in patients with type 2 diabetes mellitus (T2DM) compared with dipeptidyl peptidase-4 inhibitors (DPP4i). Patients who were newly initiated on GLP1-RA were propensity score matched to patients who were newly initiated on DPP4i. We evaluated the primary outcome, composite VTE, identified using ICD-10 (International Classification of Diseases, Tenth Revision) codes, within 12 months of the initiation of GLP1-RA or DPP4i. The study cohort comprised 540 258 patients with 270 129 individuals receiving either GLP1-RA or DPP4i. Over 12 months of follow-up, patients who received GLP1-RA had a lower incidence of VTE compared with patients who received DPP4i (6.1 vs 7.6 events per 1000 patient-years; hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.73-0.83). This was similar for pulmonary embolism (2.9 vs 3.8 events per 1000 patient-years; HR, 0.74; 95% CI, 0.68-0.82) and deep vein thrombosis (3.9 vs 4.7 events per 1000 patient-years; HR, 0.81; 95% CI, 0.75-0.88). In this propensity score-matched, TTE study, patients with T2DM who received a GLP1-RA had a lower risk of VTE at 1 year compared with patients who received DPP4i.
Background
Venous thromboembolism (VTE) affects ∼1 to 3 in 1000 adults each year and contributes to substantial morbidity and mortality.1 In the United States, VTE accounts for ∼100 000 deaths annually.2 VTE can lead to severe long-term complications such as post-thrombotic syndrome as well as recurrent episodes of VTE, underscoring the need for effective strategies for VTE prevention.3,4 Current guidelines recommend individualized approaches to thromboprophylaxis, including the use of pharmacologic anticoagulants for high-risk patients and mechanical prophylaxis, such as graduated compression stockings or intermittent pneumatic compression devices, for certain surgical and hospitalized populations.5,6
Obesity is a well-established risk factor for VTE due to its association with a proinflammatory and prothrombotic state.7 It is estimated that obesity accounts for up to 30% of the population's risk of VTE.8 The rising prevalence of obesity is an important contributor to the population-level increase in VTE.9,10 Glucagon-like peptide 1 receptor agonists (GLP1-RA) are antihyperglycemic agents originally approved for patients with type 2 diabetes mellitus (T2DM). Newer, high-intensity GLP1-RAs produce substantial weight loss and are also approved for weight management in patients with overweight or obesity, also without T2DM.11 Among patients with T2DM, the use of GLP1-RA improves long-term cardiovascular outcomes, but prior studies have predominantly focused on atherosclerotic disease and heart failure.11-16 Weight loss has been associated with a significant reduction in VTE risk,17,18 although this has not been studied in large, randomized trials. Moreover, in preclinical studies, GLP1-RA therapy attenuates thromboxane and thrombin-induced platelet aggregation.19,20 Given these observations, we hypothesized that GLP1-RA use may be associated with a reduced risk of VTE. To test this hypothesis, we conducted a target trial emulation (TTE) study by leveraging a large global database to assess the association between GLP1-RA and VTE risk in patients with T2DM.
Methods
Overview of study design
We used a TTE framework to evaluate the comparative efficacy of GLP1-RA vs dipeptidyl peptidase-4 inhibitors (DPP4i) on the risk of VTE in patients with T2DM.21 The TTE framework strengthens causal inference by emulating the design of a randomized controlled trial (RCT) using observational data, thus addressing biases that arise in nonrandomized settings.22,23 TTE is a framework designed to simulate the design and analysis of an RCT using observational data.21-23 Unlike traditional administrative database analyses, which may lack clearly defined treatment strategies or temporal relationships, TTE explicitly specifies key components of a hypothetical RCT, including eligibility criteria, treatment initiation, follow-up periods, and outcome measures. This approach minimizes biases such as immortal time bias by ensuring that the timing of treatment initiation and follow-up aligns with real-world clinical scenarios. Additionally, the use of propensity score matching may be used to balance baseline characteristics between treatment groups, mimicking the randomization process of RCTs. By structuring observational data analysis within this framework, TTE enhances the validity of causal inferences and reduces the methodological limitations often associated with administrative database studies. We selected patients on DPP4i as the control cohort as both GLP1-RA and DPP4i enhance the incretin effect to increase insulin secretion and achieve glycemic control.24 However, DPP4i are not associated with weight loss or VTE risk.25,26 Supplemental Table 1 lists the key protocol components.
Data source
The TriNetX Analytics Network contains deidentified electronic health records of >250 million patients receiving health care at >140 health care institutions including academic medical centers, community hospitals, and specialty physician practices in 21 countries.27 The dataset includes comprehensive information on inpatient and outpatient clinical encounters, including diagnosis codes, procedures performed, medication prescriptions, and laboratory values.27 Patient-level analyses were conducted on the TriNetX Analytic platform, with aggregated results available to investigators. The TriNetX platform is widely used in clinical research and has been validated in numerous studies for its reliability in supporting observational research and TTE analyses.27-30 The study was deemed exempt by the institutional review board at Beth Israel Deaconess Medical Center.
Study population
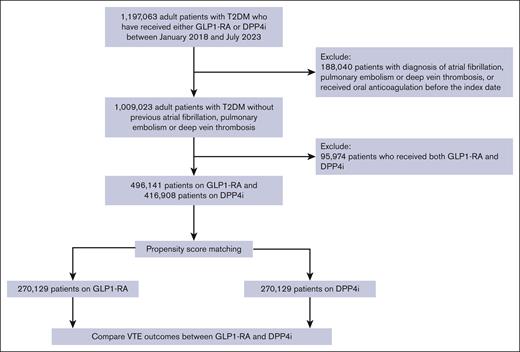
We included patients with a diagnosis of T2DM who received one or more prescriptions of either GLP1-RA or DPP4i between January 2018 and July 2023 (supplemental Table 2). We excluded patients with a prior prescription of either GLP1-RA or DPP4i, concomitant use of GLP1-RA and DPP4i, as well as those with any prior known history of VTE, atrial fibrillation, or any previous or current use of oral anticoagulation to focus on the effect of GLP1-RA on incident VTE (supplemental Table 3).
Treatment strategies
The treatment strategies were the initiation of GLP1-RA (intervention) or DPP4i (control). The list of GLP1-RA and DPP4i medications are summarized in supplemental Table 4. Consistent with an intention-to-treat design, the treatment strategy was assigned at baseline regardless of study medication (GLP1-RA or DPP4i) switch or add-on.
Outcomes
The primary outcome was the occurrence of VTE, defined as a composite of pulmonary embolism (PE) and deep vein thrombosis (DVT). The secondary outcomes were individual components of PE and DVT. These outcomes were identified using ICD-10 (International Classification of Diseases, Tenth Revision) codes (supplemental Table 2).31
Follow-up
Each eligible patient contributed data until the occurrence of the outcomes, death, or 12 months after initiation of treatment, whichever occurred first. Patients were censored at the time of disenrollment or the last recorded encounter in the TriNetX database, allowing for time-to-event analyses. This approach accounts for varying durations of follow-up among patients, with data considered up to the last available data point.
Statistical analysis
The GLP1-RA and DPP4i cohorts were propensity score matched in a 1:1 ratio using predetermined variables that included age, sex, race, underlying comorbidities based on components of the Charlson Comorbidity Index, hemoglobin A1c (HbA1c), body mass index (BMI), glomerular filtration rate (GFR), socioeconomic status, health care utilization (as measured by outpatient visits and hospitalization episodes), and the use of cardiovascular and other diabetes medications (supplemental Table 5).32 We selected these variables because they alter the likelihood of receiving treatment, may be associated with the outcomes, and may be potential confounders.33 Propensity score matching was performed using a nearest neighbor greedy matching algorithm with a caliper of 0.1 times the pooled standard deviation. Patients with missing data were still included in the propensity score matching. The missing values did not contribute to the matching process for the affected variables as propensity scores were calculated using available data. We compared the differences in baseline characteristics between the GLP1-RA and DPP4i cohorts using the standardized mean differences. We considered a standardized mean difference <10% as an acceptably balanced distribution of covariates between the cohorts.34 Kaplan-Meier survival curves were plotted to visualize the survival differences between the 2 cohorts. Survival distributions of the outcomes between the 2 groups were compared using the log-rank test. We estimated the effect of GLP1-RA use on each outcome using the Cox proportional hazards model. The proportional hazards assumption for the Cox models was tested using Schoenfeld residuals. Continuous variables were expressed as mean ± standard deviation, while categorical variables were reported as proportions. A 2-sided P value <.05 was considered statistically significant.
Subgroup and sensitivity analyses
We performed the following additional subgroup and sensitivity analyses:
Subgroup analysis stratified by the presence of obesity at baseline: BMI ≥30 kg/m2 (patients with obesity) and BMI <30 kg/m2 (patients with normal weight or overweight). BMI data were captured within 3 months of the index date. Patients without BMI data were excluded from the analyses.
Subgroup analysis stratified by BMI categories (18.5-24.9, 25.0-29.9, 30.0-34.9, 35.0-39.9, ≥40 kg/m2).
Subgroup analysis according to age (≥ 65 or <65 years old) and sex (male or female).
Sensitivity analysis restricted to patients with BMI, HbA1c, and GFR data available within 3 months prior to the index date.
Sensitivity analysis by relaxing the exclusion criteria and allowing patients with a prior history of VTE, atrial fibrillation, or received oral anticoagulation.
Sensitivity analysis by combining VTE and mortality as a composite outcome to address the possible competing risk of death.
Sensitivity analysis comparing GLP1-RA with other antidiabetic medications including metformin, insulin, sodium-glucose cotransporter-2 inhibitors, sulfonylureas, and thiazolidinediones.
Falsification analyses to test for residual confounding. We evaluated the effect of GLP1-RA on the incidence of appendicitis and dermatitis (identified using ICD-10 codes) which were treated as negative controls (supplemental Table 2).
All analyses were conducted on the TriNetX platform, which utilizes Survival 3.2 to 3 in R 4.0.2 for survival analysis and incorporates data science and statistical libraries in Python 3.7 and Java 11.0.16 for various analytical functions, including propensity score matching and comparative cohort analysis.
Results
Among 913 049 patients with T2DM between January 2018 and July 2023, originating from 140 health care organizations from 21 countries, 496 141 patients received 1 or more prescriptions for GLP1-RA and 416 908 patients received one or more prescriptions for DPP4i (Figure 1). Before propensity score matching, the GLP1-RA and DPP4i cohorts differed by age, sex, race, mean BMI, and the use of other diabetes medications. In particular, patients receiving GLP1-RA were younger (mean age, 56.7 ± 12.9 vs 63.8 ± 13.1) and tended to be obese (mean BMI, 36.0 ± 8.1 vs 31.0 ± 7.4). After propensity score matching, the study cohort included 540 258 patients (270 129 in each treatment strategy), with well-balanced demographic and clinical characteristics (Table 1). The mean age at index date was 60.1 ± 12.1 and 60.2 ± 12.8 years for the cohorts receiving GLP1-RA and DPP4i, respectively. The mean BMI (33.8 ± 7.7 vs 32.9 ± 7.3 kg/m2) and the mean HbA1c (8.4 ± 2.0% vs 8.4 ± 2.0%) were similar between the 2 cohorts. Approximately 14.5% of the patients had a diagnosis of neoplasm, 21.2% were on aspirin, and 2.5% were on hormonal contraceptives for both cohorts. Approximately 60% of patients had BMI data, 60% had HbA1c data, and 65% had GFR data within 1 year of the index date. Supplemental Table 6 summarizes the list of GLP1-RA and DPP4i after propensity score matching.
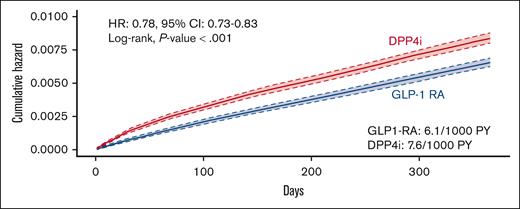
The incidence of VTE was 6.1 events per 1000 patient-years among patients receiving GLP1-RA compared with 7.6 events per 1000 patient-years among patients receiving DPP-4i (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.73-0.83; log-rank test, P < .001) (Figures 2 and 3). The incidence of PE was 2.9 events per 1000 patient-years among patients receiving GLP1-RA compared with 3.8 among patients receiving DPP-4i (HR, 0.74; 95% CI, 0.68-0.82; log-rank test, P < .001). Similarly, the incidence of DVT was lower among patients receiving GLP1-RA (3.9 vs 4.7 events per 1000 patient-years; HR, 0.81; 95% CI, 0.75-0.88; log-rank test, P < .001) (Figure 2; supplemental Figures 1 and 2).
Risk of VTE in patients with T2DM receiving GLP-1RA or DPP4i therapy. PY, patient-years.
Risk of VTE in patients with T2DM receiving GLP-1RA or DPP4i therapy. PY, patient-years.
Subgroup and sensitivity analyses
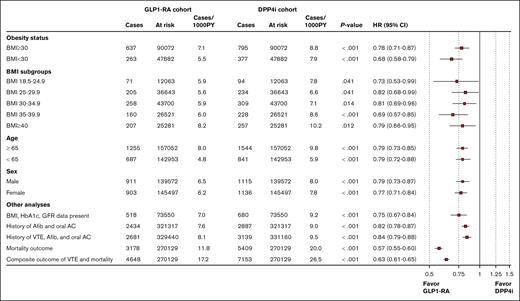
The use of GLP1-RA as compared with DPP4i significantly reduced the risk of VTE in patients with obesity (HR, 0.78; 95% CI, 0.71-0.87; log-rank test, P < .001) as well as without obesity (HR, 0.68; 95% CI, 0.58-0.79; log-rank test, P < .001). The VTE reduction associated with GLP1-RA was observed for all BMI categories with the greatest reduction observed for patients with class 2 obesity. Similar VTE reductions were observed in different subgroups according to age and sex as well as additional analyses comprising patients who had laboratory data within 3 months prior to the index date and after including patients with a history of VTE, atrial fibrillation, or having received oral anticoagulation (Figure 4). The use of GLP1-RA, as compared with DPP4i, significantly reduced all-cause mortality (HR, 0.57; 95% CI, 0.55-0.60; log-rank test, P < .001). Similarly, GLP1-RAs were associated with a lower rate of a composite outcome of all-cause mortality and VTE (HR, 0.63; 95% CI, 0.61-0.65; log-rank test, P < .001) (Figure 4). When compared to other T2DM agents including metformin, insulin, and sodium-glucose cotransporter-2 inhibitor, the use of GLP1-RA was also associated with a lower risk of VTE (Figure 5).
Subgroup and sensitivity analyses evaluating the effect of GLP1-RA on VTE. AC, anticoagulation; Afib, atrial fibrillation; PY, patient-years.
Subgroup and sensitivity analyses evaluating the effect of GLP1-RA on VTE. AC, anticoagulation; Afib, atrial fibrillation; PY, patient-years.
Sensitivity analyses comparing GLP1-RA with other antidiabetic medications on VTE. PY, patient-years; SGLT2i, sodium-glucose cotransporter-2 inhibitors; TZD, thiazolidinedione.
Sensitivity analyses comparing GLP1-RA with other antidiabetic medications on VTE. PY, patient-years; SGLT2i, sodium-glucose cotransporter-2 inhibitors; TZD, thiazolidinedione.
In falsification analyses, we observed no statistically significant differences between patients who received GLP1-RA and those who received DPP-4i in the risk of appendicitis (0.4 vs 0.4; HR, 1.01 [95% CI, 0.77-1.32]) or dermatitis (51 vs 49.6; HR, 0.99 [95% CI, 0.97-1.02]) (Supplemental Figure 3).
Discussion
Our propensity score-matched TTE evaluating the effect of GLP1-RA compared with DPP-4i on VTE risk among patients with T2DM produced 3 novel findings. First, in the 12 months following initiation of therapy, patients with T2DM who received a GLP1-RA had fewer VTE events compared with those who received DPP4i. Second, subgroup analysis suggested that VTE risk was reduced in all patients regardless of obesity status at the time of initiation. Third, sensitivity analysis showed that GLP1-RA was also associated with a lower VTE risk compared with other antidiabetic agents. To our knowledge, this is the first study to evaluate the effect of GLP1-RA on VTE outcomes among patients with T2DM and add to the growing evidence about the potential health benefits of GLP1-RA.
Although our study design does not allow us to examine the underlying mechanisms by which GLP1-RA reduces VTE risk, preliminary data from preclinical and clinical studies offer some mechanistic insights. For example, in human megakaryocyte cell lines, incubation with the GLP1-RA exenatide resulted in significant inhibition of thrombin-, adenosine 5′-diphosphate-, and collagen-induced platelet aggregation.19 In platelet-rich plasma from whole blood of adults with obesity and prediabetes stimulated with thromboxane receptor agonists, liraglutide reduced thromboxane-induced platelet aggregation by ∼50% compared with DPP4i.20 Obesity is a risk factor of VTE and weight loss resulting from bariatric surgery reduces the long-term risk of VTE by ∼40%.7,17 Taken together, these data suggest that GLP1-RA may lower VTE risk through weight-dependent and weight-independent mechanisms.
Our subgroup analyses suggested significant reductions in VTE in T2DM patients with normal weight or obesity with the greatest VTE reduction in class 2 obesity (BMI between 35 and 39.9 kg/m2). It is likely that patients with class 2 obesity experience larger absolute reductions in body weight and therefore, a greater VTE reduction. However, because we did not have data on BMI or weight changes with GLP1-RA, we were unable to directly assess the relationship between weight loss and VTE reduction. On the other hand, the distinct response to GLP1-RA seen in patients with normal weight may reflect a different underlying VTE risk profile. Patients with normal weight could have different metabolic or physiological characteristics that influence their VTE risk or their response to treatment. Supporting this hypothesis, prior studies have indicated that individuals with T2DM who have a normal weight tend to have higher rates of total, cardiovascular, and noncardiovascular mortality compared to those who are overweight.35 This “obesity paradox” in T2DM is thought to arise from differences in underlying illness severity or metabolic factors that predispose normal-weight individuals to higher mortality risk, despite having lower body mass.35 A prior study found that bariatric surgery did not reduce VTE risk in patients with BMI >40 kg/m2.17 In contrast, we found that GLP1-RA significantly lowered VTE risk in this high-risk group, supporting the potential anti-inflammatory and antithrombotic effects of GLP1-RA beyond weight loss.
Our study has several strengths. This was a large, population-based study that included a diverse cohort of patients. We used TTE to mimic the design of a RCT, with the aim to reduce selection or confounding bias commonly associated with observational studies.21 However, this study has several limitations. Because the cohorts taking or not taking GLP1-RA were not randomized, there are potentially differences in baseline characteristics between the 2 groups. We attempted to minimize these differences through propensity score matching, incorporating key variables such as BMI, HbA1c levels, and underlying comorbidities. However, the possibility of unmeasured or residual confounders that could influence the association between GLP1-RA and VTE cannot be completely excluded. We relied on ICD codes to identify VTE outcomes, as we did not have access to individual electronic health records. Based on a systematic review, ICD codes have moderate-to-high sensitivity and specificity for the identification of VTE in electronic databases.31 While the accuracy of VTE diagnosis could be improved by combining ICD codes with the use of anticoagulation, we were unable to do so given the constraints of the TriNetX platform. This introduces the possibility of misclassification of VTE outcomes. However, such misclassification would likely affect both the cohorts on GLP1-RA and DPP4i similarly and therefore reduces its potential impact on the risk estimates. Furthermore, we excluded patients with prior VTE which helps to prevent misclassification of VTE diagnoses.36 Importantly, we did not have data regarding the amount of weight loss for patients who were treated with GLP1-RA or DPP4i. Therefore, we were not able to adjust for the proportion of weight loss due to the use of GLP1-RA. It is also important to note that data from electronic health records do not capture medication adherence and therefore we were unable to assess or account for medication adherence in the analyses. Due to platform limitations, we were unable to perform a competing risk analysis. To address this, we included mortality as part of a composite outcome with VTE in our sensitivity analysis, which yielded similar results as the primary analysis. Finally, the results of this study are only applicable to patients with T2DM, and may not be generalizable to patients with obesity (without T2DM) using GLP1-RA, or other patients at high risk of VTE, such as those with prior VTE, hereditary thrombophilia or malignancies.37
In this propensity score-matched, TTE study, the use of GLP1-RA was associated with a reduction in the risk of VTE in the one year after initiating therapy among patients with T2DM. These findings add to the growing body of literature on the health benefits of GLP1-RA therapy. Further studies are needed to elucidate the mechanisms and causality underlying the association between GLP1-RA and reduction of VTE risk, and whether these findings can be extended to patients using GLP1-RA for weight control without T2DM.
Acknowledgments
R.P. was awarded the Conquer Cancer Foundation and Jennifer Luft Award by the National Blood Clot Alliance.
Authorship
Contribution: Cho-Han Chiang designed the research, performed the research, analyzed the data, and wrote the manuscript; J.S., Y.-C.C., S.O. performed the research and analyzed the data; Y.-C.L., K.-Y. Chang, K.-Y. Chi, and Cho-Hung Chiang provided database and analytical tool support; A.D., L.L., M.N.L., K.A.B., and D.S.K. interpreted the data and revised the manuscript; and R.P. designed the research, performed the research, interpreted the data, wrote and revised the manuscript, and supervised the entire project.
Conflict-of-interest disclosure: K.A.B. reports consulting fees from Sanofi, outside the submitted work. R.P. reports consulting fees from Merck Research, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Rushad Patell, Division of Hemostasis and Thrombosis, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston MA 02215; email: rpatell@bidmc.harvard.edu.
References
Author notes
Data underlying this article are available in the article and in its online supplemental Material.
The full-text version of this article contains a data supplement.