Key Points
ED visits by people with SCD declined after the PDMP started, especially after the PDMP mandate began.
30-day hospital readmissions increased at a faster rate during the PDMP mandate than before the PDMP.
Visual Abstract
Many states require prescribers to check a prescription drug monitoring program (PDMP) database before prescribing controlled substances, which may affect access and health outcomes for individuals with sickle cell disease (SCD). Our study objective was to evaluate acute care trends before and after PDMP implementation among people with SCD. We carried out a retrospective longitudinal cohort study of 13 698 individuals with confirmed or probable SCD in the Georgia Sickle Cell Data Collection program from 2010 to 2019. Primary outcomes were monthly rates of emergency department (ED) visits per 1000 people with SCD; 3-day ED revisits per 1000 visits; inpatient discharges per 1000 people with SCD; 30-day readmissions per 100 discharges; and patient days hospitalized per 100 discharges. We observed that, at the start of the PDMP mandate, the volume of ED visits was 12.7% lower during the mandatory PDMP period (relative risk, 0.873; 95% confidence interval [CI], 0.843-0.904) compared with what pre-PDMP trends would have predicted. The 3-day ED revisits followed a similar pattern. No associations were observed with inpatient hospitalization rate. There was a 0.7% monthly increase in readmissions during the PDMP mandate (incidence rate ratio [IRR], 1.007; 95% CI, 1.002-1.013) compared with 0.1% before the PDMP (IRR, 1.001; 95% CI, 1.000-1.001). The volume of patient days was higher during the PDMP phase-in period but no different during the mandate. ED use by people with SCD decreased after PDMP implementation, and there was an uptick in the monthly rate of increase in 30-day readmissions after the PDMP mandate. Further research into the impact of opioid control policies on people with SCD is warranted.
Introduction
Sickle cell disease (SCD) is a group of inherited blood disorders with a high rate of serious complications, including painful and recurrent vaso-occlusive crises resulting from tissue hypoxia distal to vessel blockages. Individuals with SCD often need timely acute care when complications arise, including rapid pain relief.1 The emergency department (ED) is an important source of care for patients with SCD in acute crisis, and inpatient (IP) hospital stays are often necessary to resolve acute events. Opioid therapy is a guideline-approved form of pain management for people with SCD in both IP and outpatient settings.2 At the same time, opioid use by individuals with SCD has come under increased scrutiny as the opioid overdose epidemic persists in the United States.3,4
One policy that many US states have implemented to curb excess opioid prescribing and dispensing in the general population is the adoption of a prescription drug monitoring program (PDMP).5 A typical PDMP is a database of controlled substance dispensing from pharmacies that health care providers are encouraged or required to check before prescribing medication with a high potential for misuse. Although PDMPs have the potential to assist health care providers in identifying multiple concurrent prescribers of opioids and possible unsafe use or misuse by patients, their overall impact is mixed.6-10 PDMPs may even have unintended effects, such as increased illicit drug mortality11 and heroin-related crime.12 There is concern that people with SCD who have legitimate and ongoing need for opioid medications may be adversely affected by PDMP implementation. A qualitative study of adults with SCD reported that opioid prescriptions had become more restrictive and were increasingly difficult to fill in the opioid epidemic era.3 One quantitative study, covering 27% of commercially insured individuals across the United States, revealed that comprehensive PDMP mandates were associated with substantial reductions in opioids dispensed to patients with SCD after ED encounters.13 Another study found that PDMP implementation was not associated with a change in opioid prescribing to patients with SCD at 2 Pennsylvania EDs, even though PDMP use was expected to increase opioid prescribing by correctly identifying patients with SCD as not having opioid misuse.14
What remains unknown is how acute care use changes in the SCD population after PDMP implementation. Reduced access to outpatient opioids after PDMP adoption could lead to increased ED use due to more unmanageable pain crises at home. On the other hand, people with SCD may defer or delay seeking emergency care if they anticipate inadequate pain management or biased treatment there. Deferred or delayed ED care, in turn, could lead to worsened complications that increase hospitalization or readmission rates or lengthen IP hospital stays. More than half of adolescents with SCD (52%) in 1 multicenter study reported that poor experiences in the ED influenced their decisions not to seek acute care.15 Once at the ED, people with SCD in acute pain crises often experience delayed analgesia,16 especially if the emergency provider reports more negative attitudes toward people with SCD.17 This concern is relevant because several previous studies found that some clinicians hold negative attitudes toward or overestimate addiction among people with SCD,17-21 despite evidence that substance abuse is no more common in patients with SCD.22 We, therefore, designed this study to address the lack of knowledge about the association between PDMP implementation and acute care use by people with SCD.
Methods
This is a retrospective cohort study of individuals with SCD in Georgia from 2010 to 2019 to evaluate trends in ED and IP use before and after implementation of a statewide PDMP. We used data from the Georgia Sickle Cell Data Collection (SCDC) program and an interrupted time series (ITS) analysis to evaluate changes in the rates of ED visits and IP stays, rates of ED revisits and IP readmission, and IP length of stay (LOS) during the following 3 time periods: (1) before PDMP implementation on 1 July 2017, (2) during the PDMP “phase-in period” (July 2017 to June 2018), and (3) after prescriber use of the PDMP became mandatory in most situations on 1 July 2018. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies. The Georgia State University Institutional Review Board approved this study with a waiver of informed consent.
Data sources and sample
This study used the Georgia SCDC program, a population-based longitudinal surveillance system consisting of administrative health care data from multiple sources for all people identified with SCD in the state.23 SCDC data sources include the state newborn screening program, state Medicaid and Children’s Health Insurance Program databases, death certificates, statewide hospital discharge and ED records, and clinical records or case reports from 3 large SCD treatment centers in Georgia.23 For each study year from 2010 to 2019, we created year-specific dynamic cohorts using the Core Surveillance Data Instrument of the SCDC Common Data Model24 to identify all individuals meeting confirmed or probable case definitions for SCD.25,26 The SCD case definition is based on an algorithm that classifies cases with laboratory confirmation as confirmed cases and those with a reported clinical diagnosis or 3 or more diagnostic codes in a 5-year period from an administrative data source as probable cases. This approach has been validated and revealed to have high positive predictive value.25,26 Once identified, year-specific index files with unique person identifiers were used to link individuals in each yearly cohort to the Georgia Discharge Data System to obtain all hospital encounters, including ED and IP discharges. To reduce the possibility of selection bias, all individuals identified with SCD in a given study year were included in each year-specific cohort. No exclusion criteria based on demographic characteristics were applied.
Exposure
Georgia implemented its PDMP in a phased approach. Therefore, changes in utilization were evaluated across 3 time periods. The pre-PDMP period was defined as 1 January 2010 to 30 June 2017. A 1-year phase-in period occurring from 1 July 2017 to 30 June 2018 accounted for the time in which dispensers were required to start entering prescription information for controlled substances into the PDMP within 24 hours. The post-PDMP period, in which prescribers were mandated to check the PDMP system before prescribing opioids in most situations, was defined as 1 July 2018 to 31 December 2019.27
Outcomes
Changes in acute care use were evaluated using 5 outcomes, all measured on a monthly basis: ED visits per 1000 people with SCD; 3-day ED revisits per 1000 ED visits; IP discharges per 1000 people with SCD; 30-day readmissions per 100 IP discharges; and patient days hospitalized per 100 IP discharges.
Statistical analysis
Demographic characteristics of the acute care encounters were analyzed using descriptive statistics. Using an ITS analysis, we fit segmented regression models, assuming a level and slope change during each of the phase-in and mandatory PDMP periods, for each of the 5 outcomes (supplemental Appendix 1). Negative binomial models were estimated to account for overdispersion when present; otherwise, Poisson regression was used (supplemental Table 1). Durbin-Watson tests and partial autocorrelation plots were used to assess for autocorrelation, and as appropriate Newey-West standard errors were used to account for identified autocorrelation of lag 1 order (supplemental Appendix 2, supplemental Table 1). All ITS models used Fourier terms to account for seasonal variation in hospital encounters (supplemental Appendix 2). Results of the ITS models were expressed as incidence rate ratios (IRRs), and relative risk (RR) values were estimated to quantify changes in the total volume of acute care visits during the phase-in and mandatory PDMP periods, relative to the pre-PDMP period (supplemental Appendix 3).28
We performed a sensitivity analysis that defined the phase-in period as 1 January 2018 to 30 June 2018, during which time prescribers were required to register for the PDMP system but not necessarily check it before prescribing, and these models were compared with the final models with Akaike and Bayesian Information Criterion measures (supplemental Figures 1-5, supplemental Tables 2-6). We also estimated alternate specifications to evaluate the robustness of our results, for example, using a linear rather than nonlinear time trend and using a 7-day rather than 3-day window for ED revisits (supplemental Figure 6, supplemental Table 7). Finally, we completed a subpopulation analysis for adults with SCD, given the progressive nature of SCD over the life span and the increase in opioid and acute care use in adulthood, which could mask the true impact of PDMP, as adults are the population most likely to be affected. Complete information for all analyses is provided in supplemental Appendices 1-3, with references in supplemental Appendix 4. All analyses were conducted in R version 4.2.1.
Results
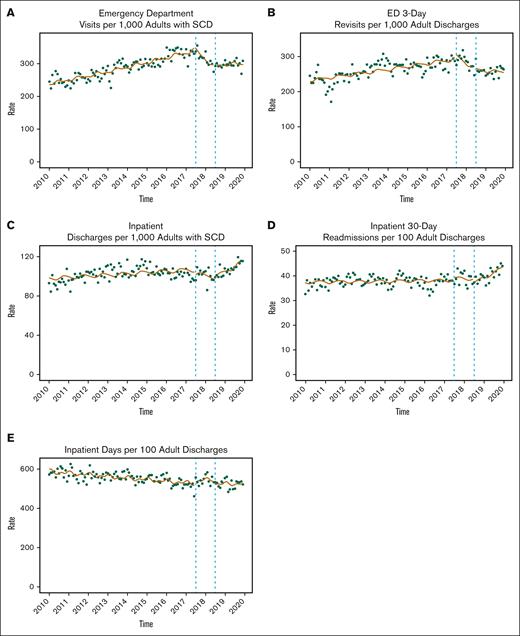
A total of 13 698 individuals with SCD were included in the cohort. Collectively, they had 318 834 acute care encounters during the study period. Demographic characteristics at the time of the acute care encounters are displayed in Table 1. Nearly three-fourths of the encounters (72.6%) were ED visits and approximately one-fourth (27.4%) were IP hospitalizations. Mean age at encounter was 29 years (standard deviation = 14 years). More encounters occurred among females (57.7%) than males (42.3%), and most encounters (93.1%) were made by Black individuals. Public insurance sources, including Medicaid (47.7%) and Medicare (24.5%), were the expected primary payer for most encounters. Abbreviated results for the ITS analysis are provided in Table 2 (see supplemental Tables 2-6 for full results) and displayed in Figure 1. Effect sizes (RRs) are provided in Table 3. The subpopulation analysis of only adults did not substantively alter the findings presented subsequently (Tables 2 and 3; Figure 2), except that the monthly rate of IP discharges for adults decreased significantly at the start of the PDMP mandate then increased faster compared to before the PDMP (Table 2).
Monthly changes in acute care use. The circles represent observed utilization data for each month. Solid lines represent ITS estimates. Dotted vertical lines represent the beginning and end of the PDMP phase-in period. Y-axis scales differ by outcome.
Monthly changes in acute care use. The circles represent observed utilization data for each month. Solid lines represent ITS estimates. Dotted vertical lines represent the beginning and end of the PDMP phase-in period. Y-axis scales differ by outcome.
Monthly changes in acute care use among adults. The circles represent observed utilization data for each month. Solid lines represent ITS estimates. Dotted vertical lines represent the beginning and end of the PDMP phase-in period. Y-axis scales differ by outcome.
Monthly changes in acute care use among adults. The circles represent observed utilization data for each month. Solid lines represent ITS estimates. Dotted vertical lines represent the beginning and end of the PDMP phase-in period. Y-axis scales differ by outcome.
ED visits per 1000 persons with SCD
Before PDMP implementation, rates of ED utilization increased by 0.4% each month (IRR, 1.004; 95% confidence interval [CI], 1.004-1.005). There was no significant immediate change in ED utilization at the start of the PDMP phase-in period (IRR, 1.059; 95% CI, 0.994-1.128). During the PDMP phase-in, rates of ED utilization decreased by 1.7% each month (IRR, 0.983; 95% CI, 0.975-0.991). At the start of the mandatory PDMP checking period (compared with the pre-PDMP period), the immediate change in ED utilization was an 11.6% reduction in visits (IRR, 0.884; 95% CI, 0.839-0.931). The trend for monthly rate of ED visits was not significant during the mandatory PDMP period (IRR, 0.999; 95% CI, 0.994-1.003; Table 2; Figure 1). In the PDMP phase-in period, the overall volume of ED visits was 4.4% lower than anticipated had the PDMP not been implemented (RR, 0.956; 95% CI, 0.923-0.996), whereas in the mandatory PDMP period, overall utilization was 12.7% lower (RR, 0.873; 95% CI, 0.843-0.904) than the pre-PDMP trends (Table 3).
ED 3-day revisits per 1000 discharges
Before PDMP implementation, 3-day ED revisits increased by 0.3% each month (IRR, 1.003; 95% CI, 1.002-1.004). There was no significant immediate change in ED revisits at the start of the PDMP phase-in period (IRR, 1.034; 95% CI, 0.918-1.164). During the PDMP phase-in, rates of ED revisits decreased by 1.4% each month (IRR, 0.986; 95% CI, 0.973-0.999). The immediate change in ED revisits at the start of the mandatory PDMP period was a 15.3% reduction in visits (IRR, 0.847; 95% CI, 0.795-0.903). During the mandatory PDMP period, monthly rates of ED revisits decreased by 0.5% (IRR, 0.995; 95% CI, 0.991-1.000; Table 2; Figure 1). In the PDMP phase-in period, the overall volume of ED revisits was no different than had the PDMP not been implemented (RR, 0.948; 95% CI, 0.895-1.005), whereas in the mandatory PDMP period, overall ED revisits were 18.9% lower (RR, 0.811; 95% CI, 0.768-0.856) than the pre-PDMP trends (Table 3).
IP discharges per 1000 persons with SCD
Before PDMP implementation, rates of IP hospitalizations increased by 0.1% each month (IRR, 1.001; 95% CI, 1.001-1.002). There was no significant immediate change in hospitalizations at the start of the PDMP phase-in period (IRR, 1.019; 95% CI, 0.958-1.083). There was no significant trend in monthly hospitalizations during the phase-in period (IRR, 0.997; 95% CI, 0.989-1.005). Similarly, there was no significant immediate change in hospitalizations during the mandatory period (IRR, 0.993; 95% CI, 0.944-1.044), and the trend for monthly rates during the mandatory period was not significant (IRR, 1.003; 95% CI, 0.999-1.007; Table 2; Figure 1) The overall volume of hospitalizations during the phase-in period (RR, 1.002; 95% CI, 0.969-1.037) and mandatory period (RR, 1.023; 95% CI, 0.989-1.059) was no different than had the PDMP not been implemented (Table 3).
IP 30-day readmissions per 100 discharges
Before PDMP implementation, rates of hospital readmissions increased by 0.1% each month (IRR, 1.001; 95% CI, 1.000-1.001). There was no significant immediate change in readmissions at the start of the PDMP phase-in period (IRR, 1.032; 95% CI, 0.952-1.118). There was no significant monthly trend in readmissions during the phase-in period (IRR, 0.997; 95% CI, 0.986-1.007). Although there was no significant immediate change in readmissions during the mandatory period (IRR, 0.957; 95% CI, 0.896-1.022), the monthly trend rate indicated an increase in readmissions by 0.7% each month during this time (IRR, 1.007; 95% CI, 1.002-1.013; Table 2; Figure 1). The overall volume of readmissions during the phase-in period (RR, 1.012; 95% CI, 0.968-1.059) and mandatory period (RR, 1.022; 95% CI, 0.977-1.070) was no different than had the PDMP not been implemented (Table 3).
IP days per 100 discharges
Before PDMP implementation, rates of patient days hospitalized decreased by 0.1% each month (IRR, 0.999; 95% CI, 0.999-1.000). There was no significant immediate change in patient days at the start of the PDMP phase-in period (IRR, 1.000; 95% CI, 0.951-1.051), and there was no significant monthly trend during the phase-in period (IRR, 1.005; 95% CI, 0.998-1.011). Similarly, there was no significant immediate change in patient days during the mandatory period (IRR, 0.995; 95% CI, 0.954-1.037), and the monthly trend for patient days during the mandatory period was also not significant (IRR, 1.002; 95% CI, 0.999-1.005; Table 2; Figure 1). The overall volume of IP days during the phase-in period was 2.9% higher than anticipated had the PDMP not been implemented (RR, 1.029; 95% CI, 1.001-1.058), but the volume of IP days was no different than anticipated during the mandatory period (RR, 1.010; 95% CI, 0.982-1.038; Table 3).
Discussion
Using longitudinal data from the Georgia SCDC program from 2010 to 2019, we found mixed results for the association of PDMP implementation with acute care use among persons with SCD: specifically, the volume of ED visits decreased significantly, the rates of IP hospitalizations and total days hospitalized remained stable, and the rate of 30-day hospital readmissions increased faster after compared with before full implementation of Georgia’s PDMP. The overall volume of ED visits was 12.7% lower in the mandatory PDMP period than would be expected had pre-PDMP trends held. Likewise, the volume of 3-day revisits to the ED was 18.9% lower than would be expected. However, overall IP days were 2.9% higher than expected during the PDMP phase-in period, though they stabilized during the mandatory period, and the rate of increase in 30-day hospital readmission was an additional 6 per 100 000 discharges each month in the mandatory period compared with before the PDMP.
Unlike ED visits for ambulatory care-sensitive conditions, where a decline in volume may be desirable, ED use for SCD complications is often medically appropriate and potentially lifesaving.1 Therefore, the 12.7% decrease in ED visits by people with SCD after the start of the PDMP mandate in Georgia is noteworthy. This decline during the mandatory period was much larger than the 4.4% decline observed in the phase-in period. Likewise, 3-day ED revisits decreased much more after the PDMP mandate (18.9%) compared with the phase-in period (5.2%), when checking the PDMP before prescribing was still optional. In the general population, the national ED visit rate also increased gradually from 2010 to 2015 but then began to decline earlier.29 In 2016, the year before the launch of Georgia’s PDMP, the national ED visit rate was already 0.2% lower than its peak in 2015,29 but it was still rising among persons with SCD in Georgia until July 2017, when the PDMP phase-in period began.
There are multiple potential reasons for the observed changes in ED utilization. Because pain is the most common reason for individuals with SCD to seek emergency care,30 and opioids are central to acute pain control in SCD, it is possible that a real or perceived decrease in the accessibility of opioids in the ED caused some patients with SCD to defer seeking ED care. It is also possible that patients increased their visits with their hematologist or primary care provider in response to decreased access to opioids at ED discharge. Equally, health care providers outside the hospital may have responded to the PDMP rollout by prescribing more outpatient prescriptions for opioids to individuals with SCD or prescriptions with higher doses or longer days’ supply. More opioid medications on hand at home could have reduced the need to seek ED care. This explanation would also be consistent with the decrease in 3-day revisits if ED-based providers wrote discharge prescriptions for greater opioid supplies after the PDMP, recognizing the challenges faced by individuals with SCD in receiving optimal pain control.
Although most IP hospitalization measures remained unchanged after PDMP adoption, it is notable that the monthly rate of increase in 30-day hospital readmissions was higher after the PDMP mandate compared with before the PDMP. More IP hospitalizations within 30 days of discharge could indicate an increase in SCD complications, as the mean LOS for patients readmitted after sickle cell crisis is significantly longer than the index hospitalization.31 Increased or worsened complications may result from delayed or deferred emergency care, and our data reveal decreased ED utilization since the PDMP. In addition, it is possible that hospital-based prescribers began writing discharge opioid prescriptions with shorter durations, given that the Georgia PDMP statute exempts prescriptions for a 3-day supply or less.27 Typically ED physicians prescribe 3 days’ worth of opioids at discharge, sending patients back to their hematologist or primary care provider for additional prescriptions, whereas 30-day prescriptions from the ED were more common before PDMPs. Fewer discharge opioids could have prompted more readmissions for pain crises within 30 days because hospitalizations in SCD are not predictable, and it might have been challenging for patients to arrange follow-up within 30 days of hospital discharge while having as few as 3 days of pain medication on hand.
It is unclear why the total volume of patient days hospitalized was lower than would be expected had the PDMP not been adopted, but only in the phase-in period and not after the start of the PDMP mandate. Owing to the importance of LOS as a measure of illness severity, researchers and policymakers should carefully observe whether total days hospitalized fluctuates for people with SCD the longer the PDMP has been in place.
Limitations and future research
This study is observational and lacks a comparison group; therefore, the findings should be considered hypothesis generating and not conclusive of causality. Our data were limited to 1 state though one with an estimated 10% of the total number of SCD cases nationally,23 and we had only 18 months of follow-up data in the mandatory period, so we cannot extrapolate nationally or infer long-term trends. This timeframe also may not have been long enough for providers to receive feedback from the program that would have affected prescribing behaviors. Alternative explanations for utilization changes, such as opioid supply shortages at retail pharmacies, are also possible. Not having broader access to health care utilization data that included outpatient clinical visits and telehealth encounters is an important limitation of this study. Moreover, outcomes may differ in areas with a comprehensive sickle cell center (eg, metro Atlanta), and looking at institution-level data may offer more granular insight. However, the agency that provides the acute care use data to the Georgia SCDC program has strict safeguards about isolating specific institutions in the data, and a simple comparison of metro Atlanta to the rest of Georgia would obscure the presence of comprehensive SCD care outside Atlanta, especially in Augusta.
There are potentially other unknown causes of the patterns described for which we could not account. For example, trends across the United States before COVID-19 were toward shorter IP LOS.32 Premature discharges could result in a rise in 30-day admissions independent of the PDMP. In response, we conducted a secondary analysis describing and visualizing average LOS in the baseline, phase-in, and mandatory PDMP periods and observed little difference in LOS trends, which would not have substantively altered the findings. In addition, we cannot rule out variations in ED volume patterns which can unexpectedly occur. Although the models included a term to adjust for seasonality and the study period ended before the COVID-19 pandemic, there could be unusually bad viral seasons or other surges of ED use not fully accounted for by the analysis. It is also unclear how many SCD providers actually checked the PDMP, and for which individuals, despite the mandate. Future research should investigate the influence of the PDMP on prescribers’ attitudes and behaviors using qualitative methods.
The inclusion of data from all identified patients with SCD with any cause for hospital interaction is both a strength and potential weakness. Although this approach maximizes representativeness, and nationally pain is still the most common patient-cited reason for ED visits by individuals with SCD (75.1% of visits from 1999 to 2020),30 not excluding the minority of patients seen for non–pain-related reasons and where opiates were not involved may have reduced observed effect sizes. As noted previously, other publications have linked PDMP use to decreased opioid prescription rates for people with SCD, but only after ED encounters.13,14 Analyzing opioid prescribing and dispensing to persons with SCD was outside the scope of this study and should be explored in future research, and incorporating prescription drug claims for Medicaid-covered patients (approximately half of the sample) is a feasible next step with Georgia SCDC data.
Conclusions
ED use (both initial and return visits) for people with SCD decreased notably after compared with before PDMP implementation in Georgia. Readmission to the hospital within 30 days increased faster during the time that checking the PDMP became mandatory than before the PDMP was implemented, and overall patient days hospitalized was higher than anticipated during the PDMP phase-in period. IP hospitalization rates remained unchanged. Because these results are observational, conclusions are only hypothesis generating and causality cannot be assumed. Therefore, research into the impact of opioid control policies on timely and effective acute care for individuals with SCD is warranted.
Acknowledgments
The authors gratefully acknowledge Mapillar Dahn, Robert Gibson, Abdullah Kutlar, André Moorer, and Betty Pace for their early feedback on this work.
This study was supported by an intramural grant from the Georgia State University Research Innovation and Scholarly Excellence challenge, also known as Project RISE. The Georgia Sickle Cell Data Collection program is funded by the Centers for Disease Control and Prevention (award number 1NU58DD000036-01-00).
The funders had no role in the design and conduct of the study; collection, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Authorship
Contribution: B.T.M. supervised the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; B.K.A. and A.B.S. had full access to all data in the study, and the other authors had access to the monthly aggregate data and all programs used to execute the data pull; B.T.M., B.K.A., and J.M. wrote the draft of the manuscript; A.B.S. and L.L.C. critically reviewed the manuscript for important intellectual content; B.K.A. performed the statistical analysis; B.A. and A.H. provided administrative, technical, or material support; and all authors contributed to the study conception and design, and acquisition, analysis, or interpretation of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Blake T. McGee, Department of Pre-Licensure Nursing, College of Nursing, Augusta University, 987 St. Sebastian Way (EC-5314), Augusta, GA 30912; email: bmcgee@augusta.edu.
References
Author notes
Presented in abstract form at the 51st annual national convention of the Sickle Cell Disease Association of America, 11-14 October 2023, Arlington, VA.
Aggregate monthly data are available on request from the corresponding author, Blake T. McGee bmcgee@augusta.edu).
The full-text version of this article contains a data supplement.