Key Points
Anticoagulation continuation vs cessation during thrombocytopenia did not affect recurrent thrombosis or bleeding after autologous HCT.
Among anticoagulated patients undergoing autologous HCT, higher platelet counts were not associated with a lower risk of bleeding.
Abstract
Management of venous thromboembolism (VTE) remains challenging in patients with hematologic malignancy who undergo hematopoietic cell transplantation (HCT) due to prolonged thrombocytopenia. This study aims to (1) determine the incidence of VTE recurrence and bleeding during autologous HCT, (2) assess the impact of continuing vs temporarily withholding anticoagulation during thrombocytopenia, and (3) explore the impact of platelet threshold among other variables on the risk of bleeding. We performed this observational study in adults with lymphoma and myeloma who underwent autologous HCT between 2006 and 2015. We selected patients with index VTE prior to HCT and assigned them to different cohorts based on antithrombotic management at the onset of thrombocytopenia. Primary outcomes included VTE recurrence and major bleeding by 30 days after HCT. Secondary outcomes included platelet and red blood cell transfusions, time to engraftment, and overall survival. Of the 1631 patients who underwent autologous HCT, 204 patients (12.5%) had preceding index VTE events, and among them, 132 patients continued and 72 patients temporarily withheld anticoagulation during thrombocytopenia. There were no significant differences in VTE recurrence (1.5% vs 1.4%) or major bleeding (3.8% vs 4.2%) between 2 groups by 30 days. The number of platelet transfusions was significantly higher in the first group. Baseline elevated bilirubin, creatinine, and prothrombin time were independently associated with increased risk in major bleeding, whereas neither platelet threshold nor average platelet count was predictive. Our findings suggest that for many patients undergoing autologous HCT, temporarily withholding anticoagulation during thrombocytopenia may offer the best risk-benefit tradeoff among available options.
Introduction
Symptomatic venous thromboembolism (VTE) occurs in ∼5% of patients with hematologic malignancies undergoing high-intensity chemotherapy or hematopoietic cell transplantation (HCT).1-3 Despite its common occurrence, VTE treatment in this population remains challenging due to the prolonged period of chemotherapy or conditioning-induced hypoproliferative thrombocytopenia. There is currently a lack of consensus on the right approach to anticoagulation during thrombocytopenia.4-6 According to the American Society of Oncology 2013 practice guideline on VTE treatment, platelets <20 × 103/μL was an absolute contraindication, whereas platelets <50 × 103/μL was a relative contraindication. In the International Society of Thrombosis and Hemostasis 2013 guideline, in patients with acute VTE that occurred <1 month prior to thrombocytopenia, full-dose anticoagulation with platelet support was recommended; in those with subacute of chronic VTE, half-dose low-molecular-weight heparin (LMWH), prophylactic LMWH, or withholding of anticoagulant therapy were all entertained. Although these recommendations seem reasonable, they are primarily based on expert opinions because there is very little published evidence that would inform these complex risk-benefit decisions in patients with both venous thrombosis and severe thrombocytopenia. Furthermore, clinical equipoise exists for the type of anticoagulant and the optimal platelet threshold required.7
Several retrospective case series attempted to address the efficacy and safety of anticoagulation in thrombocytopenic cancer and transplant patients.8-10 However, all reports are limited by the small sample size (N < 100), heterogeneous inclusion criteria, and variable duration and severity of thrombocytopenia. With these limitations in mind, we designed the current study using a large cohort of autologous HCT patients to explore antithrombotic management in patients on active anticoagulation for VTE. The objectives of the current study are threefold: (1) to determine the incidence of VTE recurrence and major bleeding in patients with lymphoma and multiple myeloma who undergo autologous HCT, (2) to assess the impact of continuing vs temporarily withholding anticoagulation during the period of thrombocytopenia, and (3) to explore the effect of platelet count on the risk of bleeding in patients who continued antithrombotic therapy.
Methods
Study design and follow-up
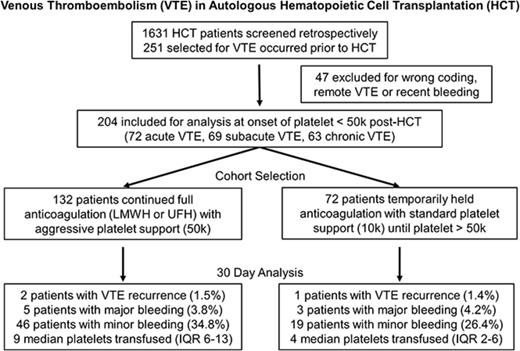
This observational study was approved by the Institutional Review Board at Fred Hutchinson Cancer Research Center (FHCRC). Consecutive adult patients with lymphoma and multiple myeloma who underwent first autologous HCT at FHCRC in Seattle, Washington between January 1, 2006 and December 31, 2015 were selected. Patients were included in the study if they had a diagnosis of VTE within the year prior to the onset of thrombocytopenia after HCT; patients were excluded if they had remote or no VTE within the same timeframe or if they had major bleeding in the month prior to HCT (Figure 1). VTE was identified by International Classification of Diseases 9 (ICD9) codes 415, 451, and 453 and confirmed by chart review.
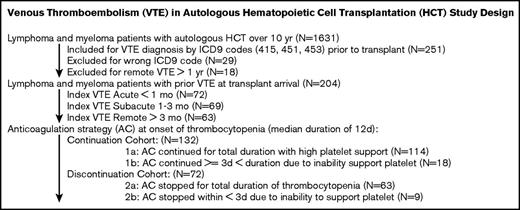
VTE in autologous HCT study design. The study design, patient selection, and cohort allocation are shown here.
VTE in autologous HCT study design. The study design, patient selection, and cohort allocation are shown here.
Patient demographics, pertinent laboratory results, admission and discharge dates, time to engraftment, survival, and transfusion data were captured using the FHCRC transplant database electronic query. Detailed information about VTE, bleeding, patient-specific risk factors, choice of anticoagulation, as well as transfusion was captured by reviewing the electronic medical records.
Study definition and outcomes
The index VTE was defined as acute if it occurred within 30 days prior to HCT, subacute if between 31 and 90 days, and remote if between 91 and 365 days. The event was considered “incidental” if it was discovered on imaging without associated clinical symptoms.
VTE was defined as pulmonary embolism (PE), lower-extremity deep vein thrombosis (DVT), or catheter-associated DVT (CA-DVT). VTE outcomes were determined by reviewing available radiology records (including Duplex ultrasound, positron emission tomography/computed tomography scan, contrast computed tomography chest scan, ventilation-perfusion scan), interim and discharge summaries, long-term follow-up clinic notes, and ICD9 coding within the first year post-HCT.
Bleeding was defined using modified World Health Organization bleeding score, with major bleeding correlating to grade 3 or 4 bleeding and overall bleeding corresponding to grade 2 to 4 bleeding.11 Bleeding was assessed by reviewing progress notes as well as interim and discharge summaries from the time of HCT until time of discharge home or death. For patients who died prior to discharging from the transplant service, postmortem analyses, if available, were reviewed for evidence of VTE or bleeding as a cause of death.
Primary outcomes included incidence of VTE recurrence and major bleeding within 30 days after HCT. Secondary outcomes included number of platelet and red blood cell (RBC) transfusions until platelet engraftment (defined as first day of a 7-day interval during which the patient maintained a platelet count >50 × 103/μL without transfusion), time to neutrophil and platelet engraftment, and overall survival.
Anticoagulation management and patient allocation
The decision and choice of anticoagulation after HCT were based on physician discretion. When enoxaparin was chosen, the dose varied between 1 mg/kg twice daily and 1.5 mg/kg daily. Doses were further adjusted with anti-Xa monitoring in patients with renal dysfunction. When heparin was chosen, the goal partial thromboplastin time (PTT) varied between 60-80 seconds and 60-100 seconds. Patients were allocated into 2 cohorts at the onset of thrombocytopenia based on whether they continued anticoagulation for 3 or more days using a high-platelet transfusion threshold of 50-70 × 103/μL, or temporarily withheld anticoagulation until platelets recovered using a standard platelet transfusion threshold of 10 × 103/μL (Figure 1). For the discontinuation group, no pharmacologic or mechanical prophylaxis was given per the institutional policy. Most patients with symptomatic PE or DVT resumed anticoagulation upon platelet recovery, although a selective cohort of CA-DVT patients stopped all anticoagulation treatment if there was imaging resolution or line removal.
Average platelet area under the curve (AUC) determination
Among patients who experienced any duration of anticoagulation during thrombocytopenia (group 1a, 1b, 2b), the treating physician decided on the necessity to transfuse platelets with the usual threshold 50 × 103/μL as inpatient and 70 × 103/μL as outpatient per FHCRC transplant department institutional policy. Each platelet was given either 4 to 6 units of pooled random donor platelets or a single donor apheresis platelet, depending on the availability of product. Given the variable response to platelet transfusion as an estimate of the degree to which the target platelet count was achieved, all platelet values from the onset of thrombocytopenia (<50 × 103/μL) until platelet engraftment were plotted against time. Platelet AUC adjusted by the time of anticoagulant exposure was calculated (AUCN = (PN+1 + PN) * (TN+1 − TN)/2; P = platelet; T = date/time). The average platelet AUC was then derived (AUCAVE = SUM(AUC1:AUCN)/(TN+1 − T1)) and separated into distinct groups (30-50, 50-60, 60-80 × 103/μL) to compare the risk of bleeding.
Statistical analysis
Categorical variables were summarized by number and percentage, and continuous variables were summarized by median and IQR. Categorical and continuous variables were compared between the 2 treatment cohorts using Fisher’s exact test and Wilcoxon rank-sum test, respectively. Median follow-up time was determined by the reverse Kaplan-Meier method. Unadjusted logistic or linear regression models were built to test the association between the 2 treatment cohorts and the binary outcomes at 30 days or continuous outcomes, respectively. A multivariate logistic regression model was further built to evaluate for VTE outcomes while controlling for index VTE acuity. To assess whether the effect of VTE recurrence or major bleeding varied by baseline risk factors, subgroup analyses were conducted using unadjusted logistic regression models to test for association between the primary composite endpoint and the 2 cohorts. Likelihood ratio test was used to test for treatment and subgroup interactions where P < .05 was considered significant. To determine the incidence of VTE recurrence during a longer follow-up period, the cumulative incidence for the 2 cohorts was plotted after accounting for the competing risk of dying from other cause. Hazard ratio with 95% confidence interval was estimated using the Fine and Gray’s proportional hazard model adjusted for the index VTE acuity. Univariate logistic regression models were built to test the association between major bleeding and all relevant demographic, clinical, and laboratory covariates to determine potential predictors of major bleeding (shown in Table 4). Multivariate logistic regression model using a stepwise estimation by backward and forward selection was then generated from variables with P < .10 from the univariate model. All statistical analyses were performed in Stata 14.2.
Results
Among 1631 patients with multiple myeloma and lymphoma who underwent first autologous HCT at FHCRC between 2006 and 2015, we identified 204 patients (12.5%) who met the inclusion and exclusion criteria (Figure 1). Patients were divided into 2 study cohorts at the onset of thrombocytopenia: 132 patients continued and 72 patients temporarily withheld anticoagulation. Baseline patient characteristics, including the type and acuity of VTE, type of hematologic malignancy, and conditioning regimen, were listed in Table 1. Apart from the index VTE acuity, there were no significant differences between the 2 cohorts. All patients had a large-bore apheresis line in place at the time of HCT. Follow-up data for primary outcomes were available in 97% of patients at 30 days. The median follow-up for VTE assessment was 361 days (44-725 days). The median duration of thrombocytopenia (platelet count <50 × 103/μL until engraftment) post-HCT was 12 days (8-16 days).
Baseline characteristics for patients with hematologic malignancy and prior VTE
| . | Overall (n = 204) . | Continuation (n = 132) . | Discontinuation (n = 72) . | P . |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 57 (48-65) | 56 (47-64) | 58 (49-65) | .34 |
| Female, n (%) | 77 (38) | 50 (38) | 27 (38) | 1.00 |
| White, n (%) | 187 (92) | 122 (92) | 65 (90) | .60 |
| Weight, kg | 84 (71-96) | 83 (71-96) | 86 (70-96) | .71 |
| Karnofsky Performance Status | 90 (80-90) | 90 (80-90) | 90 (80-90) | .55 |
| Hematologic malignancy, n (%) | ||||
| Plasma cell neoplasm | 57 (28) | 39 (30) | 18 (25) | .28 |
| Hodgkin lymphoma | 32 (16) | 20 (15) | 12 (17) | |
| Diffuse large B-cell lymphoma | 60 (29) | 33 (25) | 27 (38) | |
| Mantle cell lymphoma | 24 (12) | 19 (14) | 5 (7) | |
| Follicular lymphoma other NHL | 31 (15) | 21 (16) | 10 (14) | |
| Conditioning regimen, n (%) | ||||
| Melphalan | 68 (33) | 40 (30) | 18 (25) | .50 |
| BEAM | 58 (28) | 39 (30) | 29 (40) | |
| H-TBI/Cytoxan+/− VP16 | 47 (23) | 32 (24) | 15 (21) | |
| Other | 31 (15) | 21 (16) | 10 (14) | |
| Preconditioning laboratory tests | ||||
| WBC, 109/L | 4.6 (3.4-5.9) | 4.6 (3.4-5.9) | 4.6 (3.5-6.1) | .95 |
| Hemoglobin, g/dL | 11.3 (10.5-12.2) | 11.3 (10.5-12.3) | 11.4 (10.5-12.2) | .84 |
| Platelet, 103/μL | 181 (149-226) | 184 (149-229) | 176 (148-218) | .23 |
| Creatinine, mg/dL | 0.9 (0.8-1.0) | 0.9 (0.8-1.0) | 0.9 (0.7-1.1) | .77 |
| Bilirubin, mg/dL | 0.5 (0.4-0.6) | 0.5 (0.4-0.6) | 0.5 (0.4-0.7) | .76 |
| LDH, U/L | 179 (147-205) | 179 (152-205) | 173 (140-207) | .60 |
| PT, s | 13.0 (12.6-13.6) | 13.0 (12.5-13.6) | 13.0 (12.7-13.6) | .98 |
| Index VTE acuity, n (%) | ||||
| <1 mo | 72 (35) | 56 (42) | 16 (22) | <.01 |
| 1-3 mo | 69 (34) | 48 (37) | 20 (28) | |
| >3 mo | 63 (31) | 27 (20) | 36 (50) | |
| Index VTE type, n (%) | ||||
| CA-DVT only | 113 (55) | 68 (52) | 45 (63) | .14 |
| PE or DVT | 91 (45) | 64 (48) | 27 (38) | |
| Index VTE presentation, n (%) | ||||
| Symptomatic | 103 (50) | 66 (50) | 37 (51) | .88 |
| Incidental | 101 (50) | 66 (50) | 35 (49) | |
| Prior VTE history, n (%) | ||||
| Yes | 19 (9) | 15 (11) | 4 (6) | .21 |
| No | 185 (91) | 117 (89) | 68 (94) |
| . | Overall (n = 204) . | Continuation (n = 132) . | Discontinuation (n = 72) . | P . |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 57 (48-65) | 56 (47-64) | 58 (49-65) | .34 |
| Female, n (%) | 77 (38) | 50 (38) | 27 (38) | 1.00 |
| White, n (%) | 187 (92) | 122 (92) | 65 (90) | .60 |
| Weight, kg | 84 (71-96) | 83 (71-96) | 86 (70-96) | .71 |
| Karnofsky Performance Status | 90 (80-90) | 90 (80-90) | 90 (80-90) | .55 |
| Hematologic malignancy, n (%) | ||||
| Plasma cell neoplasm | 57 (28) | 39 (30) | 18 (25) | .28 |
| Hodgkin lymphoma | 32 (16) | 20 (15) | 12 (17) | |
| Diffuse large B-cell lymphoma | 60 (29) | 33 (25) | 27 (38) | |
| Mantle cell lymphoma | 24 (12) | 19 (14) | 5 (7) | |
| Follicular lymphoma other NHL | 31 (15) | 21 (16) | 10 (14) | |
| Conditioning regimen, n (%) | ||||
| Melphalan | 68 (33) | 40 (30) | 18 (25) | .50 |
| BEAM | 58 (28) | 39 (30) | 29 (40) | |
| H-TBI/Cytoxan+/− VP16 | 47 (23) | 32 (24) | 15 (21) | |
| Other | 31 (15) | 21 (16) | 10 (14) | |
| Preconditioning laboratory tests | ||||
| WBC, 109/L | 4.6 (3.4-5.9) | 4.6 (3.4-5.9) | 4.6 (3.5-6.1) | .95 |
| Hemoglobin, g/dL | 11.3 (10.5-12.2) | 11.3 (10.5-12.3) | 11.4 (10.5-12.2) | .84 |
| Platelet, 103/μL | 181 (149-226) | 184 (149-229) | 176 (148-218) | .23 |
| Creatinine, mg/dL | 0.9 (0.8-1.0) | 0.9 (0.8-1.0) | 0.9 (0.7-1.1) | .77 |
| Bilirubin, mg/dL | 0.5 (0.4-0.6) | 0.5 (0.4-0.6) | 0.5 (0.4-0.7) | .76 |
| LDH, U/L | 179 (147-205) | 179 (152-205) | 173 (140-207) | .60 |
| PT, s | 13.0 (12.6-13.6) | 13.0 (12.5-13.6) | 13.0 (12.7-13.6) | .98 |
| Index VTE acuity, n (%) | ||||
| <1 mo | 72 (35) | 56 (42) | 16 (22) | <.01 |
| 1-3 mo | 69 (34) | 48 (37) | 20 (28) | |
| >3 mo | 63 (31) | 27 (20) | 36 (50) | |
| Index VTE type, n (%) | ||||
| CA-DVT only | 113 (55) | 68 (52) | 45 (63) | .14 |
| PE or DVT | 91 (45) | 64 (48) | 27 (38) | |
| Index VTE presentation, n (%) | ||||
| Symptomatic | 103 (50) | 66 (50) | 37 (51) | .88 |
| Incidental | 101 (50) | 66 (50) | 35 (49) | |
| Prior VTE history, n (%) | ||||
| Yes | 19 (9) | 15 (11) | 4 (6) | .21 |
| No | 185 (91) | 117 (89) | 68 (94) |
Categorical variables were expressed as number and percentage, and continuous variables were expressed as median and interquartile range (IQR). Categorical and continuous variables were compared between the 2 treatment cohorts using Fisher’s exact test and Wilcoxon rank-sum test, respectively.
BEAM, carmustine, etoposide, cytarabine, melphalan; H-TBI, high-dose total body irradiation; LDH, lactate dehydrogenase; NHL, non-Hodgkin lymphoma; PT, prothrombin time; VP-16, etoposide; WBC, white blood cell count.
For primary outcomes, there were no significant differences in the rate of VTE recurrence (1.5% vs 1.4%) with or without adjustment for VTE acuity by 30 days (Table 2). No death was directly attributable to VTE, and the rates of VTE in both groups were low. For the 2 patients who developed recurrent VTE despite anticoagulation, 1 had recurrent PE in the setting of rapid disease progression on day 14 and also had concurrent hematuria and hematochezia while on heparin. The second patient developed asymptomatic CA-DVT despite taking enoxaparin on day 30, but he had significant structural narrowing related to prior line thrombosis. For the 1 patient who developed recurrent VTE while not on anticoagulation, he had prior subsegmental PE that was treated for >1 month prior to HCT but developed symptomatic CA-DVT on day 12 post-HCT while not taking enoxaparin. There were no significant differences in the unadjusted 30-day major bleeding rates between the 2 cohorts (3.8% vs 4.2%). Among the 5 major bleedings in the anticoagulation cohort, 4 occurred with unfractionated heparin. Specifically, 1 death was directly attributable to bleeding, where the patient was found pulseless on the floor, and autopsy revealed significant spontaneous retroperitoneal bleeding. The patient was receiving heparin with PTT of 85 seconds and had a platelet count of 66 × 103/μL 4 hours prior to the event. The other 4 patients with major bleeding required transfusion while on anticoagulation, including psoas hematoma, intramuscular hematoma, significant hematuria, and lower gastrointestinal bleeding. The 3 patients with major bleeding while not on anticoagulation included 1 hematemesis and 2 lower gastrointestinal bleeding.
Outcomes for anticoagulation vs discontinuation during thrombocytopenia
| . | Continuation (n = 132) . | Discontinuation (n = 72) . | Odds ratio . | P . |
|---|---|---|---|---|
| Primary outcomes, n (%) | ||||
| VTE recurrence by 30 d | 2 (1.5) | 1 (1.4) | 1.27 | .85* |
| Major bleeding (grade 3-4) by 30 d | 5 (3.8) | 3 (4.2) | 0.91 | .89† |
| Secondary outcomes | ||||
| Overall bleeding (grade 2-4) by 30 d, n (%) | 51 (39) | 22 (31) | 1.43 | .25† |
| Overall mortality by 30 d, n (%) | 2 (2) | 3 (4) | 0.35 | .26† |
| Number of platelet transfused | 9 (6-13) | 4 (2-6) | NA | <.01‡ |
| Number of RBC transfused | 4 (2-5) | 4 (2-6) | NA | .53‡ |
| Time to platelet >50 × 103/μL | 14 (12-17) | 15 (13-17) | NA | .61‡ |
| Time to neutrophil >500 cells/μL | 14 (12-16) | 14 (12-15) | NA | .49‡ |
| . | Continuation (n = 132) . | Discontinuation (n = 72) . | Odds ratio . | P . |
|---|---|---|---|---|
| Primary outcomes, n (%) | ||||
| VTE recurrence by 30 d | 2 (1.5) | 1 (1.4) | 1.27 | .85* |
| Major bleeding (grade 3-4) by 30 d | 5 (3.8) | 3 (4.2) | 0.91 | .89† |
| Secondary outcomes | ||||
| Overall bleeding (grade 2-4) by 30 d, n (%) | 51 (39) | 22 (31) | 1.43 | .25† |
| Overall mortality by 30 d, n (%) | 2 (2) | 3 (4) | 0.35 | .26† |
| Number of platelet transfused | 9 (6-13) | 4 (2-6) | NA | <.01‡ |
| Number of RBC transfused | 4 (2-5) | 4 (2-6) | NA | .53‡ |
| Time to platelet >50 × 103/μL | 14 (12-17) | 15 (13-17) | NA | .61‡ |
| Time to neutrophil >500 cells/μL | 14 (12-16) | 14 (12-15) | NA | .49‡ |
NA, not applicable.
Logistic regression model was used to test for association between VTE recurrence at 30 d and the 2 cohorts (adjusted by index VTE acuity).
Logistic regression model was used to test for association between bleeding or mortality at 30 d and the 2 cohorts (unadjusted).
Linear regression model was used to test for association between transfusion or engraftment time and the 2 cohorts (unadjusted).
For secondary outcomes, the median number of platelet transfusions was significantly higher in patients who continued anticoagulation (9 vs 4 units; Table 2). However, there were no differences in RBC transfusions (4 vs 4 units). Overall bleeding was numerically but not statistically increased in the cohort that continued anticoagulation (41% vs 32%). There were no differences in overall survival, time to neutrophil, or platelet engraftment.
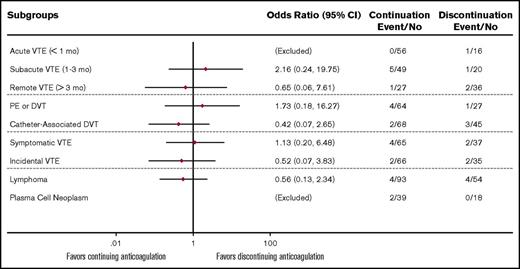
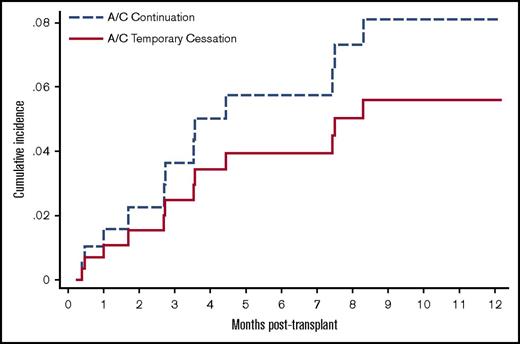
To account for differences in baseline VTE acuity and other predictable confounders, we performed subgroup analyses using a combined 30-day VTE/major bleeding outcome. There were no significant interactions (P > .05) between the treatment cohorts and each of the subgroups analyzed (VTE acuity, VTE type, VTE symptom, and type of malignancy) (Figure 2). Specifically, among patients with acute index VTE (<30 days), 41% of the 56 patients in the continuation cohort and 38% of the 16 patients in the discontinuation cohort had PE or lower-extremity DVT instead of CA-DVT. For this particular subgroup, there was no recurrent symptomatic VTE by 30 days and 1 case of major bleeding in the discontinuation cohort. To determine the incidence of VTE recurrence during a longer follow-up period, we plotted the cumulative incidence for the 2 cohorts after accounting for the competing risk of dying from alternative causes (Figure 3). In this sensitivity analysis, VTE recurred in 7% of patients with a median onset of 94 days at 1-year follow-up. There remained no significant difference in the rate of recurrence between the 2 cohorts after adjusting for index VTE acuity (hazard ratio 1.47, 95% confidence interval 0.37-5.83).
Odds ratio for composite primary outcomes (VTE or major bleeding) in autologous HCT patients. Logistic regression model was used to test for association between the primary composite endpoint and the 2 cohorts in each of the subgroup analyses. All subgroups had nonsignificant interaction; P > .05 for treatment effect by likelihood ratio test.
Odds ratio for composite primary outcomes (VTE or major bleeding) in autologous HCT patients. Logistic regression model was used to test for association between the primary composite endpoint and the 2 cohorts in each of the subgroup analyses. All subgroups had nonsignificant interaction; P > .05 for treatment effect by likelihood ratio test.
Unadjusted incidence of VTE recurrence by competing risk regression analysis. The cumulative incidence for the 2 cohorts was plotted after accounting the competing risk of dying from other cause in this sensitivity analysis. A/C, anticoagulation.
Unadjusted incidence of VTE recurrence by competing risk regression analysis. The cumulative incidence for the 2 cohorts was plotted after accounting the competing risk of dying from other cause in this sensitivity analysis. A/C, anticoagulation.
In patients who continued anticoagulation during thrombocytopenia, the inability to sustain proposed platelet threshold led to early cessation of anticoagulant in 13%, or 27 patients (group 1b and 2b). To analyze the effect of achieved platelet count with transfusion on risk of bleeding, we divided anticoagulated patients into 3 groups based on average platelet AUC (30-50, 50-60, or 60-80 × 103/μL). The rate of bleeding was not significantly different among the 3 groups (Table 3). Furthermore, we tabulated the aggregated time of sustained thrombocytopenia, defined as platelet <50 × 103/μL for at least 2 consecutive days despite transfusion for any patient on anticoagulation. During 65 patient-days of sustained thrombocytopenia, only 6 cases of grade 2 bleeding occurred and no major bleeding was reported. Finally, we performed a multivariate logistic regression model to test for association between various risk factors and major bleeding. We discovered that increasing baseline bilirubin, creatinine, and prothrombin time were independent predictors of major bleeding (Table 4).
Bleeding outcomes by average platelet AUC during anticoagulant exposure
| Average platelet AUC (n = 141) . | Major bleeding (%) . | P . | Overall bleeding (%) . | P . |
|---|---|---|---|---|
| 30-50 × 103/μL (n = 30) | 0 (0) | 11 (8) | ||
| 50-60 × 103/μL (n = 59) | 4 (3) | .28 | 27 (19) | .47 |
| 60-80 × 103/μL (n = 52) | 1 (1) | 15 (11) |
| Average platelet AUC (n = 141) . | Major bleeding (%) . | P . | Overall bleeding (%) . | P . |
|---|---|---|---|---|
| 30-50 × 103/μL (n = 30) | 0 (0) | 11 (8) | ||
| 50-60 × 103/μL (n = 59) | 4 (3) | .28 | 27 (19) | .47 |
| 60-80 × 103/μL (n = 52) | 1 (1) | 15 (11) |
As an estimate of the degree to which the target platelet count was achieved, all platelet values from the onset of thrombocytopenia until recovery were plotted against time in groups 1a, 1b, and 2b. An AUC was calculated (AUCN = (PN+1 + PN) * (TN+1 − TN)/2; P = platelet; T = date/time). The average platelet AUC value was then derived for the duration of thrombocytopenia (AUCAVE = SUM(AUC1:AUCN)/(TN+1 − T1)). If anticoagulation was stopped prior to platelet recovery, only values prior to the stop date were used in the calculation. The risk of bleeding was compared among various groups using Fisher’s exact test.
Risk factors for major bleeding in all patients by 30 days (8 events)
| . | Univariate odds . | P . | Multivariate odds . | P . |
|---|---|---|---|---|
| Patient factors | ||||
| Age (Δ10 y) | 1.15 | .65 | ||
| Weight (Δ10 kg) | 0.83 | .37 | ||
| Sex (male vs female) | 1.01 | .99 | ||
| Ethnicity (white vs nonwhite) | 0.62 | .67 | ||
| Disease (lymphoma vs myeloma) | 1.17 | .85 | ||
| Treatment factors | ||||
| Conditioning chemo (melphalan) | 1.00 | |||
| BEAM | 1.29 | .78 | ||
| H-TBI/Cytoxan+/− VP-16 | 0.61 | .69 | ||
| Other | 1.93 | .52 | ||
| Anticoagulation (discontinuation) | 1.00 | |||
| LMWH | 0.29 | .29 | ||
| Unfractionated heparin | 1.96 | .39 | ||
| Proposed platelet threshold (Δ10 × 103/μL) | 0.99 | .95 | ||
| Average platelet AUC (Δ10 × 103/μL) | 1.01 | .97 | ||
| Thrombocytopenia duration (Δ1 d) | 1.00 | .90 | ||
| Total length of stay (Δ1 d) | 1.02 | .11 | ||
| Preconditioning laboratory tests | ||||
| WBC (Δ1 × 109/L) | 1.00 | .07 | ||
| Hemoglobin (Δ1 g/dL) | 0.47 | .02 | ||
| Platelet (Δ10 × 103/μL) | 1.00 | .35 | ||
| Creatinine (Δ1 mg/dL) | 7.92 | .05 | 14.49 | .03 |
| Bilirubin (Δ1 mg/dL) | 12.16 | <.01 | 19.63 | <.01 |
| LDH (Δ100 U/L) | 1.66 | .01 | ||
| PT (Δ1 s) | 2.48 | .01 | 2.96 | <.01 |
| PTT (Δ1 s) | 1.01 | .75 |
| . | Univariate odds . | P . | Multivariate odds . | P . |
|---|---|---|---|---|
| Patient factors | ||||
| Age (Δ10 y) | 1.15 | .65 | ||
| Weight (Δ10 kg) | 0.83 | .37 | ||
| Sex (male vs female) | 1.01 | .99 | ||
| Ethnicity (white vs nonwhite) | 0.62 | .67 | ||
| Disease (lymphoma vs myeloma) | 1.17 | .85 | ||
| Treatment factors | ||||
| Conditioning chemo (melphalan) | 1.00 | |||
| BEAM | 1.29 | .78 | ||
| H-TBI/Cytoxan+/− VP-16 | 0.61 | .69 | ||
| Other | 1.93 | .52 | ||
| Anticoagulation (discontinuation) | 1.00 | |||
| LMWH | 0.29 | .29 | ||
| Unfractionated heparin | 1.96 | .39 | ||
| Proposed platelet threshold (Δ10 × 103/μL) | 0.99 | .95 | ||
| Average platelet AUC (Δ10 × 103/μL) | 1.01 | .97 | ||
| Thrombocytopenia duration (Δ1 d) | 1.00 | .90 | ||
| Total length of stay (Δ1 d) | 1.02 | .11 | ||
| Preconditioning laboratory tests | ||||
| WBC (Δ1 × 109/L) | 1.00 | .07 | ||
| Hemoglobin (Δ1 g/dL) | 0.47 | .02 | ||
| Platelet (Δ10 × 103/μL) | 1.00 | .35 | ||
| Creatinine (Δ1 mg/dL) | 7.92 | .05 | 14.49 | .03 |
| Bilirubin (Δ1 mg/dL) | 12.16 | <.01 | 19.63 | <.01 |
| LDH (Δ100 U/L) | 1.66 | .01 | ||
| PT (Δ1 s) | 2.48 | .01 | 2.96 | <.01 |
| PTT (Δ1 s) | 1.01 | .75 |
Univariate logistic regression models were built to test the association between major bleeding and all relevant covariates to determine potential predictors of major bleeding. Multivariate logistic regression model using a stepwise estimation by backward and forward selection was then generated from variables with P < .10 from the univariate model. Preconditioning creatinine, bilirubin, and prothrombin time were independently predictive of major bleeding in the final model.
Discussion
To our knowledge, this is the largest observational cohort analysis in patients with concurrent hematologic malignancies, VTE on anticoagulation, and thrombocytopenia post-HCT. In our database, 204 of 1631 adult patients with lymphoma and multiple myeloma arrived for autologous HCT had a history of VTE. Among included patients, approximately two-thirds continued antithrombotic treatment during 2 weeks of expected thrombocytopenia. Continuing anticoagulation was associated with significantly increased platelet utilization but no differences in VTE recurrence, major bleeding, or other secondary outcomes at 30 days. This result was consistent across subgroups and not changed after adjustment for index VTE acuity. The low rate of short-term VTE recurrence (1.4%) among patients who discontinued anticoagulation during the period of thrombocytopenia post-HCT suggests that temporary interruption of anticoagulation may be the better option for many patients.
Although 12.5% of index VTE pretransplant appears higher than expected, more than half of PE and CA-DVT were incidental events discovered on routine restaging scans. Fully recognizing the controversy on the optimal management, we decided to include them in our cohort analysis because our institution generally treats incidental and symptomatic VTE very similarly.12 We also chose 30 days after HCT as the cutoff for primary outcome because it represents a clinically relevant timeframe to assess a transient intervention such as interruption of anticoagulation for 1 to 2 weeks. Despite the low event rates observed at 30 days, the 7% long-term cumulative incidence of VTE recurrence shown in our sensitivity analysis is similar to the event rate reported in the tinzaparin group for cancer VTE in the CATCH trial.13 The 4% major bleeding at 30 days is also comparable to several other case series.8 Therefore, we believe our findings are likely to be reproducible. To put these numbers in perspective and highlight the intricate nature of anticoagulation management in this group of high-risk patients, the VTE and bleeding risks that we observed are both twice as high as those reported in autologous HCT patients without prior VTE.14-16
In patients with a higher risk of VTE recurrence needing full-dose anticoagulation during the period of thrombocytopenia, current guidelines recommend maintaining platelet transfusion >50 × 103/μL during the acute period but alternately considering half-dose or withholding anticoagulation for subacute to chronic VTE.4 In reality, this was not strictly adhered at our institution, and a great majority of patients with nonacute VTE nonetheless received full-dose anticoagulation with aggressive platelet support. This prompted us to examine the pattern of bleeding according to the platelet levels achieved. We were unable to find an association between platelet threshold and risk of major bleeding based on average platelet AUC comparisons and multivariate modeling. Instead, our results suggest an association between baseline hepatic (bilirubin), renal (creatinine), or hemostatic (prothrombin time) impairment and increased bleeding risk. The lack of association between platelet threshold and risk of bleeding among anticoagulated patients in our analysis is consistent with a previously published retrospective case series, where a total of 31 patients receiving enoxaparin appeared to be safe with a platelet range of 22 to 55 × 103/μL post-HCT.10,17 If the relative safety of a lower platelet transfusion threshold (30 vs 50 × 103/μL) can be confirmed in a prospective setting, a change in clinical practice could lead to fewer transfusions, conservation of donated platelets, and reduced cost.
The strengths of our study include a large patient population, uniform inclusion criteria, similar duration of thrombocytopenia with consecutive platelet values, and excellent short-term follow-up. We believe in the methodology and data accuracy because we correlated all ICD9-coded events with individual chart review and that our findings are reproducible compared with published evidence. Autologous HCT serves as an excellent real-world model to study the effect of hypoproliferative thrombocytopenia on anticoagulation for VTE. This patient population is affected by lymphoma and multiple myeloma, has a high risk of thrombosis, but experiences relatively few clinical events that could be confounders, such as recurrent cancer, failure of engraftment, or graft-versus-host disease.18,19
Similar to other retrospective studies, our results are limited by potential selection bias and possible unforeseeable differences between the groups. There are potential baseline VTE risk factors that we could not capture, such as personal or family history of thrombophilia. Despite our best attempt to minimize the differences by conducting subgroup analysis, sensitivity analysis, and adjusting for index VTE acuity, we recognize that patients with acute VTE are more likely to continue anticoagulation than patients with remote VTE, and this is an important factor that needs to be addressed by a randomized controlled trial in the future. Furthermore, the small number of events at 30 days and the lack of uniform exit screening procedure may limit the power to detect any difference, especially for asymptomatic VTE recurrence, that might exist among the acute VTE cohort for the primary endpoint. Finally, the lack of difference in bleeding risk for different platelet AUC thresholds is exploratory because we did not further control or adjust for potential confounders such as baseline bleeding risk or physician effort or platelet refractoriness.
In conclusion, in lymphoma and myeloma patients with prior VTE undergoing autologous HCT, the risk of recurrent VTE during the 30 days after HCT appears to be low. Our findings suggest that temporarily withholding anticoagulation during the period of thrombocytopenia post-HCT, especially in patients whose prior VTE has been treated for >1 month, may offer the best risk-benefit tradeoffs. For patients with higher thrombotic risks not well captured or underrepresented in our dataset, such as patients with known hereditary thrombophilia or recent symptomatic VTE, it remains critical to consider individualized recommendation. Among patients entering HCT with a recent VTE, we were unable to detect any platelet threshold below which continuous, full-dose anticoagulation becomes more dangerous. Future prospective clinical trials should include anticoagulation interruption as a management option for patients undergoing autologous HCT with prior VTE. For patients in whom briefly interrupting anticoagulation is not feasible (eg, persons with very recent and symptomatic VTE), studies that explore different platelet thresholds and/or different intensities of anticoagulation during the period of thrombocytopenia are warranted.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health under award numbers T32HL007093 (A.L.), National Cancer Institute, National Institutes of Health 5K24CA184039 (A.K.G.), and a gift from Frank and Betty Vandermeer (A.K.G.).
Authorship
Contribution: A.L. designed research, collected clinical data, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; C.D. and M.F.K. collected and analyzed institutional and claims data; Q.W. performed statistical analysis; S.L. collected clinical data; A.K.G. and L.A.H. analyzed and interpreted data; and D.A.G. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ang Li, Division of Hematology, University of Washington, 1100 Fairview Ave N, D5-100, Seattle, WA 98109; e-mail: ali2015@uw.edu.
References
Author notes
A.K.G. and D.A.G. contributed equally to this work as joint senior authors.