Key Points
Exosomal miR-340 derived from young BMSCs inhibited tumor angiogenesis via the HGF/c-MET signaling pathway.
The anti-angiogenic effect of exosomes from older BMSCs was restored by direct transfection of young BMSC-derived exosomal miRNAs.
Abstract
The study of bone marrow stromal cells (BMSCs) and the exosomes they secrete is considered promising for cancer therapy. However, little is known about the effect of donor age on BMSCs. In the present study, we investigated the therapeutic potential of BMSC exosomes derived from donors of different ages using an in vivo model of hypoxic bone marrow in multiple myeloma (MM). We found that donor age was strongly related to senescent changes in BMSCs. Exosomes derived from young BMSCs significantly inhibited MM-induced angiogenesis in Matrigel plugs. The exosomal microRNA (miRNA) expression profile was different between young and older BMSCs, despite similarities in the size and quantity of exosomes. Of note was the observation that the antiangiogenic effect of older BMSCs was enhanced by direct transfection of miR-340 that was preferentially expressed in exosomes derived from young BMSCs. We found that miR-340 inhibited angiogenesis via the hepatocyte growth factor/c-MET (HGF/c-MET) signaling pathway in endothelial cells. Our data provide new insights into exosome-based cancer therapy by modification of BMSC-derived exosomes.
Introduction
The tumor microenvironment has gradually been recognized as a key contributor to cancer progression, angiogenesis, and metastasis1 and to the development of drug resistance.2 Therefore, future development of anticancer therapies3 should consider the tumor microenvironment. Bone marrow stromal cells (BMSCs) interact with multiple myeloma (MM) cells in the bone marrow (BM). BMSCs also create a permissive microenvironment for MM cell growth and survival.4 An increase of angiogenesis is involved in the progression of MM and the cell-to-cell contact between MM cells, whereas BMSCs regulate the secretion of key mediators of angiogenesis, such as vascular endothelial growth factor and basic fibroblast growth factor.5
It is well established that MM is an incurable disease. It commonly occurs in older people with a median age at diagnosis of approximately 70 years.6,7 A previous study by Andre et al demonstrated that BMSCs in MM patients have an early senescent profile.8 Furthermore, BMSC aging results in a decrease of their ability to maintain the BM microenvironment, leading to increases in the incidence of hematological malignancies, anemia, and immune dysfunction.9,10 Recent evidence indicates that exosome-mediated cell-to-cell communication plays an important role in maintaining the BM microenvironment.11
Exosomes are 30- to120-nm membrane-bound vesicles secreted naturally by almost all cells and exist in all body fluids.12 Accumulating evidence has shown that exosomes contain proteins, lipids, DNA, messenger RNA (mRNA), microRNA (miRNA), and long noncoding RNA that can be transferred from producer cells to recipient cells, thereby facilitating cell-to-cell communication.13-16 More recent studies in animal models have indicated that healthy BMSC-derived exosomes are effective for treatment of cardiovascular disease,17 lung injury,18,19 kidney injury,20,21 and liver injury.22 These findings suggest that the contents transferred by healthy BMSC-derived exosomes have a therapeutic potential. Kordelas et al demonstrated that the clinical symptoms of graft-versus-host disease are significantly improved after treatment with healthy BMSC-derived exosomes.23 In a manner similar to that of BMSCs, BMSC-derived exosomes have lower antigenicity than do various cell types used for cell therapy, such as activated T cells.24,25 Although aging of BMSCs has been studied extensively, the effect of exosomes derived from older BMSCs on the BM microenvironment remains unclear. Therefore, we hypothesized that the therapeutic potential of healthy BMSC-derived exosomes depends largely on the donor age, possibly because of age-specific differences in their contents (eg, mRNA, miRNA, and protein).
To test this hypothesis, we investigated the donor age–related differences and therapeutic effects of healthy BMSC-derived exosomes by using a heterotransplant mouse model of chronic hypoxia-resistant (HR) MM cells.26 HR-MM cells showed continuous growth under persistent hypoxic conditions, and exosomes derived from HR-MM cells affected angiogenic functions in both in vitro and in vivo models.26 In the present study, we modified BMSC-derived exosomes from older healthy donors by directly transfecting them with miRNA expressed specifically in young BMSC exosomes. This approach resulted in exosomes derived from older BMSCs acquiring antiangiogenic properties, suggesting that exosomal miRNA replacement has a therapeutic potential.
Methods
Cell lines and culture conditions
Human MM cell lines (RPMI8226, KMS-11, and IM-9) were purchased from the Health Science Research Resource Bank (Osaka, Japan). Sublines that survived well under long-term hypoxia (HR-MM cell lines; RPMI8226-HR, KMS-11-HR, and IM-9-HR)26 were maintained under hypoxic conditions (1% O2, 5% CO2, and 94% N2) in a humidified, gas-sorted hypoxic incubator (MCO-5M; Panasonic, Osaka, Japan). Pooled human umbilical vein endothelial cells (HUVECs) and human BMSCs from young (donor ages: 19 and 20 years old; young BMSCs) and older (donor ages: 68 and 72 years old; older BMSCs) healthy donors were purchased from Lonza Inc. (Allendale, NJ). BMSCs were harvested by trypsinization and either passaged for expansion or subjected to analyses (passages 1-5). During days 1 to 5, the number of viable cells was determined using a commercial kit (Cell Counting Kit-8; Dojindo, Kumamoto, Japan). See the supplemental experimental procedures for details.
Measurement of terminal restriction fragments (TRFs)
Genomic DNA were extracted from each group of BMSCs by using the Gentra Puregene Cell kit (Qiagen, Hiden, Germany) and digested by restriction enzymes (Hinf I or Rsa I). Digested DNA were fractionated by electrophoresis on 0.7% agarose gel and then transferred to nylon membranes. Hybridization was performed with the Telo TTAGGG Telomere Length Assay kit (Roche, Mannheim, Germany) according to the manufacturer’s instructions. Smears of the developed films were captured on a VersaDoc 1000 imaging system (Bio-Rad, Hercules, CA).
Senescence-associated (SA) β-galactosidase (β-gal) staining
BMSCs were fixed in 0.2% glutaraldehyde, washed with phosphate-buffered saline (PBS), and incubated overnight at 37°C (without CO2) in staining solution (1 mg/mL of X-gal in 40 mM of citric acid/sodium phosphate, pH 6.0, 5 mM of potassium ferrocyanide, 5 mM of potassium ferricyanide, 150 mM of NaCl, and 2 mM of MgCl2). After incubation, the cells were washed with PBS and imaged for the presence of blue staining.
Isolation and characterization of exosomes
BMSCs (4 × 104 cells/cm2) were cultured in T-25 flasks (BD Biosciences, San Jose, CA) containing 5 mL of serum-free AIM-V medium (Invitrogen, Carlsbad, CA). The culture supernatants were harvested after 48 h of incubation, and the exosome fraction was purified using Exoquick-TC reagent (System Biosciences, Mountain View, CA) according to the manufacturer’s instructions. Exosomes were quantified by nanoparticle tracking analysis (NanoSight LM10; Malvern, Herrenberg, Germany) and observed under a transmission electron microscope (JEM-1200EX; JEOL, Tokyo, Japan), as described previously.27 See the supplemental experimental procedures for details.
Direct transfection of miRNA mimics into exosomes
miRNA mimics (Ambion; Invitrogen) were labeled with the Label IT small interfering RNA Tracker Cy3 kit (Mirus, Madison, WI) according to the manufacturer’s instructions. mirVana miRNA mimics (see supplemental Table 1 for mimic ID) (Applied Biosystems) were transfected directly into exosomes using Exo-fect (System Biosciences). See the supplemental experimental procedures for details.
miRNA expression profiles
Cellular and exosomal miRNAs were isolated using the miRNeasy kit (Qiagen), and miRNA profiling of both cells and exosomes was performed using the TaqMan low-density miRNA array (Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer’s recommendations. The relative expression of each gene was calculated using the comparative threshold cycle method, as described previously.28 See the supplemental experimental procedures for details.
Tube formation assay
The formation of capillary-like structures was assessed, as described previously.27 HUVECs (2 × 104 cells/well) were seeded on Matrigel (growth factor reduced, 280 µL/well; BD) in 24-well plates and treated with exosomes (1.2 × 107 particles) in endothelial basal medium-2 (480 µL/well; Lonza Inc.), supplemented with or without endothelial growth medium-2 SingleQuots and 5% fetal bovine serum for 24 h. BMSC-derived exosomes were titrated to determine the optimal concentration for tube formation assay (supplemental Figure 1). The total area of tube formation was quantified as the mean pixel density obtained from image analysis of 5 random microscopic fields using ImageJ software (http://rsb.info.nih.gov/nih-image/).
In vivo Matrigel plug assay
A mixture of growth factor–reduced Matrigel (200 µL) and 4 × 106 HR-MM cells was admixed with exosomes derived from BMSCs (4 × 107 particles). The Matrigel mixtures were injected subcutaneously into 8-week-old male nude mice (BALB/c-nu/nu; CLEA Japan, Tokyo, Japan). All experimental groups included 3 mice. Three weeks after implantation, the Matrigel plugs were harvested and processed for analysis. The Matrigel plugs were fixed with 4% paraformaldehyde and embedded in paraffin. Then, 8-µm-thick paraffin sections were prepared using a microtome (RM2245; Leica Biosystems, Heidelberg, Germany). Neovascularization was evaluated by immunohistochemistry. The total CD31-positive area was quantified as the mean pixel density obtained from image analysis of 6 random microscopic fields by using ImageJ software. See the supplemental experimental procedures for details on analysis of capillary density.
Western blotting
Western blots were probed with antibodies against cMET (rabbit polyclonal, 1:500; C-28; Santa Cruz Biotechnology, Santa Cruz, CA), phosphorylated cMET (p-cMET) (rabbit polyclonal, 1:100; ab5662; Abcam, Cambridge, MA), hepatocyte growth factor (HGF) (rabbit polyclonal, 1:100; ab83760; Abcam), and β-actin (mouse monoclonal, 1:2500; Chemicon, Temecula, CA). CD63 (mouse monoclonal anti-CD63 antibody, 1:100, sc-5275; Santa Cruz Biotechnology) and CD81 (mouse monoclonal anti-CD81 antibody; 1:100; sc-23962; Santa Cruz Biotechnology) were used as markers of exosomes. Horseradish peroxidase–conjugated secondary antibodies were purchased from GE Healthcare Life Science (Uppsala, Sweden). See the supplemental experimental procedures for details.
Ethics
All animal experiments were conducted in compliance with the institutional guidelines of the Animal Experimental Center of Tokyo Medical University/Animal Biosafety Level II Laboratory for Use of Animals. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Tokyo Medical University. The use of patient samples was approved by the Institutional Review Board of Tokyo Medical University (no. 2648, approved 22 April 2014). Written informed consent was obtained from all participants before specimen collection, according to the Declaration of Helsinki.
Statistical analyses
Data are presented as the mean ± standard deviation (SD). Comparisons between 2 groups were made with the Student t test. Multiple group comparisons were made by analysis of variance. GraphPad Prism (version 5c) for Macintosh (GraphPad Inc., La Jolla, CA) was used for statistical analyses. Results were considered statistically significant at P < .05.
Results
Donor age–dependent senescent changes in BMSCs
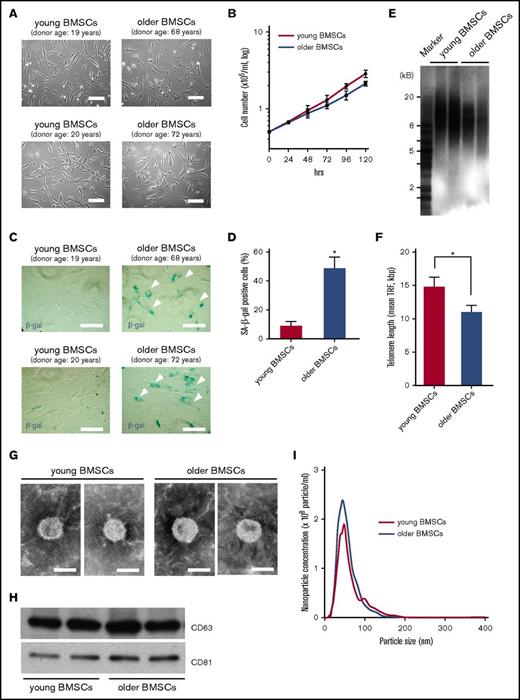
There were no morphological differences between BMSCs derived from young or older healthy donors (Figure 1A). The cell proliferation rate was not significantly different between young and older BMSCs for at least 72 h in culture, although older BMSCs had a gradual reduction in their proliferative capability (Figure 1B). The number of SA-β gal-positive cells among older BMSCs was significantly higher than that among young BMSCs at the same passage (Figure 1C-D; P < .01). Similarly, TRFs in older BMSCs (11 ± 0.99 kbp) were shorter than were those in young BMSCs (14.8 ± 1.41 kbp) (Figure 1E-F), suggesting that senescence-associated changes in BMSCs are dependent on the donor age.
Characterization of BMSCs. (A) Morphology of BMSCs derived from 2 young healthy donors (young BMSCs; passage 2; donor ages: 19 and 20 years old) and 2 older healthy donors (older BMSCs; passage 2; donor age: 68 and 72 years) by phase-contrast inverted microscopy. Scale bar, 50 µm. (B) BMSC growth measured after 24, 48, 72, 96, and 120 h in culture. Cell proliferation of young BMSCs (pink line) and older BMSCs (blue) was evaluated. Each value represents the mean ± SD (n = 6). (C-D) Representative images of SA-β-gal staining (arrows indicate positive cells) and quantification of cell senescence. Data represent the mean ± SD. *P < .01 in comparison with young BMSCs using a Student t test. Scale bar, 50 µm. (E-F) Telomere lengths in young and older BMSCs determined by Southern blotting of TRFs. Data represent the mean ± SD. *P < .05 in comparison with young BMSCs using a Student t test. (G) Transmission electron micrographs of exosomes derived from young and older BMSCs. Scale bar, 50 nm. (H) Western blots of exosomal lysates (CD63 and CD81 are common exosomal markers) derived from young and older BMSCs. Exosomes were isolated from 5 mL of culture medium collected from BMSCs (4 × 104 cells/cm2) grown for 48 h. Equal volumes of exosomal lysate (30 µL) were loaded in the lanes of gels. (I) Nanoparticle concentration and size distribution of exosomes derived from young BMSCs (pink line) and older BMSCs (blue line).
Characterization of BMSCs. (A) Morphology of BMSCs derived from 2 young healthy donors (young BMSCs; passage 2; donor ages: 19 and 20 years old) and 2 older healthy donors (older BMSCs; passage 2; donor age: 68 and 72 years) by phase-contrast inverted microscopy. Scale bar, 50 µm. (B) BMSC growth measured after 24, 48, 72, 96, and 120 h in culture. Cell proliferation of young BMSCs (pink line) and older BMSCs (blue) was evaluated. Each value represents the mean ± SD (n = 6). (C-D) Representative images of SA-β-gal staining (arrows indicate positive cells) and quantification of cell senescence. Data represent the mean ± SD. *P < .01 in comparison with young BMSCs using a Student t test. Scale bar, 50 µm. (E-F) Telomere lengths in young and older BMSCs determined by Southern blotting of TRFs. Data represent the mean ± SD. *P < .05 in comparison with young BMSCs using a Student t test. (G) Transmission electron micrographs of exosomes derived from young and older BMSCs. Scale bar, 50 nm. (H) Western blots of exosomal lysates (CD63 and CD81 are common exosomal markers) derived from young and older BMSCs. Exosomes were isolated from 5 mL of culture medium collected from BMSCs (4 × 104 cells/cm2) grown for 48 h. Equal volumes of exosomal lysate (30 µL) were loaded in the lanes of gels. (I) Nanoparticle concentration and size distribution of exosomes derived from young BMSCs (pink line) and older BMSCs (blue line).
Characterization of exosomes derived from young and older BMSCs
The size and structure of exosomes appeared to be similar between young and older BMSCs (Figure 1G). Additionally, the expression of common exosomal markers (CD63 and CD81) was similar between young and older BMSCs (Figure 1H). The particle size of BMSC-derived exosomes was approximately 50 nm. On average, after 48 h of culture, 1.9 × 108/mL exosomes and 2.3 × 108/mL exosomes were found in the culture supernatants of young BMSCs and older BMSCs, respectively (Figure 1I).
In vivo model of the MM hypoxic niche
We previously established HR-MM cells derived from MM cell lines (RPMI8226-HR, KMS-11-HR, and IM-9-HR) to evaluate the potential role of MM cells in the hypoxic BM environment. We found that HR-MM cells induce tumor angiogenesis under chronic hypoxic conditions.26 In this study, we used an in vivo Matrigel plug angiogenesis assay to analyze the angiogenic responses to HR-MM cells in vivo (supplemental Figure 2). HR-MM cells formed tumors with increased amounts of CD31-positive endothelial cells in the Matrigel plug (supplemental Figure 2A,C), whereas parental cells did not grow (supplemental Figure 2B-C). Among them, RPMI8226-HR cells induced higher levels of angiogenesis in comparison with other HR-MM cell lines (supplemental Figure 2A). Therefore, we primarily used RPMI8226-HR cells in this study.
Antiangiogenic effects of BMSC-derived exosomes from young donors
BALB/c nude mice were injected subcutaneously with HR-MM cells (RPMI8226-HR) seeded in Matrigel plugs containing exosomes (4 × 107 particles per 200 µL Matrigel) derived from young BMSCs (young BMSC exosomes) or older BMSCs (older BMSC exosomes) (Figure 2A). We observed hyperproliferative CD138-positive RPMI8226-HR cells and CD31-positive endothelial cells attracted into the Matrigel plug (Figure 2B, left). However, older BMSC exosomes inhibited the proliferation of RPMI8226-HR cells and angiogenesis in the Matrigel plug (Figure 2B, right) in comparison with the control (RPMI8226-HR + PBS) (Figure 2B-C; P < .05). In addition, the antiangiogenic effect was more evident with young BMSC exosomes than with older BMSC exosomes (Figure 2B, middle, and Figure 2C; P < .001).
Healthy BMSC exosomes inhibit HR-MM cell-induced angiogenesis in Matrigel plugs. (A) Schematic of the Matrigel plug assay. A mixture of Matrigel and 4 × 106 RPMI8226-HR cells was admixed with exosomes derived from young BMSCs (young BMSC exosomes; 4 × 107 particles per 200 µL Matrigel) or older BMSCs (older BMSC exosomes; 4 × 107 particles per 200 µL Matrigel). (B) After 3 weeks, the Matrigel plugs were harvested and photographed. Paraffin-embedded sections of Matrigel plugs were stained with hematoxylin and eosin and then subjected to immunostaining for CD138 (brown) and CD31 (red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 500 µm. (C) Quantification of vessel density in Matrigel plugs by pixel density. Young and older BMSC exosomes inhibited tumor angiogenesis in Matrigel plugs compared with the control (RPMI8226-HR only) (*P < .01; **P < .001 vs control, Student t test, n = 6). Values represent the mean ± SD. (D) Effect of BMSC exosomes on the viability of HR-MM cells. RPMI8226-HR cells were cultured with young or older BMSC exosomes (2.5 × 107 particles/mL). Cell viability was assayed after 48 h using Cell Counting Kit-8. Values represent the mean ± SD. (E) The formation of tube-like structures was observed using the cell-permeable dye Calcein AM (green). Endothelial tube formation of HUVECs treated with young BMSC exosomes, older BMSC exosomes, or the control (without exosomes). Scale bar, 500 µm. (F) Quantification of tube-like structures by pixel density. Young and older BMSC exosomes significantly inhibited HUVEC tube formation in comparison with the control (control vs young BMSC exosomes; P < .001, control vs older BMSC exosomes; P = .038, Student t test). Values represent the mean ± SD.
Healthy BMSC exosomes inhibit HR-MM cell-induced angiogenesis in Matrigel plugs. (A) Schematic of the Matrigel plug assay. A mixture of Matrigel and 4 × 106 RPMI8226-HR cells was admixed with exosomes derived from young BMSCs (young BMSC exosomes; 4 × 107 particles per 200 µL Matrigel) or older BMSCs (older BMSC exosomes; 4 × 107 particles per 200 µL Matrigel). (B) After 3 weeks, the Matrigel plugs were harvested and photographed. Paraffin-embedded sections of Matrigel plugs were stained with hematoxylin and eosin and then subjected to immunostaining for CD138 (brown) and CD31 (red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 500 µm. (C) Quantification of vessel density in Matrigel plugs by pixel density. Young and older BMSC exosomes inhibited tumor angiogenesis in Matrigel plugs compared with the control (RPMI8226-HR only) (*P < .01; **P < .001 vs control, Student t test, n = 6). Values represent the mean ± SD. (D) Effect of BMSC exosomes on the viability of HR-MM cells. RPMI8226-HR cells were cultured with young or older BMSC exosomes (2.5 × 107 particles/mL). Cell viability was assayed after 48 h using Cell Counting Kit-8. Values represent the mean ± SD. (E) The formation of tube-like structures was observed using the cell-permeable dye Calcein AM (green). Endothelial tube formation of HUVECs treated with young BMSC exosomes, older BMSC exosomes, or the control (without exosomes). Scale bar, 500 µm. (F) Quantification of tube-like structures by pixel density. Young and older BMSC exosomes significantly inhibited HUVEC tube formation in comparison with the control (control vs young BMSC exosomes; P < .001, control vs older BMSC exosomes; P = .038, Student t test). Values represent the mean ± SD.
To test whether BMSC exosomes directly affect the cell viability of HR-MM cells, we measured the cell proliferation of RPMI8226-HR cells treated with young or older BMSC exosomes (2.5 × 107 particles/mL). Neither older BMSC exosomes nor young BMSC exosomes affect the survival of RPMI8226-HR cells (Figure 2D). We assessed effector caspase activity in RPMI8226-HR cells treated with BMSC exosomes (2.5 × 107 particles/mL). There was no significant difference in caspase 3/7 activity in RPMI8226-HR cells treated with and without BMSC exosomes (supplemental Figure 3B). Furthermore, we performed experiments to assess nonapoptotic cell death mechanisms in HR-MM cells, including autophagic cell death. The expression of p62 protein showed no significant difference between RPMI8226-HR cells treated with BMSC exosomes and without (supplemental Figure 3B).
We next performed an endothelial tube formation assay with HUVECs treated with young or older BMSC exosomes (2.5 × 107 particles/mL). Consistent with the results obtained from the in vivo Matrigel plug assay, HUVEC tube formation was inhibited to a higher degree by young BMSC exosomes than they were by older BMSC exosomes (young BMSC exosomes vs older BMSC exosomes; P = .007) (Figure 2E-F).
Identification of young BMSC exosome-specific miRNAs
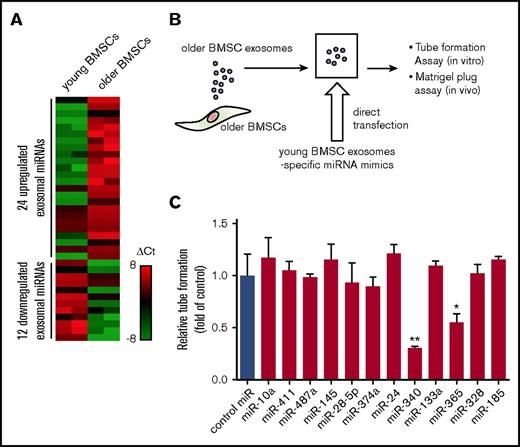
To identify the exosomal miRNAs associated with the antiangiogenic effect of young BMSC exosomes, we first compared exosomal miRNA expression profiles between young and older BMSCs (National Center for Biotechnology Information [NCBI], gene expression omnibus; GSE78865). A subset of 12 miRNAs was significantly downregulated in older BMSC exosomes. These miRNAs were specifically expressed in young BMSC exosomes (Figure 3A). Subsequently, we replenished the 12 miRNAs in older BMSC exosomes by direct transfection with miRNA mimics (Figure 3B) and investigated whether young BMSC exosome-specific miRNAs affect HUVEC tube formation. Exosomes transfected with miR-340 or miR-365 mimics significantly inhibited angiogenesis in comparison with scrambled control miRNA-containing exosomes (control miR exosomes) (Figure 3C; P < .01, P < .001).
Differential miRNA expression profiles of exosomes derived from young and older BMSCs. (A) Differential miRNA expression of young and older BMSC exosomes was determined by TaqMan miRNA array analysis. (B) Schematic of the modification of older BMSC exosomes. Young BMSC exosome-specific miRNA mimics were transfected directly into older BMSC exosomes by Exo-fect. (C) Quantification of tube-like structures to determine the antiangiogenic effect of modified exosomes. Older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes) and older BMSC exosomes transfected with miR-365 mimics (miR-365 exosomes) significantly inhibited HUVEC tube formation in comparison with the control (older BMSC exosomes transfected with negative control miR; control miR) (control vs miR-340 exosomes; *P < .01, Student t test; **P < .001, control vs miR-365 exosomes). Values represent the mean ± SD. Ct, comparative threshold cycle.
Differential miRNA expression profiles of exosomes derived from young and older BMSCs. (A) Differential miRNA expression of young and older BMSC exosomes was determined by TaqMan miRNA array analysis. (B) Schematic of the modification of older BMSC exosomes. Young BMSC exosome-specific miRNA mimics were transfected directly into older BMSC exosomes by Exo-fect. (C) Quantification of tube-like structures to determine the antiangiogenic effect of modified exosomes. Older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes) and older BMSC exosomes transfected with miR-365 mimics (miR-365 exosomes) significantly inhibited HUVEC tube formation in comparison with the control (older BMSC exosomes transfected with negative control miR; control miR) (control vs miR-340 exosomes; *P < .01, Student t test; **P < .001, control vs miR-365 exosomes). Values represent the mean ± SD. Ct, comparative threshold cycle.
Enrichment of older BMSC exosomes with young BMSC exosome-specific miRNAs inhibits angiogenesis in vivo
Older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes) exhibited a 6-fold increase in the expression of miR-340 in relation to young BMSC exosomes (Figure 4A). We also visualized with an in vitro model the uptake of exosomal Cy3-labeled miR-340 into HR-MM cells (RPMI8226-HR; Figure 4B) and HUVECs (Figure 4C) via exosomes. Matrigel plug assays were performed using Cy3-labeled miR-340. We checked the colocalization of Cy3-labeled miR-340 and HR cells in the Matrigel plug. Cy3-miR-340 signals were detected in not only the cytoplasm of HR cells (supplemental Figure 4A-B) but also the cytoplasm of endothelial cells (supplemental Figure 4C-F).
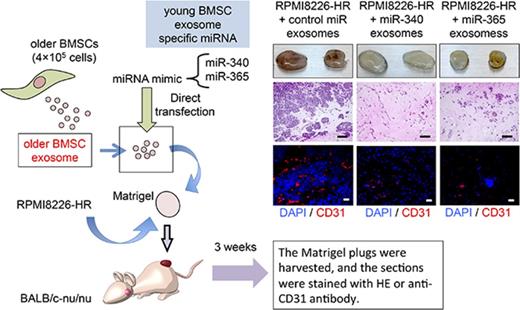
Antiangiogenic effects of modified exosomes in vivo. (A) Exosomal miR-340 expression levels in young BMSC exosomes, older BMSC exosomes, and miR-340 exosomes were quantified by reverse transcription polymerase chain reaction (young BMSC exosomes vs older BMSC exosomes; *P < .01, young BMSC exosomes vs miR-340 exosomes; **P < .001, Student t test). Values represent the mean ± SD. (B-C) Recipient cells were treated with exosomes directly transfected with Cy3-miR-340 mimics. Cy3-miR-340 signals were detected in the cytoplasm of RPMI8226-HR cells (B) and HUVECs (C). Nuclear and cytoplasmic staining were performed with DAPI (blue) and Calcein AM (green), respectively. Scale bar, 25 µm. (D) A mixture of Matrigel and 4 × 106 RPMI8226-HR cells was admixed with older BMSC exosomes directly transfected with miR-340 mimics (miR-340 exosomes; 4 × 107 particles per 200 µL Matrigel), miR-365 mimics (miR-365 exosomes; 4 × 107 particles per 200 µL Matrigel), or negative control miR (control miR exosomes). The Matrigel mixture was injected subcutaneously into nude mice. Three weeks after implantation, the Matrigel plugs were harvested. Paraffin-embedded sections of Matrigel plugs were stained with hematoxylin and eosin and then subjected to immunostaining for CD31 (red). Scale bar, 500 µm. (E) Quantification of vessel density in Matrigel plugs by pixel density. Both mir-340 and miR-365 exosomes inhibited tumor angiogenesis in Matrigel plugs in comparison with the control (*P < .001 vs control, Student t test, n = 6). Values represent the mean ± SD. Exo, exosomes.
Antiangiogenic effects of modified exosomes in vivo. (A) Exosomal miR-340 expression levels in young BMSC exosomes, older BMSC exosomes, and miR-340 exosomes were quantified by reverse transcription polymerase chain reaction (young BMSC exosomes vs older BMSC exosomes; *P < .01, young BMSC exosomes vs miR-340 exosomes; **P < .001, Student t test). Values represent the mean ± SD. (B-C) Recipient cells were treated with exosomes directly transfected with Cy3-miR-340 mimics. Cy3-miR-340 signals were detected in the cytoplasm of RPMI8226-HR cells (B) and HUVECs (C). Nuclear and cytoplasmic staining were performed with DAPI (blue) and Calcein AM (green), respectively. Scale bar, 25 µm. (D) A mixture of Matrigel and 4 × 106 RPMI8226-HR cells was admixed with older BMSC exosomes directly transfected with miR-340 mimics (miR-340 exosomes; 4 × 107 particles per 200 µL Matrigel), miR-365 mimics (miR-365 exosomes; 4 × 107 particles per 200 µL Matrigel), or negative control miR (control miR exosomes). The Matrigel mixture was injected subcutaneously into nude mice. Three weeks after implantation, the Matrigel plugs were harvested. Paraffin-embedded sections of Matrigel plugs were stained with hematoxylin and eosin and then subjected to immunostaining for CD31 (red). Scale bar, 500 µm. (E) Quantification of vessel density in Matrigel plugs by pixel density. Both mir-340 and miR-365 exosomes inhibited tumor angiogenesis in Matrigel plugs in comparison with the control (*P < .001 vs control, Student t test, n = 6). Values represent the mean ± SD. Exo, exosomes.
Matrigel plug assays confirmed that older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes) or miR-365 mimics (miR-365 exosomes) significantly inhibited angiogenesis in the Matrigel plug in comparison with scrambled control miRNA-containing exosomes (control miR exosomes) (Figure 4D-E; P < .001). We also observed inhibition of the hyperproliferation of HR-MM cells in conjunction with the antiangiogenic effect of older BMSC exosomes transfected with miR-340 and miR-365 mimics (Figure 4D). These findings suggested that the antiangiogenic effect of older BMSC exosomes was restored by transfection with young BMSC exosome-specific miRNAs (ie, miR-340 and miR-365). We performed Matrigel plug assays with KMS-11-HR and IM-9-HR cells and primary myeloma cells of MM patients. As in the experiment using RPMI8226-HR cells, miR-340 exosomes significantly inhibited angiogenesis induced by HR-MM cells (supplemental Figure 5A) or CD138+ cells from a MM patient with a high tumor burden in the Matrigel plug (supplemental Figure 5B).
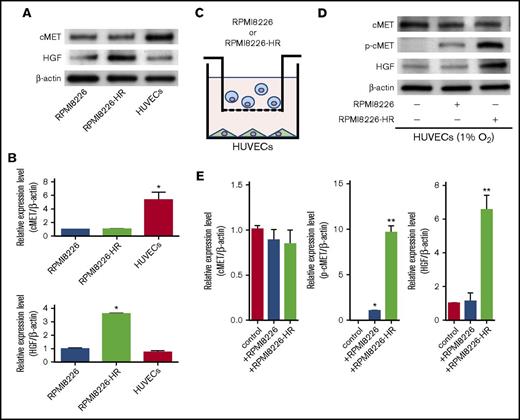
Phosphorylated cMET and HGF protein levels vary in HUVECs cocultured with HR-MM cells
We selected various confirmed targets of miR-340 and miR-365 according to reporter assays or Western blotting using miRTarBase.29 Within this list, we searched for a candidate target of miR-340 with a high predicted efficacy as calculated by the context scores. We focused on the cMET tyrosine kinase receptor that exerts context-dependent effects on endothelial cells. cMET expression was significantly higher in HUVECs than in MM cells (RPMI8226 and RPMI8226-HR) (Figure 5A). There was no difference in cMET expression between parental and HR-MM cells (RPMI8226-HR) (Figure 5A-B; P < .01). HGF protein expression was higher in HR-MM cells than in parental and endothelial cells (Figure 5A-B; P < .01). We next investigated changes in the expression of cMET, phosphorylated cMET (p-cMET), and HGF in HUVECs cultured with or without MM cells using a noncontact coculture system (Figure 5C). Although there were no significant differences in the levels of cMET, p-cMET was significantly upregulated in HUVECs cocultured with HR-MM cells (Figure 5D-E; P < .01, P < .001). Additionally, HGF expression in HUVECs was induced by coculture with HR-MM cells (Figure 5D-E; P < .001).
p-cMET and HGF protein levels vary in HUVECs cocultured with HR-MM cells. (A) Western blots showing the expression of cMET and HGF in MM cells (RPMI8226 and RPMI8226-HR) and HUVECs. (B) The intensities of cMET and HGF bands in panel A were quantified and normalized to the levels of β-actin (*P < .01 vs RPMI8226, Student t test, n = 3). (C) Schematic of the nonadherent coculture system of MM cells (blue) and HUVECs (green). MM cells and HUVECs were cocultured separately using a Transwell filter (polycarbonate membrane insert, 0.45-µm pores). (D) cMET, p-cMET, and HGF levels in HUVECs were measured by Western blotting after coculture with RPMI8226 or RPMI8226-HR cells under hypoxic conditions (1% O2). (E) The intensities of cMET, p-cMET, and HGF bands in panel D were quantified and normalized to the levels of β-actin (*P < .01; **P < .001 vs control (HUVECs only), Student t test, n = 3).
p-cMET and HGF protein levels vary in HUVECs cocultured with HR-MM cells. (A) Western blots showing the expression of cMET and HGF in MM cells (RPMI8226 and RPMI8226-HR) and HUVECs. (B) The intensities of cMET and HGF bands in panel A were quantified and normalized to the levels of β-actin (*P < .01 vs RPMI8226, Student t test, n = 3). (C) Schematic of the nonadherent coculture system of MM cells (blue) and HUVECs (green). MM cells and HUVECs were cocultured separately using a Transwell filter (polycarbonate membrane insert, 0.45-µm pores). (D) cMET, p-cMET, and HGF levels in HUVECs were measured by Western blotting after coculture with RPMI8226 or RPMI8226-HR cells under hypoxic conditions (1% O2). (E) The intensities of cMET, p-cMET, and HGF bands in panel D were quantified and normalized to the levels of β-actin (*P < .01; **P < .001 vs control (HUVECs only), Student t test, n = 3).
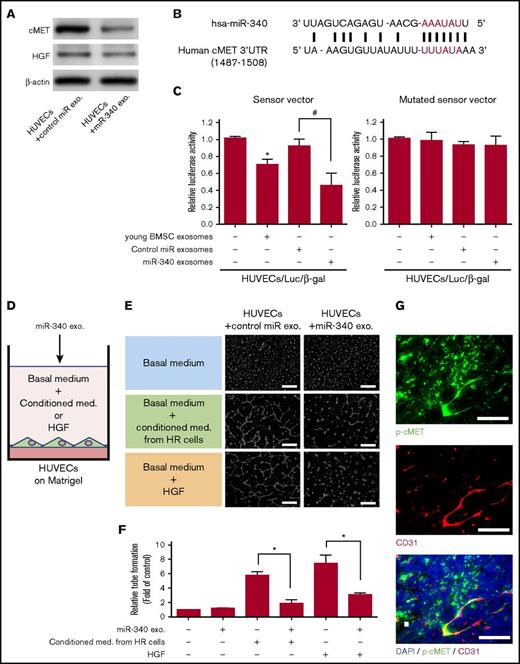
Exosomal miR-340 suppresses tube formation via inhibition of the cMET signaling pathway
To determine whether cMET expression was suppressed by the addition of older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes), we examined cMET protein levels in HUVECs treated with miR-340 exosomes (Figure 6A). cMET protein levels were reduced by approximately 60% in HUVECs treated with miR-340 exosomes in comparison with control miR exosomes (Figure 6A). These results indicated that exogenous miR-340 interacted with cMET for translational suppression of cMET in HUVECs via exosomes, whereas inhibition of cMET expression by miR-340 exosomes did not affect the expression of HGF (Figure 6A). To confirm direct binding between miR-340 and the cMET 3′-untranslated region (UTR), we performed a luciferase reporter assay using a reporter plasmid containing the cMET 3′-UTR with the miR-340-binding site (Figure 6B). A reduction of the luciferase activity from the pLuc-cMET-3′-UTR plasmid was observed in HUVECs treated with miR-340 exosomes (older BMSC exosomes directly transfected with miR-340 mimics; 45% reduction) in comparison with the control (HUVECs only) (Figure 6C).
Regulation of HGF/cMET signaling in endothelial cells by exosomal miR-340 derived from young BMSCs. (A) Representative Western blots of the expression of cMET and HGF in HUVECs after treatment with older BMSC exosomes transfected with miR-340 mimics (miR-340 exo.) or negative control miR (control miR exo.). (B) The hsa-miR-340-binding site spanning nucleotides 1487–1508 (accession no. NM_000245) of the human cMET 3′-UTR was predicted to be evolutionarily conserved. The seed sequence of hsa-miR-340 (AAAUAU) is shown in red. (C) Luciferase reporter assay using a reporter plasmid containing the cMET 3′-UTR with the miR-340-binding site. The luciferase reporter vector to assess miR-340-specific activity contained complementary miR-340 sequences in its 3′-UTR. Normalized firefly luciferase activity was obtained according to firefly luciferase activity/β-gal activity. Sensor vector: the luciferase activity of HUVECs/Luc/β-gal (HUVECs co-transfected with luciferase reporter and β-gal control vectors) treated with young BMSC exosomes was significantly reduced in comparison with control cells (HUVECs only, *P < .01, n = 3). miR-340 exosomes (older BMSC exosomes transfected with miR-340 mimics) also reduced the luciferase activity in HUVECs in comparison with control miR exosomes (older BMSC exosomes transfected with negative control miR) (#P < .01, Student t test, n = 3). Using the mutated sensor vector, there was no difference in luciferase activity with or without exosomes. (D) Schematic of the endothelial cell tube formation assay to confirm the antiangiogenic effect of miR-340 exosomes. HUVECs were cultured on Matrigel in 3 types of medium (basal medium, basal medium supplemented with conditioned medium from RPMI8226-HR cells, or basal medium supplemented with HGF). (E) Endothelial tube formation in response to older BMSC exosomes transfected with miR-340 mimics (miR-340 exo.) observed under a bright field by phase-contrast microscopy. Scale bar, 500 µm. (F) Quantification of tube formation as shown in panel E. The conditioned medium from HR-MM (RPMI8226-HR) cells and HGF induced endothelial tube formation in vitro. Induction of tube formation by conditioned medium of HR-MM cells and HGF was inhibited by older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes) in comparison with the control (older BMSC exosomes transfected with negative control miR; control miR exosomes) (*P < .01, Student t test). Values represent the mean ± SD. (G) Immunohistochemical staining of CD31 (red) and p-cMET (green) in Matrigel plugs seeded with RPMI8226-HR cells at 3 weeks after transplantation into nude mice. Scale bar, 200 µm. med., medium.
Regulation of HGF/cMET signaling in endothelial cells by exosomal miR-340 derived from young BMSCs. (A) Representative Western blots of the expression of cMET and HGF in HUVECs after treatment with older BMSC exosomes transfected with miR-340 mimics (miR-340 exo.) or negative control miR (control miR exo.). (B) The hsa-miR-340-binding site spanning nucleotides 1487–1508 (accession no. NM_000245) of the human cMET 3′-UTR was predicted to be evolutionarily conserved. The seed sequence of hsa-miR-340 (AAAUAU) is shown in red. (C) Luciferase reporter assay using a reporter plasmid containing the cMET 3′-UTR with the miR-340-binding site. The luciferase reporter vector to assess miR-340-specific activity contained complementary miR-340 sequences in its 3′-UTR. Normalized firefly luciferase activity was obtained according to firefly luciferase activity/β-gal activity. Sensor vector: the luciferase activity of HUVECs/Luc/β-gal (HUVECs co-transfected with luciferase reporter and β-gal control vectors) treated with young BMSC exosomes was significantly reduced in comparison with control cells (HUVECs only, *P < .01, n = 3). miR-340 exosomes (older BMSC exosomes transfected with miR-340 mimics) also reduced the luciferase activity in HUVECs in comparison with control miR exosomes (older BMSC exosomes transfected with negative control miR) (#P < .01, Student t test, n = 3). Using the mutated sensor vector, there was no difference in luciferase activity with or without exosomes. (D) Schematic of the endothelial cell tube formation assay to confirm the antiangiogenic effect of miR-340 exosomes. HUVECs were cultured on Matrigel in 3 types of medium (basal medium, basal medium supplemented with conditioned medium from RPMI8226-HR cells, or basal medium supplemented with HGF). (E) Endothelial tube formation in response to older BMSC exosomes transfected with miR-340 mimics (miR-340 exo.) observed under a bright field by phase-contrast microscopy. Scale bar, 500 µm. (F) Quantification of tube formation as shown in panel E. The conditioned medium from HR-MM (RPMI8226-HR) cells and HGF induced endothelial tube formation in vitro. Induction of tube formation by conditioned medium of HR-MM cells and HGF was inhibited by older BMSC exosomes transfected with miR-340 mimics (miR-340 exosomes) in comparison with the control (older BMSC exosomes transfected with negative control miR; control miR exosomes) (*P < .01, Student t test). Values represent the mean ± SD. (G) Immunohistochemical staining of CD31 (red) and p-cMET (green) in Matrigel plugs seeded with RPMI8226-HR cells at 3 weeks after transplantation into nude mice. Scale bar, 200 µm. med., medium.
In the in vitro assay (Figure 6D), endothelial cell tube formation was enhanced by treatment with conditioned medium derived from HR-MM cells or HGF. Such enhanced tube formation was inhibited by the addition of miR-340 exosomes (Figure 6E-F; P < .01). Furthermore, we assessed endothelial cMET activation in vivo in Matrigel plugs by immunohistochemistry. In Matrigel plugs, CD31-positive endothelial cells attracted by HR-MM cells expressed p-cMET (Figure 6G).
Discussion
The advantages of exosome therapy are its stability, biocompatibility, biological barrier permeability, low toxicity, and native mechanism of cell-to-cell transfer. Exosomes derived from BMSCs have low inherent immunogenicity because of composition similar to that of the body’s own cells.30 The use of BMSC exosomes is therefore especially promising for exosome-based cancer therapy. There are 2 purposes in our study. One is to clarify the difference in BMSC and BMSC exosome changes by aging. The other is to find exosomes with therapeutic effects and develop exosome-based therapy.
Target miRNA-overexpressing cells have generally been used as exosome-producing cells to obtain target miRNA-enriched exosomes. However, the expression of exosomal factors other than the target miRNA also changes using this method, because the biology of exosome-producing cells is modified by either transfected miRNA or electroporation.31 To overcome this problem, we adopted the “Exo-fect” system, which enables direct transfection of miRNAs into isolated exosomes. The transfection efficiencies achieved by Exo-fect were approximately 75% (supplemental Figure 6). As a result, we found that only 1 exosomal miRNA of young healthy donors restored the antiangiogenic function of exosomes derived from older healthy donors.
We initially hypothesized that the function of exosomes is highly dependent on the BMSC donor age. We found that young BMSC exosomes and miRNA had significant antiangiogenic effects. In this study, we first investigated the differential gene expression profiles of young BMSCs and older BMSCs (NCBI, gene expression omnibus; GSE78235) and found that 128 genes were upregulated and 117 genes were downregulated in older BMSCs in comparison with young BMSCs (supplemental Figure 7A). By performing gene ontology (GO) term analysis using the Functional Annotation Tool of the Database for Annotation, Visualization, and Integrated Discovery (http://david.ncifcrf.gov), we identified major changes between the functions of young and older BMSCs, such as developmental processes and cell proliferation and adhesion (supplemental Figure 7B). Although we did not identify GO terms related to cellular senescence, we found that older BMSCs displayed downregulation of aldehyde dehydrogenase 1, which is involved in self-renewal and differentiation, indicating that the donor age might affect the differentiation potential of BMSCs. Previous studies on age-related changes in BMSCs clearly show that the age of the BMSC donor directly influences BMSC differentiation, proliferation, and metabolic profiles.32,33 Furthermore, the immunoregulatory potential of young BMSCs is higher than that of older BMSCs.34 Age-related increases in the expression of apoptotic and senescent genes with a concomitant decrease in Sirt1 gene expression also inhibits stem cell function to some degree.35 These findings suggest that the age of BMSC donors should be considered in regard to their therapeutic efficacy. This interference further suggests that the loss of young BMSC exosome-specific miRNAs is related to cancer, because the development of the majority of cancers is considered to be related to age.
In the present study, we extracted several exosomal miRNAs specific to young BMSCs and identified 2 miRNAs (miR-340 and miR-365) that exerted an antiangiogenic effect (Figures 3C and 4D). Our in silico analysis showed that miR-365 targeted interleukin-6 (IL-6), and we conducted a luciferase reporter assay with a reporter vector containing the 3′-UTR of IL-6 with the predicted miR-365 binding site (supplemental Figure 8A). Unexpectedly, overexpression of miR-365 did not decrease the activity of the luciferase reporter (supplemental Figure 8B).
We therefore focused on miR-340, which restored and even increased the antiangiogenic effect of older BMSC exosomes. The first identified target of miR-340 was microphthalmia-associated transcription factor, a master regulator of melanocyte development and melanogenesis in melanoma cells.36 Although several miR-340 target genes have been identified to date, the HGF receptor, cMET, has been reported as a target of miRNA several times.37 It is known that the HGF/cMET interaction plays a key role in the regulation of angiogenesis, and high levels of HGF expression and cMET activation (p-cMET) have been found in BM endothelial cells of MM patients in comparison with normal endothelial cells of healthy donors.38 Consequently, miR-340 might act as a negative regulator of angiogenesis by inhibiting cMET expression. On the basis of a previous report,37 we assessed direct binding between the cMET 3′-UTR and miR-340 by a luciferase reporter assay (Figure 6C). Furthermore, Moschetta et al demonstrated that resistant R5 cells and plasma cells from MM patients at relapse with drug resistance have a high HGF-independent p-cMET content with constitutive activation of the cMET receptor.39 The expression of cMET and p-cMET did not show a difference between parental and HR-MM cells. We also did not observe constitutive activation of cMET in CD138+ cells obtained from MM patients in this study. Unfortunately, using our system, we could not analyze the effect of miR-340 on overcoming drug resistance by inhibition of cMET expression.
One of the reasons why we replenished miR-340 in older BMSC exosomes was to use older BMSC exosomes as an experimental control (miR-340-insufficient exosomes) to demonstrate miR-340 functions. However, the principal reason for modifying exosomes by direct transfection of miRNA was establishment of novel strategies for exosome-based therapy. An important issue that should be addressed is whether miR-340-enriched exosomes have a direct inhibitory effect on tumor growth. Although we cannot completely rule out the possibility of either direct growth inhibition of tumor cells or remaining natural killer cell activity in the nude mouse, miR-340-enriched exosomes did not induce HR cell proliferation in vitro (Figure 2D). We believe the inhibition of tumor angiogenesis by miR-340-enriched exosomes was due to tumor growth suppression in the Matrigel plug, although further studies are required to test this hypothesis.
In conclusion, we provide new information on the age-related changes of BMSC-derived exosomes and the inhibitive effect of exosomal miRNA on tumor angiogenesis. The present study provides evidence of the therapeutic potential of exosomal miRNAs derived from young BMSCs and offers a foundation for the development of novel strategies for exosome-based therapy via replenishment of exosomal miRNAs.
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported in part by the Private University Strategic Research-Based Support Project (S1311016); grants-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15H04303, 15K06841, and 16K07183); and the Tokyo Biochemical Research Foundation.
Authorship
Contribution: T.U., K.O., and J.H.O. designed the study and wrote the manuscript; T.U., S.I., K.A., and C.K. performed experiments and analyzed data; and S.Y. provided patient samples.
Conflict of interest disclosure: K.O. received research support from Celgene KK, Chugai Pharmaceutical KK, and Janssen Pharma KK. The remaining authors declare no competing financial interests.
Correspondence: Tomohiro Umezu, Tokyo Medical University, 6-7-1 Nishi-shinjuku, Shinjuku, Tokyo 160-0023, Japan; e-mail: t_umezu@tokyo-med.ac.jp.