Key Points
In steady state, where no IgGs against RBCs are present, macrophages are the primary phagocytes of RBCs.
In conditions where RBCs are IgG-opsonized, neutrophils can have a major effect on RBC clearance.
Abstract
Red blood cell (RBC) clearance is known to occur primarily in the spleen, and is presumed to be executed by red pulp macrophages. Erythrophagocytosis in the spleen takes place as part of the homeostatic turnover of RBCs to remove old RBCs. It can be strongly promoted by immunoglobulin G (IgG) opsonization of RBCs, a condition that can occur as a consequence of autoantibody or alloantibody formation. The purpose of our study was to investigate which phagocytes are involved in IgG-mediated RBC clearance in the human spleen. We developed a highly specific in vitro assay to monitor RBC phagocytosis in total human splenocytes. Surprisingly, we have found that whereas homeostatic clearance of RBCs is primarily a task for splenic macrophages, neutrophils and, to a lesser extent, also monocytes can be a major factor in clearance of IgG-opsonized RBCs. Erythrophagocytosis by neutrophils is strongly dependent on the degree of opsonization of the RBCs. Additionally, the process is enhanced after blocking the “do not eat me” signal CD47 on the opsonized RBCs, which binds signal regulatory protein α, a myeloid inhibitory receptor that restricts phagocytosis. Moreover, RBCs isolated from autoimmune hemolytic anemia patients, opsonized by auto-IgGs, were shown to be readily phagocytosed by neutrophils. Finally, priming of neutrophils by inflammatory mediators such as tumor necrosis factor α and lipopolysaccharide further increases the magnitude of erythrophagocytosis. Collectively, our data suggest that neutrophils contribute significantly to the phagocytosis of antibody-opsonized RBCs, especially under inflammatory conditions. This indicates a hereto unanticipated contribution of neutrophils in RBC phagocytosis, especially under pathological conditions such as alloimmunization or autoimmunization.
Introduction
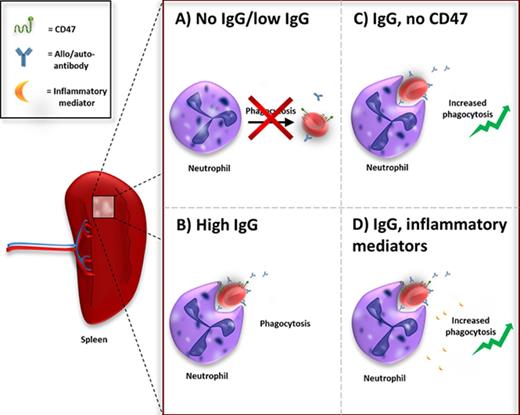
The spleen is the biggest lymphoid organ of the body and acts primarily as a blood filter. It is the main site of red blood cell (RBC) clearance.1 Erythrophagocytosis is important for RBC turnover and is believed to be executed by the red pulp macrophages or Kupffer cells in the liver.1,2 The mechanism of RBC clearance under homeostatic conditions is not fully understood and there is no consensus about the removal signals on the RBCs that trigger phagocytosis by macrophages.2 In general, it is accepted that an accumulation of “eat me” signals triggers phagocytosis of the RBC at the end of its lifespan.2-4 Additionally, CD47 is an important regulator of RBC homeostasis by functioning as a “do not eat me” signal.3-9 CD47 on red cells inhibits phagocytosis of erythrocytes by binding the inhibitory myeloid receptor signal regulatory protein α (SIRPα). CD47-SIRPα interactions negatively control effector functions such as phagocytosis.3,5-9 CD47 acts as a molecular switch for erythrophagocytosis. Through a conformational modification, the protein can change from a “do not eat me” signal to an “eat me” signal.3 In a general sense, the balance between “eat me” and “do not eat me” signals seem to determine whether a RBC is cleared from the bloodstream.
Autoantibody formation against RBCs can occur in conditions such as autoimmune hemolytic anemia (AIHA) or upon alloantibody formation as a result of blood transfusion. Immunoglobulin G (IgG) opsonization of RBCs promotes phagocytosis of the RBCs through Fc-receptor activation on phagocytes.
Studies investigating RBC clearance have mainly focused on the role of macrophages. In this study, we have investigated the role of different populations of human spleen leukocytes in phagocytosis of antibody-opsonized RBCs. Surprisingly, we found that, depending on the conditions, neutrophils have a prominent role in phagocytosis of opsonized RBCs. So far, there have been no studies on erythrophagocytosis by neutrophils in humans. However, there are several case reports of patients with hemolytic conditions such as AIHA in which circulating neutrophils are identified that carry internalized RBCs.10-17 Nevertheless, this phenomenon has remained unexplained, as it seems highly unlikely that neutrophils capture RBCs in the bloodstream where the shear force is markedly high. Furthermore, it is not known whether neutrophils, under these and other conditions, have a substantial contribution to erythrocyte clearance in humans. If so, this would certainly contrast with the current dogma that neutrophils do not play a major role in the clearance of IgG-opsonized RBCs. Here, we demonstrate in vitro and in vivo that neutrophils from the spleen can make a significant contribution to phagocytosis of IgG-opsonized RBCs.
Methods
Antibodies
Information on antibodies used for phagocytosis assays can be found in supplemental Figure 2. The following antibodies were used for opsonization of human RBCs: anti-RhD (human polyclonal antibody, RheDQuin; Sanquin), anti-glycophorin A (anti-GPA) (CD235a, mouse monoclonal IgG1; Sanquin Reagents), isotype control monoclonal mouse IgG1 (Ref: MG100; ThermoFisher), anti-CD47 (mouse monoclonal IgG1, clone B6H12; generously provided by E. Brown, University of California, San Francisco, San Francisco, CA),18 anti-CR1 (rabbit polyclonal antibody; a kind gift from M. R. Daha, Leiden University Medical Center, Leiden, The Netherlands), anti-human CD147 (mouse monoclonal IgG2a, conjugate phycoerythrin, clone 8D12; eBioscience), anti-CD55 (mouse monoclonal IgG1, conjugate fluorescein isothiocyanate [FITC]; Pelicluster), anti-CD59 (mouse monoclonal IgG2a, conjugate FITC; Pelicluster). B6H12 F(ab′)2 fragments were generated by pepsin digestion. Antibody opsonization was measured using goat-anti-human (conjugate Alexa 488; Invitrogen), goat-anti-mouse (conjugate Alexa 488; Invitrogen), or goat-anti-rabbit (conjugate Alexa 488; Invitrogen). Complement deposition was measured using iC3b antibody (Quidel) or C3 antibody (clone anti-C3-19; a kind gift from Diana Wouters, Department of Immunopathology, Sanquin, Amsterdam, The Netherlands). Monocytes or macrophages were identified by anti-CD14 (conjugate FITC; Santa Cruz Biotechnology) and anti-CD163 (conjugate phycoerythrin; Trillium Diagnostics) staining.
Patient material
Blood samples of 6 AIHA patients were provided by the treating physician or by the diagnostic department of Sanquin Amsterdam (excess of blood for diagnostic procedures was used in an anonymous fashion for research in the present study, in accordance with the Dutch law regarding the use of rest material for research purposes). Patients were selected based on the clinical diagnosis and a positive Coombs test for IgG at time of sample collection. Patients were tested negative for IgM and IgA. RBCs of the patients were used in the phagocytosis assay with neutrophils isolated from 2 healthy donors.
Human spleen tissue
Splenocytes were isolated as previously described.19 Splenic material was obtained from organ transplant donors without clinical signs of infection or inflammation. Written informed consent for organ donation was obtained according to national regulations regarding organ donation. Splenic tissue of the donors was collected during transplantation surgery as part of the standard diagnostic procedure for HLA typing. Excess splenic material for diagnostic procedures was used in an anonymous fashion and used for research purposes.
Isolation of RBCs
RBCs were isolated as previously described3 from blood from healthy RhD+ donors, after obtaining informed consent. Studies on human blood samples were approved by the Sanquin Research institutional medical ethical committee, in accordance with the standards laid down in the 1964 Declaration of Helsinki.
Isolation of human neutrophils and monocytes
Neutrophils (polymorphonuclear neutrophils) or monocytes were isolated as previously described.20,21 All blood was obtained after informed consent and according to the 1964 Declaration of Helsinki.
Human spleen phagocytosis assay
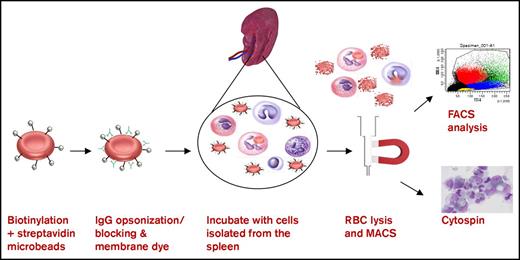
RBCs were biotinylated by incubating cells in 6 μg/mL biotin (Thermo Scientific) at 37°C for 30 minutes and then washed and incubated with streptavidin MicroBeads (Miltenyi Biotec) for 30 minutes at 4°C. RBCs were washed and stained with DiD lipophilic membrane dye (1:100 for 108 RBCs, 20 minutes, 37°C; Life Technologies). The RBCs were incubated for 1 hour at 37°C with cells isolated from the spleen (1:2 ratio, 80 × 106 RBCs + 40 × 106 splenocytes) under various conditions. After incubation of the RBCs with the splenocytes for 1 hour at 37°C in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) medium,20 remaining RBCs were lysed using an isotonic ammonium chloride buffer for 10 minutes at 4°C followed by a second lysis step of 5 minutes at 4°C. After washing, the phagocytotic cells were selected using a LS magnetic-activated cell sorting (MACS) column (Miltenyi Biotec) analyzed by cytospin followed by May-Grünwald Giemsa staining and flow cytometry using Canto II or LSRII + HTS (BD Biosciences) equipped with FACSDiva software (BD Biosciences) (for gating strategy, see supplemental Figure 1).
Human blood neutrophil phagocytosis assay
RBCs were stained with DiD lipophilic membrane dye (Life Technologies). Subsequently, RBCs were incubated with neutrophils isolated from blood for 1 hour at 37°C (10:1 ratio, 5 × 106 RBCs + 0.5 × 106 neutrophils). Next, RBCs were lysed using an isotonic ammonium chloride buffer for 10 minutes at 4°C followed by a second lysis step of 5 minutes at 4°C. Cells were washed and resuspended in stop buffer containing 0.5% (wt/vol) paraformaldehyde, 1% (wt/vol) bovine serum albumin, and 20 mM NaF in phosphate-buffered saline. Phagocytosis was measured using flow cytometry.
Mice
Male and female CD47-deficient (CD47KO) mice on the Balb/c background,22 SIRPα-mutant mice (lacking most of the SIRPα cytoplasmic domain) on the C57BL/6 background,23,24 and their respective homozygous wild-type (Wt) littermates were used. Mice were kept in accordance with local guidelines, maintained in a specific pathogen-free barrier facility, and experiments were performed in compliance with relevant Swedish and institutional laws and guidelines and approved by the Umeå research animal ethics committee (A14-12).
Phagocytosis experiments in mice in vivo
RBCs were isolated from the blood of Wt or CD47KO mice, labeled with PKH26, and IgG-opsonized with the anti-murine RBC monoclonal antibody (mAb) 34-3C (mouse IgG2a; kindly provided by Shozo Izui, University of Geneva, Geneva, Switzerland) at 1 µg/1 × 108 RBCs, as previously described.8 After washing, 150 µL of RBCs at 10% hematocrit was injected IV into a lateral tail vein of anesthetized mice (isofluorane). At 90 minutes, mice were euthanized, blood was harvested by heart puncture into heparin (5000 IU/mL), and spleens were removed and kept on ice. Whole blood was incubated with Fc block (mAb 2.4G2), followed by addition of allophycocyanin-conjugated anti-mouse Gr-1 and FITC-conjugated anti-mouse CD11b at room temperature for 15 minutes. After washing and RBC lysis, samples were analyzed by flow cytometry (FACSCalibur; BD Biosciences) to quantify the fraction of PKH26+Gr-1hiCD11b+ neutrophils. Single-cell suspensions were prepared from spleens by mechanical disruption, as previously described.25 Following incubation in Fc block, splenocytes were labeled with mAbs against Gr-1 and CD11b followed by fluorescence-activated cell sorter (FACS) analysis of the Gr-1hiCD11b+ neutrophil population, as described for neutrophils from whole blood.
Phagocytosis experiments in mice in vitro
Bone marrow neutrophils were isolated from femurs of 2- to 4-month-old mice, using a Percoll gradient as previously described,26 and were resuspended at 5 × 106/mL in cold Hank balanced salt solution (HBSS) containing Ca2+ and Mg2+ (HBSS++). RBCs were isolated from the blood of Wt or CD47KO mice and opsonized with 5 µg of mAb 34-3C/1 × 108 RBC as described in the previous section. Neutrophils were incubated with IgG-opsonized RBCs at a 1:20 ratio for 60 minutes at 37°C. Following lysis of noningested RBCs using ice-cold sterile H2O, cells were resuspended in phosphate-buffered saline/1% human serum albumin (Octapharma) and cytospun onto glass slides at 200g for 5 minutes. The slides were stained with May-Grünwald Giemsa and mounted using DPX (BDH Prolabo). A phagocytosis index was calculated by counting ingested RBCs in 200 to 300 neutrophils per slide, and expressed as number of RBCs per 100 neutrophils.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.02. For comparison of phagocytosis levels, the 1-way analysis of variance test was used followed by the Sidak post hoc test for correction of multiple comparison (****P < .0001; ***P < .001; **P < .01; *P < .05).
Results
Unopsonized RBCs are primarily phagocytosed by macrophages
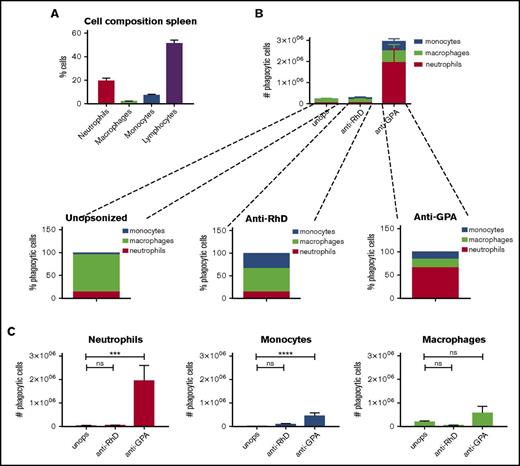
We wanted to identify, in an unbiased fashion, the relevant phagocytic cells in the human spleen that engulf IgG-opsonized RBCs. To examine this, we developed an in vitro assay to monitor RBC phagocytosis in total human splenocytes (Figure 1). Instead of isolating specific subsets of phagocytic cells, such as the red pulp macrophages, we used all nucleated cells from the spleen. As a result, the different subsets of splenocytes are present in their different natural ratios in our phagocytosis assay. Approximately 2% of the cells isolated from human spleen (n = 9) are macrophages (high autofluorescence), 7% are monocytes (side scatter low [SSClo]–forward scatter high [FSChi]), 19% are neutrophils (SSChi-FSCint), and 51% are lymphocytes (SSClo-FSClo) (supplemental Figure 1; Figure 2A).
Magnetic sorting of RBC-phagocytosing cells from the human spleen. The following assay was used to investigate erythrophagocytosis by cells of the human spleen in vitro. RBCs were biotinylated and coupled to magnetic beads. RBCs were opsonized and stained with the membrane dye DiD. Cells of the spleen were isolated and incubated with the RBCs for 1 hour. RBCs were lysed and the remaining spleen cells were put over a MACS column. Cells elutriated from the column were analyzed by flow cytometry and cytospin (original magnification ×50; May-Grünwald Giemsa stain).
Magnetic sorting of RBC-phagocytosing cells from the human spleen. The following assay was used to investigate erythrophagocytosis by cells of the human spleen in vitro. RBCs were biotinylated and coupled to magnetic beads. RBCs were opsonized and stained with the membrane dye DiD. Cells of the spleen were isolated and incubated with the RBCs for 1 hour. RBCs were lysed and the remaining spleen cells were put over a MACS column. Cells elutriated from the column were analyzed by flow cytometry and cytospin (original magnification ×50; May-Grünwald Giemsa stain).
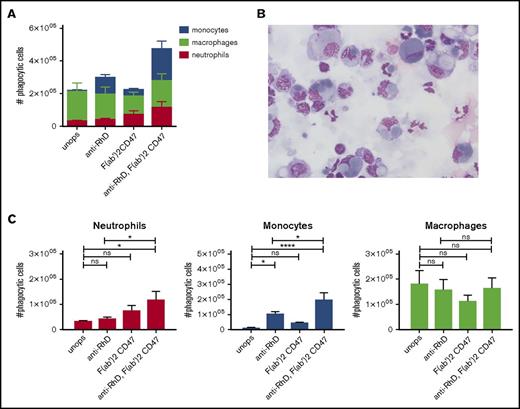
IgG opsonization of RBCs induces phagocytosis by neutrophils and monocytes. (A) Percentages of cell types in the spleen (n = 9). (B) RBCs were unopsonized (unops), anti-RhD-opsonized, or anti-GPA-opsonized. Splenocytes and RBCs were incubated for an hour and the phagocytic fraction was obtained using MACS. The absolute numbers of phagocytes are shown. Anti-GPA opsonization causes a drastic increase of phagocytosis. (C) The absolute number of splenocytes that take up RBCs per cell type. Neutrophils are the primary phagocytes in case of anti-GPA opsonization (n = 5-12). ns, nonsignificant difference.
IgG opsonization of RBCs induces phagocytosis by neutrophils and monocytes. (A) Percentages of cell types in the spleen (n = 9). (B) RBCs were unopsonized (unops), anti-RhD-opsonized, or anti-GPA-opsonized. Splenocytes and RBCs were incubated for an hour and the phagocytic fraction was obtained using MACS. The absolute numbers of phagocytes are shown. Anti-GPA opsonization causes a drastic increase of phagocytosis. (C) The absolute number of splenocytes that take up RBCs per cell type. Neutrophils are the primary phagocytes in case of anti-GPA opsonization (n = 5-12). ns, nonsignificant difference.
In our phagocytosis assay, we evaluated the heterogeneous splenocyte population for their phagocytic capacity with magnetic RBCs. After incubation, RBCs were lysed and the remaining cells were selected by magnetic sorting and analyzed (Figure 1). This allowed us to extract the phagocytes that had taken up a magnetic RBC. First, we determined phagocytosis of unopsonized RBCs representative for homeostatic erythrophagocytosis (Figure 2B-C; supplemental Figure 3). Using unopsonized RBCs as targets, analysis by flow cytometry showed that the phagocytes isolated in this manner contained 81.6% macrophages, 14.3% neutrophils, and 4% monocytes, consistent with the idea that splenic macrophages represent the primary phagocytes for homeostatic turnover of RBCs in vivo.1,2
RBC opsonization leads to phagocytosis by spleen neutrophils and monocytes
We next determined the effect of IgG opsonization of RBCs on phagocytosis. Using anti-RhD, which causes a low level of opsonization (Figure 3A), there was a slight but significant increase of phagocytosis by monocytes (Figure 2B-C; supplemental Figure 3). Compared with the unopsonized condition there was no substantial increase in phagocytosis in the total number of phagocytes. Of note, there was a shift toward the monocytes within the phagocytic fraction, now representing 34% of the phagocytic fraction, which occurred at the expense of the phagocytosis by macrophages, suggesting that anti-RhD opsonization selectively promoted monocyte phagocytosis (Figure 2B). However, using anti-GPA, which leads to a very high level of opsonization (Figure 3A), we observed a significant increase in the total number of RBCs that were phagocytosed. In absolute terms, the phagocytosis by neutrophils was most notably enhanced (up to 65.7% of the phagocytic cells) and monocyte phagocytosis was too, albeit to a lesser extent, by opsonization with the anti-GPA antibody. Thus, opsonization of RBCs leads to a gradual recruitment of first monocytes (at low opsonizing levels) and later neutrophils (at higher levels) to the pool of spleen erythrophagocytic cells (supplemental Figure 4A). This indicates that there are different thresholds for erythrophagocytosis among the different phagocytes, and that antibody opsonization is a particularly powerful trigger for lowering the threshold in neutrophils.
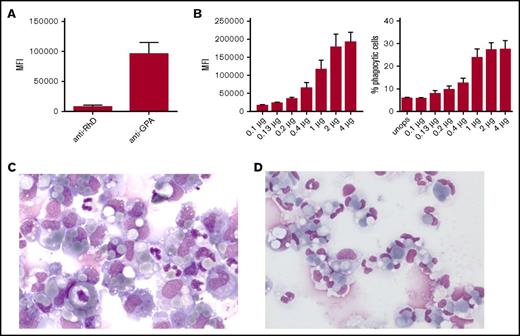
Erythrophagocytosis by neutrophils is strongly dependent on the degree of opsonization of the RBCs. (A) Anti-GPA shows a much higher mean fluorescence intensity (MFI) compared with anti-D after secondary antibody staining (n = 3). (B) Phagocytosis by neutrophils depends on the degree of opsonization; extent of phagocytosis explained as MFI of anti-GPA-opsonized RBCs (left) or the percentage of phagocytic neutrophils (right) (n = 10). Error bars denote the standard error of the mean. (C-D) Cytospins of the phagocytic fraction using (C) anti-RhD-opsonized RBCs (original magnification ×50; May-Grünwald Giemsa stain) and (D) anti-GPA-opsonized RBCs (original magnification ×50; May-Grünwald Giemsa stain). These images were blindly chosen and are representative for 5 different experiments.
Erythrophagocytosis by neutrophils is strongly dependent on the degree of opsonization of the RBCs. (A) Anti-GPA shows a much higher mean fluorescence intensity (MFI) compared with anti-D after secondary antibody staining (n = 3). (B) Phagocytosis by neutrophils depends on the degree of opsonization; extent of phagocytosis explained as MFI of anti-GPA-opsonized RBCs (left) or the percentage of phagocytic neutrophils (right) (n = 10). Error bars denote the standard error of the mean. (C-D) Cytospins of the phagocytic fraction using (C) anti-RhD-opsonized RBCs (original magnification ×50; May-Grünwald Giemsa stain) and (D) anti-GPA-opsonized RBCs (original magnification ×50; May-Grünwald Giemsa stain). These images were blindly chosen and are representative for 5 different experiments.
Phagocytosis of IgG-opsonized RBCs by neutrophils is dependent on the degree of opsonization
Because neutrophils are the most abundant phagocyte in the spleen, their contribution to RBC phagocytosis can be quite substantial once they participate in the process. Therefore, we subsequently explored factors that are of potential influence on RBC phagocytosis by neutrophils. First, we investigated how the degree of opsonization influences RBC phagocytosis by neutrophils. Therefore, we opsonized RBCs with increasing amounts of anti-GPA and determined the effect on phagocytosis by neutrophils from blood (Figure 3B-C). The results indicate that with increasing amounts of antibody deposited on the RBC, phagocytosis by neutrophils increases as well. An isotype control did not induce phagocytosis (supplemental Figure 4B). The effect of antibody opsonization was not restricted to anti-GPA antibodies. Using antibodies directed against various other RBC antigens, phagocytosis by neutrophils was found to correlate with the degree of antibody binding (supplemental Figure 5A-B). We also determined the effect of different IgG subclasses on RBC phagocytosis by neutrophils. Using monoclonal IgG1, IgG2, or IgG3 anti-RhD for RBC opsonization, we found that the IgG subclass of anti-RhD does not influence RBC phagocytosis (supplemental Figure 5C). Our anti-GPA is from murine origin so we next investigated whether other mouse monoclonal IgG1 antibodies could be as potent in inducing phagocytosis of RBCs by neutrophils. Phagocytosis of RBCs opsonized with anti-CD44 or anti-CD55 showed a far lower level of phagocytosis compared with RBCs opsonized with anti-GPA (supplemental Figure 5D). This coincided with the lower level of opsonization of these 2 antibodies (supplemental Figure 5E) which suggests that not antibody origin but rather the level of opsonization is the determining factor for RBC phagocytosis by neutrophils.
Blocking of CD47 increases erythrophagocytosis by neutrophils and monocytes
Antibody-dependent phagocytosis of RBCs is mediated through Fc-receptor signaling. To counterbalance prophagocytic signals, RBCs display the “do not eat me” signal CD47 on their cell membrane. Interaction of CD47 with the inhibitory myeloid receptor SIRPα inhibits phagocytosis.5,6,27 Using F(ab′)2 blocking antibodies against CD47, we determined the role of CD47 in IgG-mediated phagocytosis of RBCs in the spleen. For these studies, we used low opsonizing anti-RhD that by itself does not induce erythrophagocytosis. The data show that merely blocking CD47 is not enough to induce RBC phagocytosis by neutrophils. However, the combination of anti-RhD opsonization and blocking of CD47 enhances phagocytosis by neutrophils (Figure 4A,C). With a conventional phagocytosis assay using stained RBCs instead of magnetic RBCs the same results were obtained (supplemental Figure 6A). Moreover, CD47 blocking was able to further augment phagocytosis of anti-GPA-opsonized RBCs (supplemental Figure 6B). In conclusion, CD47 blocking is able to lower the threshold for neutrophil phagocytosis, which enables them to phagocytose RBCs opsonized with low levels of IgG. Thus, IgG opsonization and CD47 are major counterbalancing determinants for phagocytosis and, by lowering the threshold for phagocytosis through CD47 blocking, neutrophils can also be engaged in erythrophagocytosis.
Erythrophagocytosis is greatly enhanced after blocking the “do not eat me” signal CD47 on the opsonized RBCs in vitro. (A) CD47-SIRPα interaction was blocked on unopsonized and IgG-coated RBCs with F(ab′)2 CD47. Splenocytes and RBCs were incubated for an hour and the phagocytic fraction was obtained using MACS. Absolute amounts of phagocytic cells are shown (n = 7-10). (B) Cytospins of the phagocytic fraction using anti-D-opsonized RBCs with CD47 blocking. These images where blindly chosen and are representative for 5 different experiments (original magnification ×50; May-Grünwald Giemsa stain). (C) F(ab′)2 fragments of anti-CD47 do not significantly increase RBC phagocytosis by neutrophils. However, F(ab′)2 CD47 in combination with anti-RhD opsonization does significantly increase RBC phagocytosis (n = 7-10). Error bars denote the standard error of the mean. Asterisks represent highly significant differences (*P < .05, ****P < .0001).
Erythrophagocytosis is greatly enhanced after blocking the “do not eat me” signal CD47 on the opsonized RBCs in vitro. (A) CD47-SIRPα interaction was blocked on unopsonized and IgG-coated RBCs with F(ab′)2 CD47. Splenocytes and RBCs were incubated for an hour and the phagocytic fraction was obtained using MACS. Absolute amounts of phagocytic cells are shown (n = 7-10). (B) Cytospins of the phagocytic fraction using anti-D-opsonized RBCs with CD47 blocking. These images where blindly chosen and are representative for 5 different experiments (original magnification ×50; May-Grünwald Giemsa stain). (C) F(ab′)2 fragments of anti-CD47 do not significantly increase RBC phagocytosis by neutrophils. However, F(ab′)2 CD47 in combination with anti-RhD opsonization does significantly increase RBC phagocytosis (n = 7-10). Error bars denote the standard error of the mean. Asterisks represent highly significant differences (*P < .05, ****P < .0001).
Neutrophils of the blood and spleen phagocytose IgG-opsonized Wt or CD47-deficient RBCs in vivo
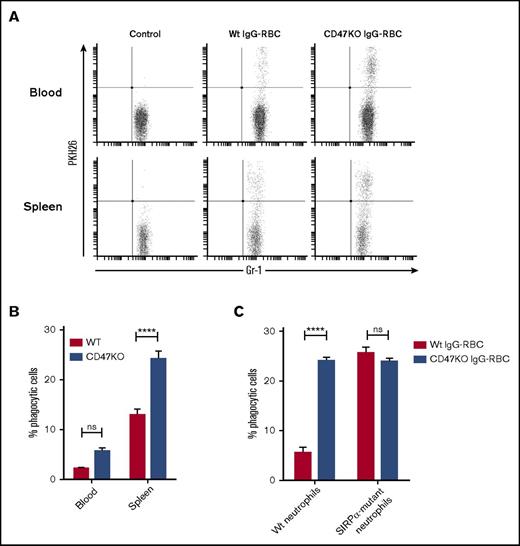
Our in vitro experiments indicate an important role for human neutrophils in IgG-mediated RBC clearance in the spleen. To establish the potential of neutrophils to phagocytose IgG-opsonized RBCs in vivo, we used a murine model. For this, labeled IgG-opsonized RBCs were injected IV into Wt Balb/c mice and neutrophils in blood and spleens were analyzed for RBC uptake at 90 minutes after injection, using flow cytometry (Figure 5A-B; supplemental Figure 7). Because murine neutrophils were found to phagocytose IgG2a-opsonized, but not IgG1-opsonized, RBCs in vitro (data not shown), an IgG2a antibody was used to opsonize RBCs in the present study. Our results showed that IgG-opsonized RBCs were taken up by neutrophils in the spleen (supplemental Figure 7B) and were also found, albeit to a much lesser extent, in neutrophils in the blood. Additionally, erythrophagocytosis was substantially enhanced when RBCs lacked CD47 (Figure 5A-B). Collectively, these data show that neutrophils can significantly contribute to erythrophagocytosis, also in vivo, and thus support our in vitro findings with human splenocytes. To further pinpoint whether the ability of CD47 to regulate neutrophil phagocytosis required SIRPα signaling in neutrophils, we investigated in vitro phagocytosis of IgG-opsonized RBCs by neutrophils from Wt or SIRPα-mutant mice. In SIRPα-mutant mice, the extracellular SIRPα-domain is expressed whereas the cytoplasmic signaling domain is deleted.23,24 Although phagocytosis of CD47KO RBCs by Wt neutrophils was about 4 times more efficient than that for equally opsonized Wt RBCs, CD47KO and Wt RBCs were phagocytosed equally by SIRPα-mutant neutrophils (Figure 5C). These data therefore show that the phagocytosis-inhibitory function of CD47 on RBCs requires SIRPα signaling in neutrophils.
The CD47-SIRPα interaction regulates neutrophil phagocytosis of syngeneic RBCs in vivo. (A-B) Neutrophil uptake of IgG-opsonized Wt or CD47-deficient RBCs in vivo. PKH26-labeled IgG-opsonized RBCs were injected IV into Wt recipient mice and neutrophils in blood or spleen were analyzed for RBC uptake at 90 minutes after injection using flow cytometry (n = 4-5). (C) Phagocytosis of equally IgG-opsonized CD47 Wt or CD47-deficient (CD47KO) RBCs by Wt or SIRPα mutant bone marrow neutrophils in vitro in the presence of 1 µM fMLF. Error bars denote the standard error of the mean. Asterisks represent highly significant differences (****P < .0001).
The CD47-SIRPα interaction regulates neutrophil phagocytosis of syngeneic RBCs in vivo. (A-B) Neutrophil uptake of IgG-opsonized Wt or CD47-deficient RBCs in vivo. PKH26-labeled IgG-opsonized RBCs were injected IV into Wt recipient mice and neutrophils in blood or spleen were analyzed for RBC uptake at 90 minutes after injection using flow cytometry (n = 4-5). (C) Phagocytosis of equally IgG-opsonized CD47 Wt or CD47-deficient (CD47KO) RBCs by Wt or SIRPα mutant bone marrow neutrophils in vitro in the presence of 1 µM fMLF. Error bars denote the standard error of the mean. Asterisks represent highly significant differences (****P < .0001).
RBCs of AIHA patients are more readily phagocytized by neutrophils compared with RBCs of healthy individuals
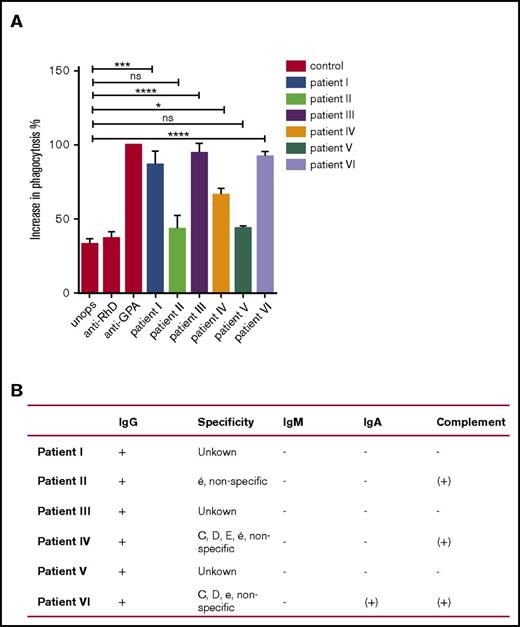
To further address whether neutrophils may play a role in erythrophagocytosis in AIHA, we tested whether RBCs of AIHA patients (n = 6) could be phagocytosed by neutrophils (Figure 6). To compare different patients tested on different occasions, we used RBCs of healthy controls opsonized with anti-RhD or anti-GPA as a reference for IgG-induced phagocytosis, and normalized the data of each patient to the phagocytic response seen with anti-GPA on the same day. All patients showed a positive Coombs test (direct antiglobulin test) for IgG, but with varying levels of total IgG observed by flow cytometry (supplemental Figure 8A). Furthermore, the IgGs were shown to be of different specificities (supplemental Figure 8D). Nevertheless, in 4 of 6 patients, the overall opsonization appeared sufficient to induce neutrophil erythrophagocytosis, and in 3 of 6 patients the level of phagocytosis was close to that seen with anti-GPA-opsonized healthy control RBCs (Figure 6). This was striking because the level of opsonization with anti-GPA appeared substantially higher (Figure 3A) than that with the endogenous autoantibodies of AIHA patients. As expected, based on the data in the previous 2 sections, blocking of CD47 could further enhance neutrophil phagocytosis of AIHA RBCs, but not of RBCs from a healthy control in 2 of 3 cases (supplemental Figure 8E). Taken together, these data show that the prophagocytic signals are certainly sufficient, in the majority of AIHA patients tested here, to induce neutrophils to phagocytose RBCs from AIHA patients.
RBCs of AIHA patients show increased phagocytosis compared with RBCs from healthy individuals. (A) RBCs of an AIHA patient with auto-IgG antibodies and RBCs of a healthy control were labeled with DiD and incubated with neutrophils of 2 to 3 healthy donors. Erythrophagocytosis of patient I, III, and VI RBCs was comparable to erythrophagocytosis of anti-GPA-opsonized RBCs from a healthy control. Phagocytosis of RBCs of patient II and V by neutrophils is not significantly increased compared with RBCs of a healthy control. Phagocytosis of RBCs of patient IV by neutrophils is increased compared with RBCs of a healthy control. Error bars denote the standard error of the mean. Asterisks represent highly significant differences (*P < .05, ***P < .001, ****P < .0001). (B) Results of the Coombs test (direct antiglobulin test). All patients were tested positive for IgG and negative for IgM and IgA in the Coombs test performed by diagnostics. Patient II and IV showed low positivity for complement and remaining patients were tested negative for complement (+, positive; −, negative; (+), low positivity). Error bars denote the standard error of the mean.
RBCs of AIHA patients show increased phagocytosis compared with RBCs from healthy individuals. (A) RBCs of an AIHA patient with auto-IgG antibodies and RBCs of a healthy control were labeled with DiD and incubated with neutrophils of 2 to 3 healthy donors. Erythrophagocytosis of patient I, III, and VI RBCs was comparable to erythrophagocytosis of anti-GPA-opsonized RBCs from a healthy control. Phagocytosis of RBCs of patient II and V by neutrophils is not significantly increased compared with RBCs of a healthy control. Phagocytosis of RBCs of patient IV by neutrophils is increased compared with RBCs of a healthy control. Error bars denote the standard error of the mean. Asterisks represent highly significant differences (*P < .05, ***P < .001, ****P < .0001). (B) Results of the Coombs test (direct antiglobulin test). All patients were tested positive for IgG and negative for IgM and IgA in the Coombs test performed by diagnostics. Patient II and IV showed low positivity for complement and remaining patients were tested negative for complement (+, positive; −, negative; (+), low positivity). Error bars denote the standard error of the mean.
Neutrophil priming enhances erythrophagocytosis
In 20% to 80% of the cases, AIHA is secondary to an underlying illness such as an infection, a lymphoproliferative disorder, or an autoimmune disease.28,29 In idiopathic as well as in secondary AIHA, the production of proinflammatory cytokines has been reported to be increased.30-33 We hypothesized that the immunological status of the patient can be of influence on the degree of erythrophagocytosis by neutrophils. For this reason, we investigated the effect of neutrophil priming on erythrophagocytosis. Neutrophil priming prior to incubation with unopsonized RBCs or anti-RhD-opsonized RBCs did not have any effect on phagocytosis (Figure 7A-B). Yet, depending on the stimulus, phagocytosis of anti-GPA-opsonized RBCs was significantly increased after stimulating neutrophils (Figure 7C). FSL-1 (ligand for Toll-like receptor 2 [TLR2] and TLR6), tumor necrosis factor α (TNFα; TNFR1 agonist), and lipopolysaccharide (LPS; TLR4 agonist) all induce phagocytosis of IgG-opsonized RBCs by neutrophils (Figure 7A). Priming with C5a (C5aR agonist), interferon γ, or interleukin-8 (IL-8) did not increase phagocytosis. Neutrophil priming using LPS in combination with CD47 blocking resulted in augmented phagocytosis of IgG-opsonized RBCs by neutrophils (Figure 7D). Although Bian et al showed this same effect in vivo in mice for multiple stimuli,34 in our assay other stimuli did not have this effect. Overall, our data demonstrate that various inflammatory mediators can lower the threshold for phagocytosis of IgG-opsonized RBCs by neutrophils.
Neutrophil priming enhances erythrophagocytosis. (A-C) Stimulation of human neutrophils isolated from the blood. Stimuli can enhance phagocytosis of anti-GPA-opsonized RBCs but not of unopsonized RBCs or anti-RhD-opsonized RBCs. (D) CD47 blocking combined with neutrophil priming using LPS enhances phagocytosis by neutrophils. Other stimuli do not have this effect. Error bars denote the standard error of the mean. Asterisks represent highly significant differences (****P < .0001). INF, interferon.
Neutrophil priming enhances erythrophagocytosis. (A-C) Stimulation of human neutrophils isolated from the blood. Stimuli can enhance phagocytosis of anti-GPA-opsonized RBCs but not of unopsonized RBCs or anti-RhD-opsonized RBCs. (D) CD47 blocking combined with neutrophil priming using LPS enhances phagocytosis by neutrophils. Other stimuli do not have this effect. Error bars denote the standard error of the mean. Asterisks represent highly significant differences (****P < .0001). INF, interferon.
Discussion
In this study, we took an unbiased approach to investigate phagocytosis of IgG-opsonized RBCs in the human spleen. The aim of the study was to examine the role of different phagocytes in the process of RBC clearance in the presence of antibodies. The spleen is the primary site for IgG-mediated RBC clearance whereas IgM-associated extravascular hemolysis occurs predominantly in the liver.33 For our study, the spleen is therefore the organ of interest.
Using magnetic RBCs, we have developed a unique assay to investigate the role of different cell types in RBC phagocytosis in the human spleen (Figure 1). Previous methods for studying erythrophagocytosis have difficulties differentiating between RBC uptake, RBC adherence, and trogocytosis. By lysing remaining RBCs after incubating RBCs with splenocytes and subsequently selecting for magnetic splenocytes, we efficiently obtain the cells that have taken up RBCs. In line with the current literature, we found that under steady-state conditions (in the absence of anti-RBC antibodies) macrophages are responsible for RBC clearance.2,35 For IgG-opsonized RBCs, as in AIHA, the mechanism of RBC clearance appears different. Namely, neutrophils, and to a lesser extent monocytes, can contribute significantly to IgG-opsonized RBC clearance not only in our in vitro assay, but also in a murine model of AIHA. These data fit with the current opinion that tissue-resident macrophages, such as the red pulp macrophages, have a homeostatic function in clearance whereas infiltrating myeloid cells take over this function under pathological conditions.36-38
The threshold for neutrophils to phagocytose RBCs seems to be much higher than that of macrophages (whereas monocytes have an intermediate one). Different factors were shown to determine whether the threshold to phagocytose IgG-opsonized RBCs by neutrophils is reached. First, erythrophagocytosis by neutrophils was found to be strongly dependent on the degree of opsonization of the RBCs. Second, activation of the neutrophils, which can be increased by different stimuli, such as ligands for TLRs (FSL-1 and LPS) and TNFα, increases phagocytosis of IgG-opsonized RBCs. Lastly, RBC phagocytosis by neutrophils is under negative control by the inhibitory CD47-SIRPα pathway.
Our data indicate that once the threshold for RBC phagocytosis by neutrophils has been reached, neutrophils may become an important factor in the process of RBC clearance. To investigate the possibility that neutrophils participate in the clearance of RBCs in AIHA, we studied phagocytosis by neutrophils using RBCs from AIHA patients. RBCs from AIHA patients were more readily phagocytosed than RBCs of healthy controls. This is despite the fact that in vivo IgG opsonization of patient RBCs is always lower than that of in vitro–opsonized RBCs. An explanation for this may be that RBCs of AIHA can have additional “eat me” signals besides the IgG opsonization that enhances phagocytosis by the neutrophils, such as complement. Even though the Coombs tests for complement deposition were negative or low in all cases, in some patients, C3 fragments were detectable by flow cytometry (supplemental Figure 8B). It could also be that the specificity of the antibody has an effect on the type of immune activation. Anti-GPA is highly potent in inducing phagocytosis because GPA is the most abundant protein of the cell surface of the RBC membrane. Besides the abundance of the epitope, the density may also affect phagocytosis. Additionally, the glycosylation status of the antibody may also be of influence. Low IgG-Fc fucosylation has been shown to enhance platelet phagocytosis by neutrophils and monocytes via increased binding to FcγRIIIa/b.39 Moreover, low IgG fucosylation has been found to correlate with hemoglobin levels and disease severity in hemolytic disease of the fetus and newborn.40,41
This project has focused on FcγR-mediated phagocytosis, yet in vivo antibody-mediated RBC clearance can also be promoted by complement, which induces phagocytosis via complement receptors.42 Both Fcγ- and complement receptor–mediated phagocytosis are regulated by CD47-SIRPα interactions.6 It would be interesting to investigate the role of neutrophils in complement receptor–mediated RBC clearance.
In vitro, RBC phagocytosis by neutrophils can further be potentiated by inflammatory mediators, indicating that in AIHA the inflammatory status of the patient may also contribute to the severity of RBC clearance and hence anemia. Indeed, there is a large body of evidence that AIHA can be induced by an underlying disease in the patient which may lower the threshold of the neutrophils to phagocytose IgG-opsonized RBCs, and thus exacerbate the hemolytic anemia. It is important to keep in mind that during these pathological conditions the cellular composition of the spleen can change43,44 which subsequently may influence phagocytosis. Additionally, IV immunoglobulin administration, a common therapy in AIHA can change cellular distribution in the spleen which may affect erythrophagocytosis.45
Overall, our data show that neutrophils residing in the spleen can contribute significantly to the destruction of IgG-opsonized RBCs. We have identified IgG levels on the RBC as well as the presence of inflammatory mediators to be important factors to drive phagocytosis of IgG-opsonized RBC by neutrophils. Therefore, in a clinical setting where antibody opsonization of RBCs and inflammatory mediators are present, neutrophils may strongly contribute to RBC clearance. Due to their abundance, the impact of erythrophagocytosis by neutrophils may be of great significance in conditions such as AIHA or blood transfusion reactions.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Swi Fan Cheung (Sanquin, Amsterdam, The Netherlands) for providing tissue samples, Conny Brouwer (Sanquin, Amsterdam, The Netherlands) for collecting patient material, and Peter Ligthart (Sanquin, Amsterdam, The Netherlands) for providing human monoclonal anti-RhD antibodies.
S.M.M. was supported by a grant from the Dutch Ministry of Health awarded to T.K.v.d.B. P.-A.O. was supported by the Swedish Research Council (2012-2702) and the Faculty of Medicine Foundations at Umeå University.
Authorship
Contribution: S.M.M. performed the experiments, analyzed the data, and wrote the manuscript; B.M.B. and T.R.L.K. performed additional experiments; P.-A.O. and J.J. performed the animal experiments, analyzed the data, and wrote the manuscript; T.M. provided the SIRPα mutant mice; T.W.K., E.J.H., and M.d.H. enrolled patients; T.K.v.d.B., R.v.B., and S.M.M. designed the study; T.K.v.d.B. and R.v.B. supervised the study, edited the manuscript, and served as principal investigators; and all authors revised and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robin van Bruggen, Department of Blood Cell Research, Sanquin Research and Landsteiner Laboratory, Academic Medical Center, University of Amsterdam, Plesmanlaan 125, 1066CX Amsterdam, The Netherlands; e-mail: r.vanbruggen@sanquin.nl.
References
Author notes
T.K.v.d.B. and R.v.B. contributed equally to this study.