Key Points
The proportion of PCNSL patients receiving chemotherapy increased, but remains 31% lower in community than in academic cancer programs.
Estimated overall survival in PCNSL is 37.7% at 3 years and reaches 51.8% in the subgroup treated with multiagent chemotherapy.
Abstract
Although the role of radiation therapy and chemotherapy in primary central nervous system lymphoma (PCNSL) has evolved considerably over the past decade, the application of treatment modalities in the community has not been evaluated. We analyzed the use of chemotherapy, radiation therapy, and associated overall survival, among 9165 HIV-negative PCSNL cases reported to the US National Cancer Database in 2004-2013. During this time, the proportion of patients receiving chemotherapy significantly increased from 65.6% to 78.8% (P for trend <.0001), whereas the proportion receiving radiation therapy decreased from 37.6% to 18.8% (P < .0001). Adjusting for the varying distribution of clinical and sociodemographic characteristics by type of treating facility, the risk of not receiving chemotherapy was significantly lower in academic/research cancer programs compared with community programs (adjusted relative risk, 0.69; 95% confidence interval [CI], 0.62-0.76; P < .0001). Furthermore, omission of chemotherapy was associated with increasing age, comorbidities, black race, and indicators of poor socioeconomic status. Overall survival at 3 years was 37.7% (95% CI, 36.6-38.8) and ranged from 14.1% for patients treated with radiation therapy alone to 51.8% for those who received multiagent chemotherapy. There was evidence of improved survival over time (P for trend =.0002). The disparities in application of chemotherapy for PCNSL underscore the need to provide access to expert management for this rare disease and improve safe delivery of systemic treatment in the community setting, where most older patients receive their care.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare, clinically and biologically distinct subtype of non-Hodgkin lymphoma, with a high incidence among immune-compromised patients, including those with advanced HIV infection.1,2 Over 90% of cases are classified as diffuse large B-cell lymphoma (DLBCL), and the stereotactic brain biopsy is the method of choice to confirm the diagnosis. Management of PCNSL has undergone a major evolution over the past 15 years. Historically, whole brain radiation therapy (WBRT) was the mainstay of treatment, associated with high response rate, but significant toxicity and little to no curative potential.3,4 Since the early 2000s, high-dose methotrexate (HDMTX)-based chemotherapy, with or without radiation, has demonstrated high efficacy and established HDMTX as a standard therapeutic approach in PCNSL, endorsed by multiple guidelines.5-7 However, neurotoxicity of multimodality treatment with chemotherapy and WBRT remained a major concern, especially among patients over the age of 60, who constitute a majority of cases.8 More recently, randomized and single-arm clinical trials provided supportive evidence for using upfront chemotherapy without radiation, and for combining HDMTX with other systemic agents that cross the blood-brain barrier in immunocompetent patients, or consolidation using high-dose therapy with autologous stem cell transplantation.9-13
Because of the rarity of PCNSL and expertise required to deliver HDMTX-based regimens, it is uncertain how much of the accumulating knowledge about its optimal management has disseminated into the community. HDMTX requires inpatient delivery with IV hydration and monitoring of plasma methotrexate levels to guide leucovorin rescue and avoid prohibitive nephrotoxicity. Observational studies of PCNSL are limited to tightly controlled, centralized settings, whereas population-based analyses have focused on disease incidence and survival.14-17 Those studies are further complicated by difficulties in identifying cases associated with HIV infection and recipients of solid organ transplants or other immunosuppressive therapy, as well as by the lack of records pertaining to lymphoma-directed treatment. Recent data from the US Surveillance, Epidemiology, and End Results (SEER) registry indicate that the proportion of HIV-associated cases decreased from 64% in the 1990s to only ∼13% in 2007-2011 and that 5-year overall survival (OS) of immunocompetent patients significantly improved, but could not place this trend in the context of evolving therapeutic approaches.16 We have shown that HIV-positive patients have experienced an increase in chemotherapy application (from 29% to 47%), correlating with improved survival over the past 10 years.18
We now hypothesized that delivery of chemotherapy in HIV-negative PCNSL may differ according to the type of practice setting and that chemotherapy-based management may be associated with better outcomes in the community. Our objective in this study was to use a contemporary, nationwide dataset to study those associations and analyze the trends in the application of treatment modalities for PCNSL in the United States.
Patients and methods
Data source
We obtained data from the National Cancer Database (NCDB) Participant Use File, a hospital-based oncology outcomes registry encompassing over 1500 US cancer programs, as a part of an exploratory research proposal for the study of treatments and outcomes in rare subtypes of aggressive B-cell lymphomas.19 The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The registry captures ∼70% of all newly diagnosed cancer cases in the United States, including >80% of lymphomas. Participating facilities are accredited by the Commission on Cancer and execute data use agreements with the American College of Surgeons. Available data include patient demographics, HIV status (for lymphomas only), cancer histology, stage of disease, presence of B symptoms, as well as treatment modalities used for the initial management of cancer: surgery, radiation therapy (with radiation dose, modality, and field), and chemotherapy. Chemotherapy regimens are identified as single- or multiagent, but specific drugs, route of administration, doses, number of cycles, and response to treatment are unrecorded. All reporting facilities must collect follow-up survival data on a minimum of 90% of cases within 5 years from diagnosis. The data on individual patients and cancer programs are deidentified for research purposes, and this study was deemed exempt from human protection oversight by our local institutional review board.
Cohort selection
We selected patients who were 18 years or older with histologically confirmed PCNSL reported to the NCDB between 2004 and 2013. Cases were identified by a combination of the International Classification of Diseases in Oncology, 3rd edition codes for histology (DLBCL, 9680/3, including immunoblastic lymphoma, 9884/3, or unspecified malignant lymphoma, 9590/3 or 9591/3) and primary anatomical site (central nervous system, C71.0-C72.9).20 Of the 11 306 cases, we sequentially excluded those without histologic confirmation (N = 690, 6.1%) and those with recorded positive HIV status (N = 878, 8.3%), who were analyzed in our prior study.18 Other forms of immune deficiency or immunosuppression were not distinguished in the data and could not be analyzed as a variable in this study. We also excluded patients whose treatment decisions were all made outside of the reporting facility (N = 573, 5.9%), because the NCDB does not require documentation of their treatment and outcomes. According to the NCDB policy, survival was not available for cases diagnosed in 2013 because of potentially incomplete reporting.
Variables
We categorized age at diagnosis as 18-50, 51-60, 61-70, 71-80, and >80 years, and race as white, black, or other. We used median income in the county of residence (according to the 2008-2012 American Community Survey) and individual type of health insurance as proxies for patients’ socioeconomic status. Health insurance was classified as private (typically provided by the employer or self-purchased), Medicare (government-sponsored and available to all Americans who are older than 65 years or disabled), Medicaid (state-sponsored and available primarily to low-income individuals), other (provided by military or other governmental agencies), or no insurance. Patients’ underlying comorbidities were estimated by the Charlson-Deyo comorbidity index, a weighted score measuring the number and type of comorbid conditions documented as hospital diagnoses.21 We did not use the Ann Arbor staging variable, as it is not consistently used for PCNSL in clinical practice.2 We identified the receipt of radiation therapy alone, single-agent chemotherapy, or multiagent chemotherapy as the first course of therapy. The chemotherapy regimen was designated as “multiagent” if 2 or more cytotoxic or immunotherapeutic agents (excluding steroids, but including for example rituximab or intrathecal agents) were administered. Treatments delivered upon recurrence or progression of disease were not recorded in the NCDB. Participating hospitals were classified as “community” or “academic/research” programs according to the Commission on Cancer designation, which depends on facility case volume and available oncology services. To illustrate trends in OS, we grouped patients into 3 roughly equal groups diagnosed in 2004-2006, 2007-2009, or 2010-2012.
Statistical analysis
Patient characteristics were compared by χ2 test (for categorical variables) or Kruskal-Wallis test (for continuous variables). We expressed linearized trends in the use of treatment modalities as average annual percent change (APC), calculated by log-binomial regression, using the treated proportion as dependent variable and calendar year as a continuous independent variable. We investigated factors associated with nonreceipt of chemotherapy in a multivariable modified Poisson model, which provides direct estimates of adjusted relative risk (RR).22 The 10 relevant variables included in multivariable models were selected on the basis of their clinical relevance, regardless of statistical significance, and without any stepwise selection. In order to account for guarantee-time bias resulting from the fact that patients who died soon after diagnosis could not receive any therapy, we employed the landmark analysis method, requiring at least 3 months of survival for inclusion of a subject in the treatment selection model.23 We used OS at 3 years from PCNSL diagnosis, estimated using the Kaplan-Meier method, as the main survival endpoint. The association of mortality with treatment was analyzed in a multivariable Cox model. The proportional hazard assumption was evaluated using a plot of Schoenfeld residuals against time. Missing data on race (1.8% of cases), insurance (2.3%), median income (2.3%), B symptoms (13.8%), use of radiation (0.8%), and chemotherapy (2.1%) were addressed by multiple imputation using chained equations.24 This method of handling missing data is superior to alternatives (for example, case deletion) by minimizing bias under the assumption of data missing at random. The imputation models incorporated all dependent and independent variables from the outcome models, including the Nelson-Aalen estimate of cumulative hazard of death. Coefficients and confidence intervals (CI) in outcome models were averaged using Rubin’s rules, accounting for variation between 20 imputed datasets.24 We reported all estimates with 95% CI using Stata/SE v. 14.0 (StataCorp LP, College Station, TX). Because of the size of the dataset, and because up to 11 variables were included in the models, only P values <.0045 were considered statistically significant.
Results
Patient characteristics
The study cohort included 9165 PCNSL patients diagnosed between 2004 and 2013, with median age at diagnosis of 67 years and equal proportions of men and women (Table 1). Half of patients were treated in cancer programs designated as academic/research. Those patients were on average younger (median age 65 years vs 68 years, P < .0001) and more commonly had no recorded comorbidities (69.4% vs 63.2%, P < .0001, supplemental Table 1). Academic/research programs had a higher average PCNSL case volume (median 2 cases per year, compared with 1 per year, P = .0001) and a higher proportion of programs reporting >3 cases per year (25.0% compared with 2.4%, P < .0001).
Characteristics of patients with PCNSL in the NCDB, 2004-2013
| Variable . | N . | % or IQR . |
|---|---|---|
| Number of cases | 9165 | 100.0 |
| Age, median, y, IQR | 67 (58-75) | |
| Age group, y | ||
| ≤60 | 2910 | 31.8 |
| >60 | 6255 | 68.2 |
| Sex | ||
| Male | 4586 | 50.0 |
| Female | 4579 | 50.0 |
| Race | ||
| White | 8135 | 88.8 |
| Black | 436 | 4.8 |
| Asian/other | 425 | 4.6 |
| Unrecorded | 169 | 1.8 |
| Comorbidity index | ||
| 0 | 6093 | 66.5 |
| 1 | 1904 | 20.8 |
| ≥2 | 1168 | 12.7 |
| Prior malignancy | ||
| No | 7766 | 84.7 |
| Yes | 1399 | 15.3 |
| Health insurance | ||
| Private insurance | 3195 | 34.9 |
| Medicare | 4780 | 52.2 |
| Medicaid | 555 | 6.1 |
| Uninsured | 298 | 3.3 |
| Other | 129 | 1.4 |
| Unrecorded | 208 | 2.3 |
| Median income* | ||
| <$38 000 | 1444 | 15.8 |
| $38 000-$47 999 | 2090 | 22.8 |
| $48 000-$62 999 | 2438 | 26.6 |
| ≥$63 000 | 2979 | 32.5 |
| Unrecorded | 214 | 2.3 |
| Histology | ||
| DLBCL | 7603 | 83.0 |
| PCNSL, NOS | 1562 | 17.0 |
| Anatomical location | ||
| Brain | 7682 | 83.8 |
| Spinal cord | 483 | 5.3 |
| Unspecified | 1000 | 10.9 |
| B symptoms | ||
| Absent | 7026 | 76.7 |
| Present | 875 | 9.5 |
| Unrecorded | 1264 | 13.8 |
| Cancer program designation | ||
| Community | 4321 | 47.1 |
| Academic/research | 4844 | 52.9 |
| Variable . | N . | % or IQR . |
|---|---|---|
| Number of cases | 9165 | 100.0 |
| Age, median, y, IQR | 67 (58-75) | |
| Age group, y | ||
| ≤60 | 2910 | 31.8 |
| >60 | 6255 | 68.2 |
| Sex | ||
| Male | 4586 | 50.0 |
| Female | 4579 | 50.0 |
| Race | ||
| White | 8135 | 88.8 |
| Black | 436 | 4.8 |
| Asian/other | 425 | 4.6 |
| Unrecorded | 169 | 1.8 |
| Comorbidity index | ||
| 0 | 6093 | 66.5 |
| 1 | 1904 | 20.8 |
| ≥2 | 1168 | 12.7 |
| Prior malignancy | ||
| No | 7766 | 84.7 |
| Yes | 1399 | 15.3 |
| Health insurance | ||
| Private insurance | 3195 | 34.9 |
| Medicare | 4780 | 52.2 |
| Medicaid | 555 | 6.1 |
| Uninsured | 298 | 3.3 |
| Other | 129 | 1.4 |
| Unrecorded | 208 | 2.3 |
| Median income* | ||
| <$38 000 | 1444 | 15.8 |
| $38 000-$47 999 | 2090 | 22.8 |
| $48 000-$62 999 | 2438 | 26.6 |
| ≥$63 000 | 2979 | 32.5 |
| Unrecorded | 214 | 2.3 |
| Histology | ||
| DLBCL | 7603 | 83.0 |
| PCNSL, NOS | 1562 | 17.0 |
| Anatomical location | ||
| Brain | 7682 | 83.8 |
| Spinal cord | 483 | 5.3 |
| Unspecified | 1000 | 10.9 |
| B symptoms | ||
| Absent | 7026 | 76.7 |
| Present | 875 | 9.5 |
| Unrecorded | 1264 | 13.8 |
| Cancer program designation | ||
| Community | 4321 | 47.1 |
| Academic/research | 4844 | 52.9 |
IQR, interquartile range; NOS, not otherwise specified.
In the patient’s zip code of residence, according to the 2012 American Community Survey data and grouped into population quartiles.
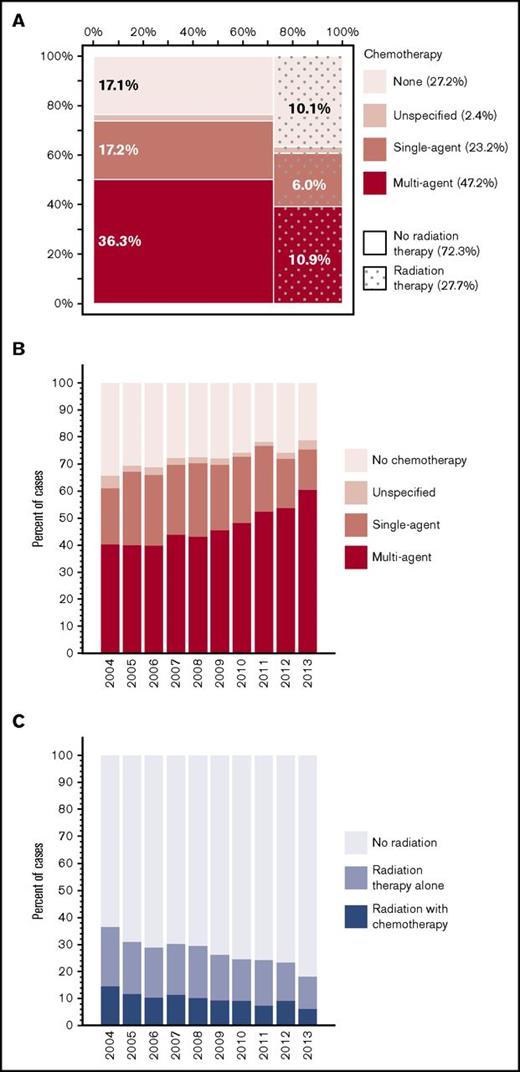
Among the 8924 patients with non–missing treatment records, 1523 (17.1%) did not receive any initial therapy for their PCNSL, 900 (10.1%) received radiation therapy alone, and 6501 (72.8%) received some chemotherapy (Figure 1A). Patients who received no treatment were on average older (median age 73 years, compared with 66 years for treated patients), more often with comorbidities (41% vs 32%, respectively), and less likely managed in academic centers (45% vs 55%, respectively), and they had extremely poor outcome (median OS, 1.9 months; 95% CI, 1.7-2.0). Median time from diagnosis to start of treatment was 19 days (IQR, 10-32 days). Among recipients of chemotherapy, 24.2% underwent radiation therapy as well, at median 72 days from the first chemotherapy (IQR, 15-111 days). Median dose of radiation was 39.6 Gy (IQR, 30.0-45.0 Gy), delivered over median 20 fractions (IQR, 13-25). Chemotherapy regimen was recorded as single agent in 31.9%, multiagent in 64.7%, and unspecified in 3.4% of cases where any chemotherapy was delivered. In 2013, the specific use of rituximab was distinguished as “immunotherapy” and was recorded in 43.8% of patients receiving chemotherapy.
Use of chemotherapy and radiation for management of PCNSL. (A) Matrix plot illustrating proportions of patients receiving chemotherapy and/or radiation therapy; percentages indicating cases treated with unspecified chemotherapy were omitted for clarity. (B) Yearly trend in the proportion of cases receiving chemotherapy. (C) Yearly trend in the proportion of cases receiving radiation therapy (with or without chemotherapy).
Use of chemotherapy and radiation for management of PCNSL. (A) Matrix plot illustrating proportions of patients receiving chemotherapy and/or radiation therapy; percentages indicating cases treated with unspecified chemotherapy were omitted for clarity. (B) Yearly trend in the proportion of cases receiving chemotherapy. (C) Yearly trend in the proportion of cases receiving radiation therapy (with or without chemotherapy).
Trends in the use of treatment modalities
There was a significant upward trend in the proportion of patients who received chemotherapy, which increased steadily from 65.6% in 2004 to 78.8% in 2013 (APC, 1.7%; 95% CI, 1.2-2.1; P < .0001; Figure 1B). There was also a significant increase in the proportion of patients receiving multiagent regimens (from 40.3% to 60.4%, respectively). The increasing trend in multiagent chemotherapy use occurred principally after 2009 (APC, 7.1%; 95% CI, 5.0-9.2; P < .0001), whereas the increase was not significant before (APC, 2.4%; 95% CI, −0.2 to 5.1; P = .07). Conversely, the proportion treated with radiation therapy decreased from 37.6% to 18.8%, respectively (APC, −5.7%; 95% CI, −6.8 to −4.6; P < .0001; Figure 1C). This downward trend was evident for radiation delivered as a sole modality or in combination with chemotherapy. The overall trends were similar in academic and community cancer programs for both chemotherapy (P for interaction =.77) and radiation therapy use (P = .84).
Factors associated with nonreceipt of chemotherapy
We evaluated factors associated with nonreceipt of chemotherapy for PCNSL in a subpopulation of patients who had at least 3 months of survival (N = 7068) to avoid guarantee-time bias. In a multivariable model, advanced age, black race, high comorbidity index, unspecified PCNSL histology, Medicaid coverage, and lack of health insurance were significantly associated with not receiving chemotherapy (Table 2; see supplemental Table 2 and supplemental Figure 1 for an analogous model using restricted cubic splines for age modeling). After adjusting for those factors, the risk of not receiving chemotherapy was 31% lower in academic/research hospitals compared with other types of cancer programs. The unadjusted proportions of patients receiving chemotherapy were 86.3% and 78.1%, respectively. Patients receiving chemotherapy in academic/research centers were also relatively more likely to receive a multiagent regimen (67.9% compared with 60.4%, P < .0001), and, for the subgroup diagnosed in 2013, more likely to receive immunotherapy (49.7% vs 31.8%, P < .0001). Moreover, the proportion receiving both chemotherapy and radiation was significantly lower in academic/research centers (20.6% vs 28.8%; adjusted RR, 0.70; 95% CI, 0.64-0.76; P < .0001).
Factors associated with nonreceipt of chemotherapy among patients with PCNSL who survived at least 3 months from diagnosis (N = 7068)
| Variable . | % not receiving chemotherapy . | Adjusted RR . | 95% CI . | P . |
|---|---|---|---|---|
| Age group, y | ||||
| 18-50 | 10.9 | Reference | <.0001 | |
| 51-60 | 10.1 | 0.96 | 0.77-1.21 | |
| 61-70 | 14.4 | 1.29 | 1.04-1.60 | |
| 71-80 | 23.3 | 2.01 | 1.60-2.54 | |
| >80 | 44.1 | 3.81 | 3.00-4.84 | |
| Sex | ||||
| Male | 16.7 | Reference | .98 | |
| Female | 18.1 | 1.00 | 0.91-1.10 | |
| Race | ||||
| White | 17.3 | Reference | <.0001 | |
| Black | 23.2 | 1.61 | 1.31-1.96 | |
| Asian/other | 14.1 | 1.00 | 0.77-1.31 | |
| Comorbidity index | ||||
| 0 | 15.8 | Reference | .0001 | |
| 1 | 19.2 | 1.07 | 0.95-1.21 | |
| ≥2 | 24.3 | 1.34 | 1.17-1.53 | |
| Prior malignancy | ||||
| No | 16.9 | Reference | .86 | |
| Yes | 20.7 | 1.01 | 0.89-1.15 | |
| Health insurance | ||||
| Private insurance | 10.9 | Reference | .0005 | |
| Not insured | 18.9 | 1.68 | 1.27-2.22 | |
| Medicaid | 15.7 | 1.43 | 1.12-1.82 | |
| Medicare | 22.9 | 1.22 | 1.04-1.42 | |
| Other | 17.6 | 1.29 | 0.81-2.05 | |
| Median income* | ||||
| <$30 000 | 19.8 | Reference | .06 | |
| $30 000-$35 999 | 18.3 | 0.93 | 0.79-1.09 | |
| $36 000-$45 999 | 17.7 | 0.88 | 0.76-1.03 | |
| ≥$46 000 | 15.5 | 0.82 | 0.70-0.95 | |
| Histology | ||||
| DLBCL | 15.9 | Reference | <.0001 | |
| PCNSL, NOS | 24.7 | 1.52 | 1.36-1.70 | |
| Anatomical location | ||||
| Brain | 17.9 | Reference | .013 | |
| Spinal cord | 15.8 | 0.79 | 0.63-0.99 | |
| Unspecified | 15.0 | 0.82 | 0.70-0.97 | |
| Type of cancer program | ||||
| Community | 21.1 | Reference | <.0001 | |
| Academic/research | 14.0 | 0.69 | 0.62-0.76 |
| Variable . | % not receiving chemotherapy . | Adjusted RR . | 95% CI . | P . |
|---|---|---|---|---|
| Age group, y | ||||
| 18-50 | 10.9 | Reference | <.0001 | |
| 51-60 | 10.1 | 0.96 | 0.77-1.21 | |
| 61-70 | 14.4 | 1.29 | 1.04-1.60 | |
| 71-80 | 23.3 | 2.01 | 1.60-2.54 | |
| >80 | 44.1 | 3.81 | 3.00-4.84 | |
| Sex | ||||
| Male | 16.7 | Reference | .98 | |
| Female | 18.1 | 1.00 | 0.91-1.10 | |
| Race | ||||
| White | 17.3 | Reference | <.0001 | |
| Black | 23.2 | 1.61 | 1.31-1.96 | |
| Asian/other | 14.1 | 1.00 | 0.77-1.31 | |
| Comorbidity index | ||||
| 0 | 15.8 | Reference | .0001 | |
| 1 | 19.2 | 1.07 | 0.95-1.21 | |
| ≥2 | 24.3 | 1.34 | 1.17-1.53 | |
| Prior malignancy | ||||
| No | 16.9 | Reference | .86 | |
| Yes | 20.7 | 1.01 | 0.89-1.15 | |
| Health insurance | ||||
| Private insurance | 10.9 | Reference | .0005 | |
| Not insured | 18.9 | 1.68 | 1.27-2.22 | |
| Medicaid | 15.7 | 1.43 | 1.12-1.82 | |
| Medicare | 22.9 | 1.22 | 1.04-1.42 | |
| Other | 17.6 | 1.29 | 0.81-2.05 | |
| Median income* | ||||
| <$30 000 | 19.8 | Reference | .06 | |
| $30 000-$35 999 | 18.3 | 0.93 | 0.79-1.09 | |
| $36 000-$45 999 | 17.7 | 0.88 | 0.76-1.03 | |
| ≥$46 000 | 15.5 | 0.82 | 0.70-0.95 | |
| Histology | ||||
| DLBCL | 15.9 | Reference | <.0001 | |
| PCNSL, NOS | 24.7 | 1.52 | 1.36-1.70 | |
| Anatomical location | ||||
| Brain | 17.9 | Reference | .013 | |
| Spinal cord | 15.8 | 0.79 | 0.63-0.99 | |
| Unspecified | 15.0 | 0.82 | 0.70-0.97 | |
| Type of cancer program | ||||
| Community | 21.1 | Reference | <.0001 | |
| Academic/research | 14.0 | 0.69 | 0.62-0.76 |
NOS, not otherwise specified.
In the patient’s zip code of residence, according to the 2012 American Community Survey data and grouped into population quartiles.
Survival analysis
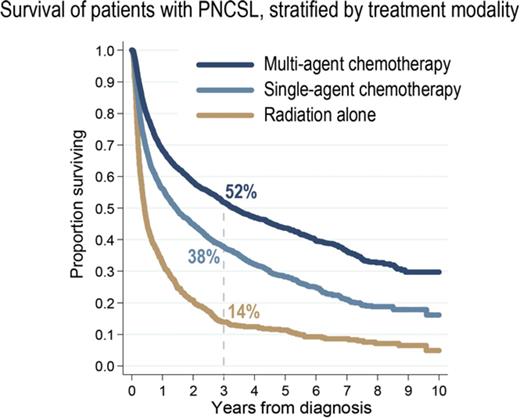
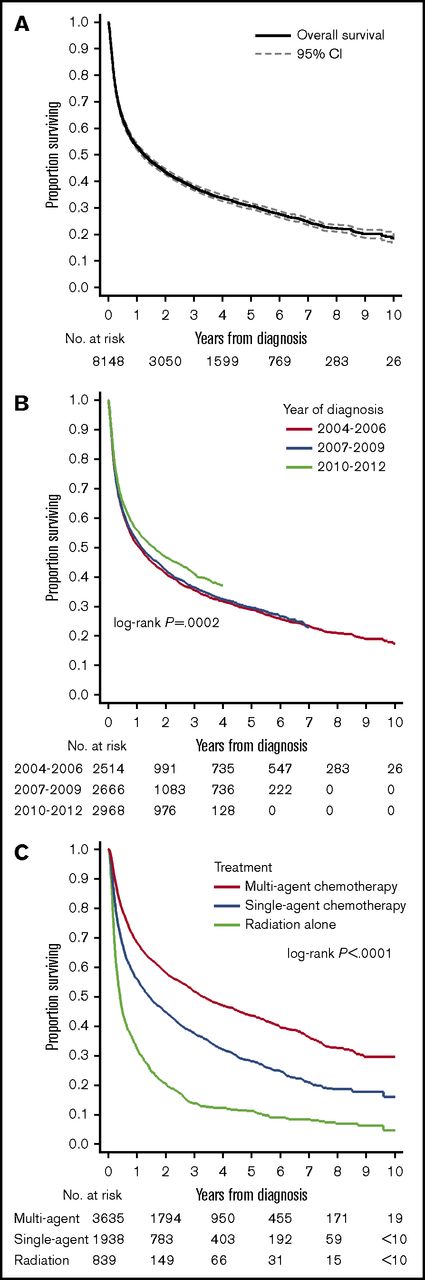
Median follow-up for censored patients was 3.7 years. Median OS for all PCNSL cases was 1.3 years (95% CI, 1.2-1.4; Figure 2A). Estimated OS was 37.7% at 3 years (95% CI, 36.6-38.8) and 30.5% at 5 years from diagnosis (95% CI, 29.4-31.6). There was evidence of improved OS in the most recent epoch (2010-2012, OS at 3 years 40.9%) compared with prior years (3-year OS of 36%; P for trend =.0002; Figure 2B, see supplemental Figure 3 for yearly trends). This difference in OS was not evident when comparing survival stratified by receipt of chemotherapy (stratified log-rank, P = .35). Unadjusted 3-year OS stratified by treatment modalities was 51.8% (95% CI, 50.1-53.5) for multiagent chemotherapy, 37.7% (95% CI, 35.5-39.9) for single-agent chemotherapy, and 14.1% (95% CI, 11.7-16.7) for radiation therapy alone (Figure 2C).
OS outcomes in PCSNL. (A) Survival in the entire cohort (2004-2012). (B) Survival stratified by year of diagnosis; P is derived from a log-rank test for trend. (C) Survival stratified by upfront treatment modality. Cell sizes <10 were suppressed according to the NCDB policy.
OS outcomes in PCSNL. (A) Survival in the entire cohort (2004-2012). (B) Survival stratified by year of diagnosis; P is derived from a log-rank test for trend. (C) Survival stratified by upfront treatment modality. Cell sizes <10 were suppressed according to the NCDB policy.
Adjusting for available confounding factors (Table 3), receipt of multiagent chemotherapy was associated with better OS than receipt of single-agent chemotherapy (hazard ratio, 1.35; 95% CI, 1.26-1.45) or radiation therapy alone (hazard ratio, 2.18; 95% CI, 1.98-2.39). Other factors associated with worse OS included older age, male sex, higher Charlson-Deyo comorbidity index, lack of private insurance, residence in poorer areas, and location of the tumor in the brain. Accounting for the type of treatment modality, the difference in mortality between academic and community centers was minor (hazard ratio, 0.92; 95% CI, 0.86-0.98) and did not reach our threshold for statistical significance (see supplemental Table 3 and supplemental Figure 2 for an analogous survival model using restricted cubic splines for modeling of age).
Factors associated with OS among patients with PCNSL who received upfront treatment with chemotherapy or radiation
| Variable . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Treatment modality | |||
| Multiagent chemotherapy | Reference | <.0001 | |
| Single-agent chemotherapy | 1.35 | 1.26-1.45 | |
| Radiation therapy alone | 2.17 | 1.98-2.39 | |
| Age group, y | |||
| 18-50 | Reference | <.0001 | |
| 51-60 | 1.63 | 1.43-1.86 | |
| 61-70 | 2.13 | 1.87-2.42 | |
| 71-80 | 2.98 | 2.59-3.43 | |
| >80 | 3.88 | 3.29-4.57 | |
| Sex | |||
| Male | Reference | .003 | |
| Female | 0.91 | 0.85-0.97 | |
| Race | |||
| White non-Hispanic | Reference | .09 | |
| Black | 0.91 | 0.77-1.07 | |
| Asian/other | 0.85 | 0.72-1.01 | |
| Charlson-Deyo index | |||
| 0 | Reference | <.0001 | |
| 1 | 1.11 | 1.03-1.20 | |
| ≥2 | 1.25 | 1.14-1.38 | |
| Health insurance | |||
| Private insurance | Reference | .0003 | |
| Not insured | 1.25 | 1.04-1.51 | |
| Medicaid | 1.26 | 1.08-1.46 | |
| Medicare | 1.16 | 1.06-1.27 | |
| Other | 1.34 | 1.02-1.76 | |
| Median income* | |||
| <$30 000 | Reference | .0001 | |
| $30 000-$35 999 | 0.93 | 0.85-1.03 | |
| $36 000-$45 999 | 0.86 | 0.78-0.95 | |
| ≥$46 000 | 0.81 | 0.74-0.89 | |
| Histology | |||
| DLBCL | Reference | .027 | |
| PCNSL, NOS | 0.91 | 0.84-0.99 | |
| Anatomical location | |||
| Brain | Reference | <.0001 | |
| Spinal cord | 0.57 | 0.49-0.67 | |
| Unspecified | 0.76 | 0.68-0.85 | |
| Type of cancer program | |||
| Community | Reference | .009 | |
| Academic program | 0.92 | 0.86-0.98 |
| Variable . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Treatment modality | |||
| Multiagent chemotherapy | Reference | <.0001 | |
| Single-agent chemotherapy | 1.35 | 1.26-1.45 | |
| Radiation therapy alone | 2.17 | 1.98-2.39 | |
| Age group, y | |||
| 18-50 | Reference | <.0001 | |
| 51-60 | 1.63 | 1.43-1.86 | |
| 61-70 | 2.13 | 1.87-2.42 | |
| 71-80 | 2.98 | 2.59-3.43 | |
| >80 | 3.88 | 3.29-4.57 | |
| Sex | |||
| Male | Reference | .003 | |
| Female | 0.91 | 0.85-0.97 | |
| Race | |||
| White non-Hispanic | Reference | .09 | |
| Black | 0.91 | 0.77-1.07 | |
| Asian/other | 0.85 | 0.72-1.01 | |
| Charlson-Deyo index | |||
| 0 | Reference | <.0001 | |
| 1 | 1.11 | 1.03-1.20 | |
| ≥2 | 1.25 | 1.14-1.38 | |
| Health insurance | |||
| Private insurance | Reference | .0003 | |
| Not insured | 1.25 | 1.04-1.51 | |
| Medicaid | 1.26 | 1.08-1.46 | |
| Medicare | 1.16 | 1.06-1.27 | |
| Other | 1.34 | 1.02-1.76 | |
| Median income* | |||
| <$30 000 | Reference | .0001 | |
| $30 000-$35 999 | 0.93 | 0.85-1.03 | |
| $36 000-$45 999 | 0.86 | 0.78-0.95 | |
| ≥$46 000 | 0.81 | 0.74-0.89 | |
| Histology | |||
| DLBCL | Reference | .027 | |
| PCNSL, NOS | 0.91 | 0.84-0.99 | |
| Anatomical location | |||
| Brain | Reference | <.0001 | |
| Spinal cord | 0.57 | 0.49-0.67 | |
| Unspecified | 0.76 | 0.68-0.85 | |
| Type of cancer program | |||
| Community | Reference | .009 | |
| Academic program | 0.92 | 0.86-0.98 |
NOS, not otherwise specified.
In the patient’s zip code of residence, according to the 2012 American Community Survey data and grouped into population quartiles.
Discussion
In this large observational study, we described the use of treatment modalities for management of HIV-negative PCNSL as recorded in the NCDB between 2004 and 2013. These contemporary, nationwide data provide novel insights into how the evolving therapeutic approaches translated into clinical practice in the United States and uncovered previously unrecognized disparities. Our main findings are that approximately a quarter of newly diagnosed patients do not receive chemotherapy, that this proportion has been decreasing over time, and that it shows a significant association with the type of treating facility, even accounting for differences in baseline characteristics of patients seen in academic and community centers. Furthermore, in this retrospective analysis, receipt of multiagent chemotherapy was associated with 3-year OS of 51.8%, which was significantly better relative to other modalities and comparable to results achieved in recent clinical trials.10,11
Therapy of PCNSL has advanced primarily through nonrandomized trials conducted in academic settings due to the rarity of the disease, which has an incidence of ∼0.5 per 100 000 person-years in the United States.25 To date, only 5 randomized trials have been published, with only 1 completed phase 3 study.6,9-11,26 However, most HIV-negative PCNSL patients are over 60 years old, and trial participants may not reflect the characteristics of populations encountered in the community setting.27 The present study, to our knowledge, is the first to evaluate PCNSL therapy and associated outcomes in a large data source. Prior observational studies were based on the US SEER registry data, demonstrating that 12.7% of PCSNL cases in 2007-2011 were among HIV-positive patients, and that survival among the HIV-uninfected cases was 30.1% at 5 years—nearly identical to our estimate of 30.5%.16 Incidence of PNCSL increased among immunocompetent individuals, particularly those older than 65 years.16,17,25 Because the NCDB is not a population-based resource (as it is limited only to accredited reporting cancer programs), it did not allow analysis of measures like incidence or mortality rate, but unlike SEER, it allowed us to place the survival analysis in the context of therapy. The proportion of patients recorded as HIV-positive in the NCDB was somewhat lower (8.3%) than in SEER, possibly reflecting a less metropolitan population in our data set. HIV-positive patients in our prior study were shown to have a much lower likelihood of receiving chemotherapy (35%) and on aggregate had 5-year OS of only 22%, although this was 40% for those who received chemotherapy.18
We demonstrated a significant increase in the application of chemotherapy for PCNSL, and a concurrent decline in the use of WBRT. Although a marked improvement in response rates and survival with HDMTX-based chemotherapy was already noted in the 1990s,28,29 concerns about neurotoxicity of combined modality therapy, particularly among older patients, limited the application of this strategy. Our data indicate that a quarter of patients older than 70 years who survive >3 months from diagnosis do not receive chemotherapy, even though HDMTX may provide survival benefit for older individuals with adequate organ function.8,30-32 Significant disparities by race and health insurance status were also evident, although the reason for them could not be elucidated from the registry data. Similar socioeconomic disparities in the application of curative therapy for hematologic malignancies have been recently reported in other settings, including use of novel agents for multiple myeloma, combined modality therapy in classical Hodgkin lymphoma, or rituximab in non-Hodgkin B-cell lymphomas.33-36 After adjusting for sociodemographic profiles of patients treated in various types of hospitals, those managed in academic/research centers had a 31% lower risk of not receiving chemotherapy. Although additional uncaptured factors (performance status, degree of neurologic impairment, renal or liver insufficiency) may confound this disparity, local expertise in this rare malignancy and ability to safely deliver HDMTX with leucovorin rescue (particularly in older patients with decreased renal function) may hypothetically explain the observed difference. Centralizing the care of PCNSL to higher-volume cancer programs with adequate resources, as well as educating patient and physicians alike about risks and benefits of chemotherapy and WBRT, may help alleviate the disparity. For patients treated with radiation therapy alone, our study, based on the largest reported cohort, demonstrates poor outcomes, with 3-year OS of only 14%, even though a small number of patients achieved long-term survival. Some of those subjects may have received salvage chemotherapy later in the course of their disease, as the NCDB only records modalities used for the initial course of therapy.
Receipt of multiagent chemotherapy was associated with significantly better survival, but we caution against interpreting this association causally as a treatment effect—it should rather be considered a description of outcomes among patients eligible to receive this modality. OS of 51.8% at 3 years is quite close to the result of the recent International Extranodal Lymphoma Study Group 32 trial of HDMTX and cytarabine-based chemotherapy, in which 52% of subjects were alive at median follow-up of 30 months.11 In that study, the rate of complete remissions (49%) was significantly higher with the 4-drug combination of HDMTX, cytarabine, thiotepa, and rituximab (MATRix regimen), as compared with HDMTX/cytarabine ± rituximab (23% to 30%). Survival outcomes were also better, although increase in toxicity was limited to hematologic adverse events. We note that, in our data, the proportion of patients receiving multiagent chemotherapy increased steeply after 2009, and this most recent cohort also showed improved survival compared with 2004-2009. The change in practice patterns coincided with the publication of the International Extranodal Lymphoma Study Group 20 trial, which demonstrated a better response rate using HDMTX with cytarabine over single-agent HDMTX in a randomized phase 2 design.9 Current National Comprehensive Cancer Network guidelines recommend HDMTX-based systemic therapy for all patients with PCNSL without contraindications.37 Although our report supports the use of multiagent chemotherapy, 1 meta-analysis found that escalating treatment beyond 2-drug combinations may not provide additional benefit for patients older than 60 years.8 However, another recent study demonstrated safety and efficacy of HDMTX combined with temozolomide, or with procarbazine, vincristine, and cytarabine in patients over 60 years of age, favoring the 4-drug regimen.10 Our inability to discern specific drugs and regimens poses a significant limitation. Although one might expect that many patients received HDMTX-based therapy according to guidelines, this assumption cannot be verified without further research to determine what specific regimens are actually prescribed in the US community. Combinations of HDMTX with cytarabine,9 with rituximab, procarbazine, and vincristine,38,39 or with ifosfamide6 may be most commonly offered. OS at 3 years in relevant clinical trials ranged from 39%6 to 77%,38 reaching 81% in trials employing consolidation autologous stem cell transplantation.39,40 The NCDB outcomes are worse, but the cohort contains subjects who would not be eligible for many clinical trials and who contribute to lower survival estimates. Interestingly, the 2013 data suggest that a large proportion of patients may be receiving rituximab-containing regimens for PCNSL. We noted a fairly long time from diagnosis to treatment initiation (median 19 days), with the caveats that the date of diagnosis in the NCDB may be assigned based on clinical findings (tumor on imaging) rather than a biopsy, and administration of steroids alone was not recorded as initiation of chemotherapy.
Additional limitations of the NCDB data are worth mentioning. The registry, although large, is hospital-based and has some demographic bias that precludes generalization to the entire population. Critically important data on progression-free survival, patients’ quality of life, time from the onset of symptoms to PCNSL diagnosis, and neuropsychological function were not available. Our OS model lacked some of the previously identified prognostic factors: baseline performance status, elevated lactate dehydrogenase level, tumor location within deep regions of the brain, cerebrospinal fluid protein level, or leptomeningeal involvement.41 Some of the detected associations were quantitatively small and may be artifacts in this very large cohort, although we guarded against it by adjusting our threshold for statistical significance. Others, like the better OS in cases of spinal cord lymphoma, although interesting, are difficult to interpret in the absence of more detailed clinical data. Therefore, we focused our discussion on the most meaningful findings. Because we excluded patients who were treated without a histologic diagnosis, cases with primary tumor located in deep cerebral structures may have been underrepresented due to difficulties in obtaining a stereotactic biopsy in these anatomical locations. Furthermore, brain biopsy may be technically challenging in some patients who have severe neurologic impairment or comorbidities.
In conclusion, we demonstrated an increasing trend in the application of chemotherapy for PCNSL in the United States, which is associated with improved survival, strongly supporting current guidelines that recommend chemotherapy-based treatment of PCNSL. Disparities in the application of those guidelines are evident, and further research should focus on determining their direct cause and possible alleviation, particularly with regard to the care of older patients treated in the community setting. As novel therapies exploring the molecular features of PCNSL, including the B-cell receptor signaling pathway or the MYD88 mutation, become available, it will be important to engage community practitioners to assure adequate dissemination of potential advances and improve outcomes for all patients with PCNSL. Finally, our study illustrates that collection of data on specific chemotherapy regimens by cancer registries is crucial if the effort of large-scale data is to advance our understanding of modern cancer therapy.42
The full-text version of this article contains a data supplement.
Acknowledgments
The data used in the study are derived from a deidentified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
A.J.O. is supported by the American Society of Hematology Scholar Award and the American Cancer Society Research Scholar Award.
Authorship
Contribution: J.F., L.Q., and A.J.O. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adam J. Olszewski, Division of Hematology-Oncology, Rhode Island Hospital, George-353, 593 Eddy St, Providence, RI 02903; e-mail: adam_olszewski@brown.edu.
References
Competing Interests
Preliminary results were presented at the 2016 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2016.