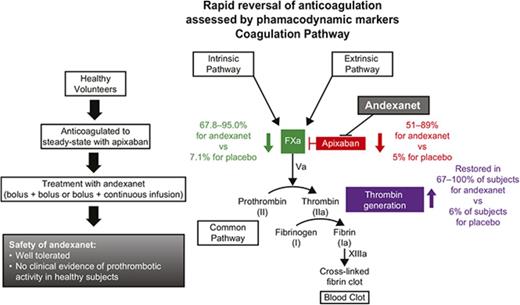
Key Points
Andexanet reversed apixaban anticoagulation in healthy volunteers within minutes after administration and for the duration of infusion.
Andexanet was generally well tolerated, with no evidence of prothrombotic activity in preclinical models and in healthy subjects.
Abstract
Direct factor Xa (FXa) inhibitors lack a specific reversal agent for emergencies such as major bleeding or urgent surgery. Andexanet alfa, a modified, catalytically inactive, recombinant human FXa derivative, reverses anticoagulant effect by binding and sequestering FXa inhibitors. This original report of safety and dose-finding, phase 1 and 2 randomized, double-blind, placebo-controlled studies, investigated various doses of andexanet in healthy volunteers. Phase 1 evaluated the safety and pharmacokinetics of andexanet (n = 24) or placebo (n = 8). In phase 2, andexanet (n = 36) or placebo (n = 18) was administered following steady-state apixaban dosing (5 mg twice daily for 6 days); safety, pharmacokinetics, and pharmacodynamics were assessed. Andexanet plasma concentration increased proportionally with dose, with rapid elimination (terminal elimination half-life, 4.35-7.5 hours). Following apixaban treatment, andexanet rapidly (≤2 minutes) and dose dependently reduced unbound apixaban concentration vs placebo (51% to 89% vs 5% reduction; all P < .05), decreased anti-FXa activity (67.8% to 95.0% vs 7.1% reduction; all P < .05), and restored thrombin generation in 67% to 100% vs 6% of subjects (all P < .01), maintaining these effects during continuous 45- and 120-minute infusions. Andexanet was well tolerated. Nine subjects had mild/moderate infusion reactions not associated with hemodynamic changes or respiratory compromise that generally resolved without intervention or dose reduction. There were no thrombotic events or other serious safety issues. In conclusion, andexanet reversed apixaban-mediated effects on pharmacodynamic markers of anticoagulation in healthy volunteers within minutes after administration and for the duration of infusion. This trial was registered at www.clinicaltrials.gov as #NCT01758432.
Introduction
Oral direct factor Xa (FXa) inhibitors (rivaroxaban, apixaban, edoxaban) bind to FXa and prevent the conversion of prothrombin to thrombin. These agents are effective anticoagulants for the prevention and treatment of thromboembolism, stroke prevention in atrial fibrillation, and treatment and prevention of deep vein thrombosis and pulmonary embolism based on the results of large clinical trials.1-14 Although reported rates of major bleeding complications are low,15,16 bleeding and its complications are the major concern with all anticoagulants, including oral FXa inhibitors. There is no approved reversal agent for the anticoagulant effects of FXa inhibitors in emergency situations such as life-threatening bleeding or urgent surgery.
Andexanet alfa (andexanet) is a modified, catalytically inactive, recombinant human FXa decoy protein that lacks pro- or anticoagulant activity of its own, but retains the ability to bind and sequester FXa inhibitors, thereby restoring activity of native FXa.17 In animals treated with rivaroxaban, andexanet reversed its effects on plasma anti-FXa activity and reduced unbound rivaroxaban concentrations.18 Furthermore, andexanet dose-dependently reduced bleeding in animals treated with enoxaparin or fondaparinux.17
Here, we report original data from the phase 1 and 2 studies of andexanet administered to healthy volunteers, with or without apixaban anticoagulation. The objectives of the phase 1 study were to evaluate safety (including immunogenicity) and pharmacokinetics (PK) of andexanet. The objectives of the phase 2 study were to evaluate the safety, tolerability, PK, and pharmacodynamic (PD) effects of andexanet in apixaban-anticoagulated subjects during and after administration by bolus, bolus followed by a second bolus, or bolus followed by a continuous infusion. The PK and PD of apixaban before, during, and after andexanet administration were also evaluated. These safety and dose-finding phase 1 and 2 studies informed the dose selections for confirmatory phase 3 studies in older healthy subjects,19 and an ongoing phase 3b/4 study (ANNEXA-4; #NCT02329327) in patients anticoagulated with an FXa inhibitor and presenting with major bleeding complications.20,21
Methods
Subjects
Adult subjects (phase 1, 18-50 years; phase 2, 18-45 years) were eligible for the study if they had an unremarkable medical history and physical examination in addition to normal electrocardiogram (ECG), vital signs, liver function, and coagulation tests. Subjects with a personal or family history of hypercoagulability or clotting, and those with abnormal protein C, protein S, and factor V Leiden testing were excluded. Randomization and treatment allocation were conducted using a randomization schedule and performed by an unblinded research pharmacist (refer to supplemental Figure 1 for CONSORT flowchart). Study protocols were approved by the Chesapeake Institutional Review Board (Columbia, Maryland), and all subjects provided written informed consent before initiating study procedures.
Study design, treatments, and assessments
The phase 1 (pharmacokinetic, not registered) study was a randomized, double-blind, placebo-controlled, single-center study, conducted June 5 to August 12, 2012, in which subjects received single ascending doses of andexanet or placebo (saline). A total of 32 subjects were randomized in a 6:2 ratio of andexanet or placebo within 1 of 4 sequential dosing cohorts: 30, 90, 300, or 600 mg. Andexanet was administered as an IV bolus over 10 minutes or, for the 600-mg dose, over 20 minutes. Blood samples for assessing andexanet plasma concentration and immunogenicity were collected predose, immediately after completion of the IV bolus, and at various time points after completion of the IV bolus. Subjects were followed for 4 weeks, with in-house observation of dosing and assessments on days 7, 14, and 28.
The phase 2 study (#NCT01758432) was a randomized, double-blind, placebo-controlled study conducted November 19, 2012 to October 21, 2013, in which healthy volunteers received 5.5 days of apixaban (5 mg orally every 12 hours; fasted state) followed by randomization to receive andexanet or placebo (2:1) 3 hours after the last dose of apixaban. Andexanet/placebo was administered in 6 sequential dosing cohorts (N = 8 per cohort) as follows: (i) cohort 1: IV bolus of 90 mg over 3 minutes (all IV bolus administered at ∼30 mg/min); (ii) cohort 2: IV bolus of 210 mg over 7 minutes; (iii) cohort 3: IV bolus of 420 mg over 15 minutes; (iv) cohort 4: IV bolus of 420 mg over ∼14 minutes followed immediately by a continuous IV infusion of 180 mg (4 mg/min) over 45 minutes; (v) cohort 5: IV bolus of 420 mg over ∼14 minutes followed after 45 minutes by a second bolus of 180 mg over ∼6 minutes; and (vi) cohort 6: IV bolus of 420 mg over ∼14 minutes followed by a continuous infusion of 480 mg for 120 minutes (4 mg/min) (supplemental Figure 2).
Samples for assessing andexanet plasma concentration were drawn predose, immediately after completion of the bolus, and at various time points after completion of the bolus. Apixaban plasma PK parameters were determined the day prior to and the day of andexanet administration. Samples for PD parameters were collected on day 5 post-apixaban (3 to 12 hours) in cohorts 1 to 3, on day 5 pre-apixaban to 3 hours post-apixaban in cohorts 4 to 6, and on day 6 from pre-andexanet/placebo until 14.5 to 16.5 hours post-andexanet/placebo. For determining apixaban and andexanet concentration, urine was collected on days 5 and 6. Subjects were observed in-house for 13 days followed by outpatient follow-up for safety and PD markers through day 48. Blood samples for PD and PK endpoints were collected into 3.2% trisodium citrate tubes and stored at −70°C for analysis in centralized laboratories.
The studies were conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice and the Declaration of Helsinki. For the phase 2 study, safety was monitored by an Independent Safety Committee comprising clinicians, including coagulation/thrombosis specialists, and a statistician, who generated unblinded data outputs for the committee.
Bioanalytical methods
Plasma and urine drug concentrations were measured using validated electrochemiluminescent (andexanet; BioAgilytix Labs, Durham, NC) or liquid chromatography-mass spectrometry (apixaban; Alturas Analytics, Inc, Moscow, ID) methods as described previously.19 The lower limit of quantitation for plasma andexanet concentrations was 12.0 ng/mL. The unbound fraction of apixaban was separated by an HTD96b high-throughput rapid equilibrium microdialysis device (HTDialysis, Gales Ferry, CT) and quantified by a validated high-performance liquid chromatography/mass spectrometry method at a central laboratory. Total plasma concentration of apixaban was quantified by high-performance liquid chromatography/mass spectrometry (lower limit of detection was 0.100 ng/mL) following solid-liquid extraction using the same validated method.
Pharmacokinetic parameters
Andexanet plasma PK parameters were calculated using a noncompartmental IV infusion model (Phoenix WinNonlin Version 6.3). PK parameters included half-life (t1/2; distribution and terminal), time to maximum plasma concentration (tmax), maximum plasma concentration (Cmax), area under the plasma concentration-time curve (AUC), systemic clearance (CL), volume of distribution (Vd), volume of distribution at steady state (Vss), and elimination rate constant.
Pharmacodynamic parameters
The PD effects of apixaban and andexanet were assessed by evaluation of anti-FXa activity, thrombin generation, prothrombin time (PT), activated partial thromboplastin time (aPTT), and activated clotting time (ACT).
A modified anti-FXa activity chromogenic assay was performed as described previously.17 Briefly, the assay was adapted from a commercial kit (Coamatic Heparin; DiaPharma, West Chester, OH) with modifications of plasma volume (75 µL), the reaction incubation conditions (5 minutes at room temperature), and the volume of exogenously added bovine FXa (25 µL). The apixaban reference standard (Bristol-Myers Squibb, New Brunswick, NJ) was prepared daily in pooled human plasma for standard curves and controls.
The thrombin generation assay was adapted from the protocol used in the phase 2 EXPERT and EXPLORE-Xa trials.22,23 Plasma volume was 75 μL in a total 100 μL volume of reaction mixture, as previously described.17 Thrombin generation was measured by addition of 0.1 nM tissue factor (Innovin, SIEMENS) and a fluorogenic substrate. The mean relative fluorescence units (RFU) at the 10-minute time point following initiation of the reaction were used for calculations. The RFU readout is equivalent to the area under the thrombin generation curve (in RFU).
ACT was tested locally using Hemochron Jr Signature Elite coagulation device according to the manufacturer’s instructions, using ACT+ cuvette and whole blood. Plasma PT and aPTT were analyzed using standard assay methodology at a local clinical laboratory.
Safety
Safety assessments in both studies included standard laboratory blood and urine tests, physical examinations, vital signs, and 12-lead ECGs. Assessments were performed at screening or admission, preandexanet dosing, immediately after andexanet dosing, and during follow-up visits until study termination. Testing for antibodies to andexanet, factor X, and FXa used a tiered approach of screening, confirmation, and titer assessment. To assess immunogenicity, blood samples for anti-andexanet, anti-factor X, and anti-FXa antibodies were collected on days 1, 20, 34, and 48 and were assessed in a central laboratory using a validated bridging immunoassay based on electrochemical luminescence. For any sample that tested positive for antibody the potential for neutralizing antibody activity was assessed by measuring the functional activity of andexanet in plasma. Adverse events (AEs) were coded with Medical Dictionary for Regulatory Activities (MedDRA) Version 15.0.
Coagulation markers were analyzed in a central laboratory using commercial kits. D-dimer was measured using the STA-Liatest D-Di assay from Diagnostica Stago; F1+2 was measured using the Enzygost monoclonal assay from Siemens Healthcare Diagnostics.
Statistical analysis
PK parameters were computed using Phoenix WinNonlin Version 6.3 (or higher) for phase 2 and Version 5.2 (or higher) for phase 1. For PK data, descriptive statistics included summary statistics mean and standard deviation (SD), or standard error of the mean, median and maximum/minimum, and coefficient of variation (%). Geometric mean and geometric coefficient of variation (%) were calculated for AUCs and Cmax. Pre-apixaban baseline values were calculated the day prior to and the day of apixaban administration. Pre-andexanet baseline values were obtained on day 6, 3 hours following apixaban administration. Analysis of covariance was used to compare results between groups inferentially after adjusting for baseline values. The least-square means (adjusted means) were reported for inferential comparisons. The paired Student t test was used for within group comparison. Statistical summaries were performed using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
Results
Baseline characteristics and disposition
The phase 1 study enrolled a total of 32 healthy volunteers, 8 in each of the 4 dosing cohorts (andexanet, n = 24; placebo, n = 8). All 32 subjects completed the study and were included in the PK and safety analyses. The demographics are summarized in Table 1.
Summary of subject demographics at baseline
| . | Phase 1 (N = 32) . | Phase 2 (N = 54) . |
|---|---|---|
| Sex, n (%) | ||
| Female | 17 (53) | 8 (15) |
| Male | 15 (47) | 46 (85) |
| Race,*n (%) | ||
| American Indian/Alaska native | 1 (3) | 0 |
| Black or African American | 2 (6) | 2 (4) |
| White | 26 (81) | 52 (96) |
| Other | 3 (9) | 0 |
| Hispanic or Latino ethnicity | 22 (69) | 43 (80) |
| Median age, y (range) | 35.0 (20-50) | 33 (19-44) |
| Median weight, kg (range) | 76 (64-109) | 77 (62-96) |
| Median height, cm (range) | 167 (150-196) | 170 (150-194) |
| Median BMI, kg/m2 (range) | 28.6 (22.2-31.9) | 27.2 (19.5-30.0) |
| . | Phase 1 (N = 32) . | Phase 2 (N = 54) . |
|---|---|---|
| Sex, n (%) | ||
| Female | 17 (53) | 8 (15) |
| Male | 15 (47) | 46 (85) |
| Race,*n (%) | ||
| American Indian/Alaska native | 1 (3) | 0 |
| Black or African American | 2 (6) | 2 (4) |
| White | 26 (81) | 52 (96) |
| Other | 3 (9) | 0 |
| Hispanic or Latino ethnicity | 22 (69) | 43 (80) |
| Median age, y (range) | 35.0 (20-50) | 33 (19-44) |
| Median weight, kg (range) | 76 (64-109) | 77 (62-96) |
| Median height, cm (range) | 167 (150-196) | 170 (150-194) |
| Median BMI, kg/m2 (range) | 28.6 (22.2-31.9) | 27.2 (19.5-30.0) |
BMI, body mass index.
Subjects were allowed to report multiple races at screening.
The phase 2 study enrolled a total of 54 healthy subjects (9 subjects in each of the 6 dosing cohorts) all of whom received apixaban 5 mg orally every 12 hours for 6 days followed by andexanet (total doses ranging from 90 to 900 mg, n = 36) or placebo (n = 18) 3 hours after the last dose of apixaban. All 54 subjects completed the study and were included in the PK, PD, and safety analyses (Table 1).
Pharmacokinetics
Phase 1.
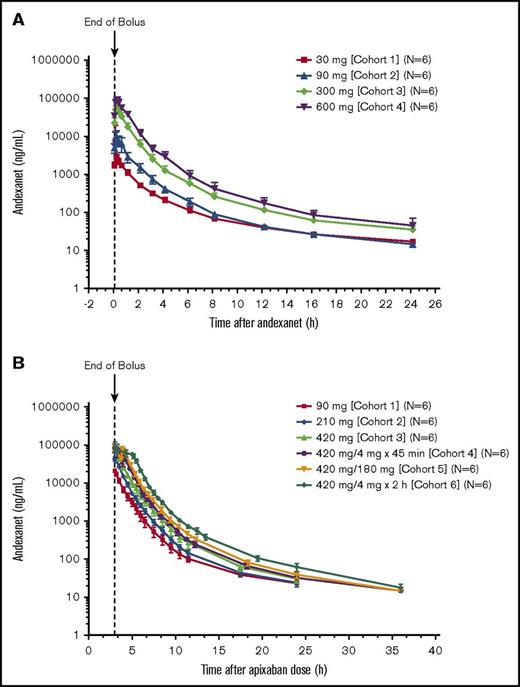
The Cmax of andexanet alone increased relatively proportionally with increasing doses (Figure 1A). At dose levels >30 mg, exposure (AUC) values also increased proportionally, and the mean volume of distribution decreased with increasing doses (Table 2). The mean terminal elimination t1/2 and CL were similar following all andexanet doses. Mean Vd and mean Vss decreased with increasing doses of andexanet. Andexanet concentrations in urine were below the limit of quantitation, and therefore, no urine PK parameters for andexanet could be calculated.
Plasma concentrations of andexanet. Plasma concentrations of andexanet alone (phase 1) (A) and in the presence of apixaban (phase 2) (B). Values are means ± SD.
Plasma concentrations of andexanet. Plasma concentrations of andexanet alone (phase 1) (A) and in the presence of apixaban (phase 2) (B). Values are means ± SD.
Andexanet PK parameters in the absence of apixaban (phase 1 study)
| Pharmacokinetic parameters (mean ± SD) . | 30 mg (n = 6) . | 90 mg (n = 6) . | 300 mg (n = 6) . | 600 mg (n = 6) . |
|---|---|---|---|---|
| Cmax, ng/mL | 3080 ± 389 | 10 800 ± 4 110 | 52 800 ± 13 200 | 93 300 ± 14 200 |
| tmax,* h | 0.21 (0.17, 0.29) | 0.23 (0.18, 0.29) | 0.26 (0.17, 0.34) | 0.51 (0.42, 0.52) |
| AUC0-last, ng*h/mL | 4510 ± 472 | 12 500 ± 3 470 | 61 000 ± 15 400 | 117 000 ± 17 400 |
| AUC0-∞, ng*h/mL | 4710 ± 508 | 12 600 ± 3 450 | 61 400 ± 15 400 | 118 000 ± 17 300 |
| t1/2, h | 7.25 ± 2.14 | 7.38 ± 2.21 | 7.46 ± 1.58 | 6.40 ± 1.88 |
| lz, 1/h | 0.10 ± 0.03 | 0.11 ± 0.06 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| CL, L/h | 6.30 ± 0.68 | 6.24 ± 0.92 | 5.13 ± 1.16 | 5.18 ± 0.64 |
| Vd, L | 65.27 ± 17.61 | 67.29 ± 23.05 | 55.39 ± 19.89 | 48.49 ± 18.46 |
| Vss, L | 24.95 ± 5.58 | 14.80 ± 4.16 | 8.27 ± 2.40 | 7.82 ± 1.81 |
| Pharmacokinetic parameters (mean ± SD) . | 30 mg (n = 6) . | 90 mg (n = 6) . | 300 mg (n = 6) . | 600 mg (n = 6) . |
|---|---|---|---|---|
| Cmax, ng/mL | 3080 ± 389 | 10 800 ± 4 110 | 52 800 ± 13 200 | 93 300 ± 14 200 |
| tmax,* h | 0.21 (0.17, 0.29) | 0.23 (0.18, 0.29) | 0.26 (0.17, 0.34) | 0.51 (0.42, 0.52) |
| AUC0-last, ng*h/mL | 4510 ± 472 | 12 500 ± 3 470 | 61 000 ± 15 400 | 117 000 ± 17 400 |
| AUC0-∞, ng*h/mL | 4710 ± 508 | 12 600 ± 3 450 | 61 400 ± 15 400 | 118 000 ± 17 300 |
| t1/2, h | 7.25 ± 2.14 | 7.38 ± 2.21 | 7.46 ± 1.58 | 6.40 ± 1.88 |
| lz, 1/h | 0.10 ± 0.03 | 0.11 ± 0.06 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| CL, L/h | 6.30 ± 0.68 | 6.24 ± 0.92 | 5.13 ± 1.16 | 5.18 ± 0.64 |
| Vd, L | 65.27 ± 17.61 | 67.29 ± 23.05 | 55.39 ± 19.89 | 48.49 ± 18.46 |
| Vss, L | 24.95 ± 5.58 | 14.80 ± 4.16 | 8.27 ± 2.40 | 7.82 ± 1.81 |
tmax is presented as median (minimum, maximum).
Phase 2.
PK parameters of andexanet administered in the presence of apixaban were similar to those measured for andexanet alone. Plasma concentrations of andexanet increased proportionally with increasing doses (Figure 1B). The mean (SD) distribution t1/2 of andexanet ranged from 0.3 (0.3) hour to 0.6 (0.1) hour across dosages in cohorts 1 to 6 (Table 3). The mean terminal elimination t1/2 and CL values were similar between andexanet doses, and mean Vss decreased with increasing doses of andexanet. Andexanet was not detected in urine samples.
Andexanet PK parameters in the presence of apixaban (phase 2 study)
| Pharmacokinetic parameters (mean ± SD) . | Andexanet dose (n = 6/cohort) . | |||||
|---|---|---|---|---|---|---|
| 90 mg bolus (cohort 1) . | 210 mg bolus (cohort 2) . | 420 mg bolus (cohort 3) . | 420/4 × 45 min (cohort 4) . | 420/180 (cohort 5) . | 420/4 × 2 h (cohort 6) . | |
| Cmax, ng/mL | 21 200 ± 2 400 | 42 800 ± 5 620 | 81 400 ± 10 900 | 93 300 ± 18 400 | 88 000 ± 9 010 | 90 800 ± 29 600 |
| tmax,* h | 0.07 (0.07, 0.08) | 0.15 (0.14, 0.30) | 0.27 (0.26, 0.29) | 0.43 (0.25, 0.52) | 0.27 (0.26, 0.31) | 0.28 (0.272, 0.29) |
| AUC0-last, ng*h/mL | 23 200 ± 3 490 | 49 100 ± 3 980 | 93 400 ± 15 500 | 131 000 ± 20 500 | 155 000 ± 16 200 | 213 000 ± 33 900 |
| AUC0-∞, ng*h/mL | 23 400 ± 3 460 | 49 200 ± 3 960 | 93 500 ± 15 500 | 131 000 ± 20 500 | 155 000 ± 16 200 | 213 000 ± 33 900 |
| t1/2, h | 5.51 ± 0.84 | 5.12 ± 1.66 | 5.02 ± 2.02 | 4.35 ± 1.40 | 5.79 ± 2.00 | 6.45 ± 0.41 |
| lz, 1/h | 0.13 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.05 | 0.17 ± 0.04 | 0.14 ± 0.06 | 0.11 ± 0.01 |
| CL, L/h | 3.93 ± 0.63 | 4.29 ± 0.33 | 4.60 ± 0.81 | 4.70 ± 0.89 | ND | 4.30 ± 0.61 |
| Vss, L | 6.97 ± 1.42 | 6.18 ± 1.31 | 6.26 ± 0.74 | 5.11 ± 1.64 | ND | 4.01 ± 0.95 |
| Pharmacokinetic parameters (mean ± SD) . | Andexanet dose (n = 6/cohort) . | |||||
|---|---|---|---|---|---|---|
| 90 mg bolus (cohort 1) . | 210 mg bolus (cohort 2) . | 420 mg bolus (cohort 3) . | 420/4 × 45 min (cohort 4) . | 420/180 (cohort 5) . | 420/4 × 2 h (cohort 6) . | |
| Cmax, ng/mL | 21 200 ± 2 400 | 42 800 ± 5 620 | 81 400 ± 10 900 | 93 300 ± 18 400 | 88 000 ± 9 010 | 90 800 ± 29 600 |
| tmax,* h | 0.07 (0.07, 0.08) | 0.15 (0.14, 0.30) | 0.27 (0.26, 0.29) | 0.43 (0.25, 0.52) | 0.27 (0.26, 0.31) | 0.28 (0.272, 0.29) |
| AUC0-last, ng*h/mL | 23 200 ± 3 490 | 49 100 ± 3 980 | 93 400 ± 15 500 | 131 000 ± 20 500 | 155 000 ± 16 200 | 213 000 ± 33 900 |
| AUC0-∞, ng*h/mL | 23 400 ± 3 460 | 49 200 ± 3 960 | 93 500 ± 15 500 | 131 000 ± 20 500 | 155 000 ± 16 200 | 213 000 ± 33 900 |
| t1/2, h | 5.51 ± 0.84 | 5.12 ± 1.66 | 5.02 ± 2.02 | 4.35 ± 1.40 | 5.79 ± 2.00 | 6.45 ± 0.41 |
| lz, 1/h | 0.13 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.05 | 0.17 ± 0.04 | 0.14 ± 0.06 | 0.11 ± 0.01 |
| CL, L/h | 3.93 ± 0.63 | 4.29 ± 0.33 | 4.60 ± 0.81 | 4.70 ± 0.89 | ND | 4.30 ± 0.61 |
| Vss, L | 6.97 ± 1.42 | 6.18 ± 1.31 | 6.26 ± 0.74 | 5.11 ± 1.64 | ND | 4.01 ± 0.95 |
ND, not determined.
tmax is presented as median (minimum, maximum); time relative to the start of the first andexanet bolus dose.
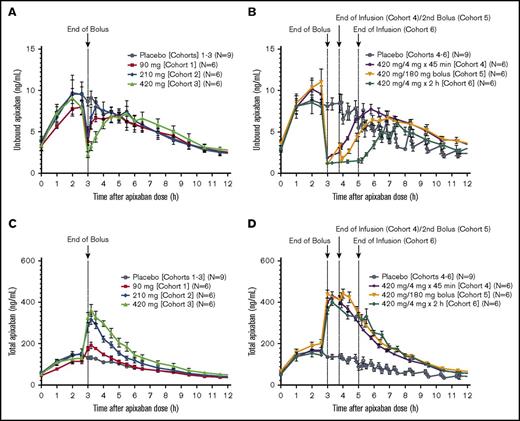
Andexanet administration reduced the unbound (pharmacologically active) apixaban plasma concentration (up to ∼90% decrease) in a dose-dependent manner within 2 minutes after the initial bolus dose for all cohorts (Figure 2A-B). The lowest concentration of unbound apixaban (2.25-1.10 ng/mL) was achieved after administration of a 420-mg andexanet bolus in cohorts 3 to 6 (supplemental Table 1). In contrast, in the placebo group, unbound apixaban concentration remained essentially unchanged at the end of the bolus dose. Administration of andexanet as a 420-mg bolus followed by a continuous 2-hour infusion (cohort 6) resulted in a sustained decrease (3 hours) in unbound apixaban concentrations (P < .05 vs pooled, cohorts 1-6 placebo groups; Figure 2B). At the end of the bolus or infusion of andexanet, the unbound apixaban concentrations gradually returned to placebo levels within 0.17 to 3.5 hours, depending on andexanet concentration (Figure 2A-B). Coincident with this decrease in the unbound fraction, the total apixaban concentration increased up to approximately threefold within 2 minutes after andexanet administration (Figure 2C-D; supplemental Table 1).
Apixaban unbound and total concentrations. Plasma concentrations of unbound apixaban before and after andexanet administration in cohorts 1 to 3 (A) and 4 to 6 (B), and total plasma concentration of apixaban in cohorts 1 to 3 (C) and 4 to 6 (D) of the phase 2 study. Values are means ± standard error of the mean.
Apixaban unbound and total concentrations. Plasma concentrations of unbound apixaban before and after andexanet administration in cohorts 1 to 3 (A) and 4 to 6 (B), and total plasma concentration of apixaban in cohorts 1 to 3 (C) and 4 to 6 (D) of the phase 2 study. Values are means ± standard error of the mean.
Pharmacodynamics
Reversal of anti-FXa activity.
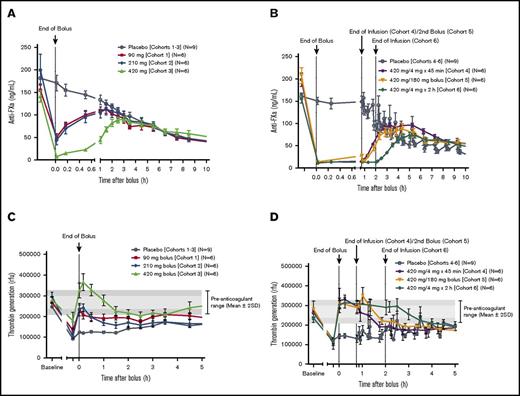
In the phase 2 study, apixaban was dosed to steady state over 5.5 days. Andexanet was administered such that the end of the bolus dose ended at 3 hours after the last dose of apixaban (Cmax). Compared with placebo, administration of andexanet resulted in a rapid (ie, within 2 minutes after the end of the bolus dose) and significant decrease in anti-FXa activity relative to the pre-andexanet/placebo baseline (all P < .05; Figure 3A-B). Both the magnitude and the duration of anti-FXa activity reversal were dose and regimen dependent. The greatest effect of andexanet on anti-FXa activity (92.8%-95.0% decrease relative to baseline; P < .05) was observed within 2 minutes after the end of the 420-mg bolus (cohorts 3-6). In comparison, in the pooled placebo group, the mean (SD) anti-FXa activity declined by 7.1% (10.8%). Following treatment with andexanet, the anti-FXa activity returned gradually to placebo levels (earliest time point at which P > .05 vs pooled placebo group) after 1 to 2.5 hours in cohorts 1 to 3, and after 3.3 to 4.3 hours in cohorts 4 to 6.
Anti-FXa activity and thrombin generation. Anti-FXa activity in cohorts 1 to 3 (A) and cohorts 4 to 6 (B). Thrombin generation in cohorts 1 to 3 (C) and cohorts 4 to 6 of the phase 2 study (D). All subjects had achieved steady-state concentrations of apixaban (5 mg twice a day) on day 6 prior to administration of andexanet. Values are means ± standard error of the mean. The pre-anticoagulant ranges on day 1 for thrombin generation are shown as mean ± 1 SD (light gray) and mean ± 2 SD (dark gray), respectively.
Anti-FXa activity and thrombin generation. Anti-FXa activity in cohorts 1 to 3 (A) and cohorts 4 to 6 (B). Thrombin generation in cohorts 1 to 3 (C) and cohorts 4 to 6 of the phase 2 study (D). All subjects had achieved steady-state concentrations of apixaban (5 mg twice a day) on day 6 prior to administration of andexanet. Values are means ± standard error of the mean. The pre-anticoagulant ranges on day 1 for thrombin generation are shown as mean ± 1 SD (light gray) and mean ± 2 SD (dark gray), respectively.
When a second bolus of andexanet (180 mg) was administered 45 minutes after the end of the first bolus (cohort 5), anti-FXa activity was reduced to levels similar to those measured after the first bolus (Figure 3B). Administration of andexanet as a 420-mg bolus followed by either a 180-mg continuous 45-minute infusion bolus (cohort 4) or a 480-mg continuous 2-hour infusion (cohort 6) resulted in a sustained decrease in anti-FXa activity (3.5 to 3.75 hours; P < .05 vs pooled placebo group). A 420-mg bolus dose of andexanet achieved >90% reduction in anti-FXa activity, which equates to a stoichiometric molar ratio of andexanet to total apixaban of ∼2.3:1 to 2.9:1.
Restoration of thrombin generation.
Thrombin generation decreased following apixaban administration and was restored to baseline (ie, within 1 SD of study population mean pre-apixaban level) within 2 minutes of the andexanet bolus, in a dose-dependent manner (Figure 3C-D). Thrombin generation was restored in 67% of subjects who received a 90-mg bolus (cohort 1), 83% of subjects who received a 210-mg bolus (cohort 2), 100% of subjects who received a 420-mg bolus (cohort 3), 83% of subjects who received a 420-mg bolus followed by a second 180-mg bolus (cohort 5), and 100% of subjects who received a 420-mg bolus followed by a 45-minute or 2-hour infusion (cohorts 4 and 6, respectively). In contrast, only 6% of pooled-placebo subjects showed restoration of thrombin generation at the end of bolus (all P < .01 for the difference from placebo).
When the 420-mg bolus was followed by a continuous 2-hour infusion (cohort 6), a prolonged, sustained restoration of thrombin generation, extending for 3 hours following the infusion, was observed (Figure 3D). Thrombin generation increased above baseline following a 420-mg bolus of andexanet (cohort 3), but not following a 420-mg bolus plus infusion (cohorts 4 and 6), and returned to baseline levels within 30 minutes (Figure 3C-D). A 420-mg bolus dose of andexanet achieved normalization of thrombin generation, which equates to a stoichiometric molar ratio of andexanet to total apixaban of approximately >2:1.
PT, aPTT, ACT.
Apixaban had no detectable effect on PT and aPTT levels (supplemental Table 2). There was a modest but not significant (up to ∼31%) dose-dependent decrease in mean ACT values at 2 minutes following the end of bolus dose in all andexanet cohorts relative to just prior to andexanet dosing (supplemental Table 2). In the placebo group, there was a slight decrease (∼3%) in mean ACT levels at the end of the bolus dose.
Safety
All subjects in the phase 1 and phase 2 studies completed the assigned treatment and follow-up as planned. Andexanet was generally well tolerated. The most frequently reported AEs were general disorders and administration site conditions (Table 4). No deaths, severe or life-threatening AEs, or thrombotic events were reported.
Summary of AEs
| MedDRA system organ class, n (%)* . | Phase 1 . | Phase 2 . | ||
|---|---|---|---|---|
| Andexanet alfa (n = 24) . | Placebo (n = 8) . | Andexanet alfa (n = 36) . | Placebo (n = 18) . | |
| Any AE | 13 (54) | 4 (50) | 27 (75) | 15 (83) |
| Gastrointestinal disorders | 3 (13) | 0 | 1 (3) | 2 (11) |
| General disorders and administration site conditions | 6 (25) | 2 (25) | 5 (14) | 4 (22) |
| Infections and infestations | 1 (4) | 1 (13) | 1 (3) | 0 |
| Injury, poisoning, and procedural complications | 3 (13) | 1 (13) | 7 (19) | 8 (44) |
| Musculoskeletal and connective tissue disorders | 2 (8) | 0 | 5 (14) | 2 (11) |
| Nervous system disorders | 5 (21) | 0 | 7 (19) | 3 (17) |
| Pregnancy, puerperium, and perinatal conditions | 1 (4) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 0 | 0 | 2 (6) | 5 (28) |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 3 (8) | 0 |
| Eye disorders | 0 | 0 | 1 (3) | 0 |
| Cardiac disorders | 0 | 0 | 0 | 1 (6) |
| MedDRA system organ class, n (%)* . | Phase 1 . | Phase 2 . | ||
|---|---|---|---|---|
| Andexanet alfa (n = 24) . | Placebo (n = 8) . | Andexanet alfa (n = 36) . | Placebo (n = 18) . | |
| Any AE | 13 (54) | 4 (50) | 27 (75) | 15 (83) |
| Gastrointestinal disorders | 3 (13) | 0 | 1 (3) | 2 (11) |
| General disorders and administration site conditions | 6 (25) | 2 (25) | 5 (14) | 4 (22) |
| Infections and infestations | 1 (4) | 1 (13) | 1 (3) | 0 |
| Injury, poisoning, and procedural complications | 3 (13) | 1 (13) | 7 (19) | 8 (44) |
| Musculoskeletal and connective tissue disorders | 2 (8) | 0 | 5 (14) | 2 (11) |
| Nervous system disorders | 5 (21) | 0 | 7 (19) | 3 (17) |
| Pregnancy, puerperium, and perinatal conditions | 1 (4) | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 0 | 0 | 2 (6) | 5 (28) |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 3 (8) | 0 |
| Eye disorders | 0 | 0 | 1 (3) | 0 |
| Cardiac disorders | 0 | 0 | 0 | 1 (6) |
Classified according to Version 15.0.
In the phase 1 study, 1 moderate serious AE (bilateral pneumonia) was reported 21 days after the 30-mg andexanet dose and was considered unrelated/unlikely related to the study drug. Another subject who received 600 mg andexanet experienced a spontaneous abortion considered possibly/probably related to the study drug. Serum pregnancy tests were negative for this subject at screening and predose. The subject’s pregnancy test was positive on day 28 follow-up visit; the pregnancy test was negative ∼2 months after the day 28 visit. Two subjects who received 90 mg andexanet required discontinuation due to infusion-related reactions (including diaphoresis, palpitations, retrosternal tightness, facial flushing, sensation of warmth, decreased breath sounds, lower back pain, sensation of pulsating lower back, and solitary cough); 2 reactions were moderate and all other reactions were mild. One subject who received 600 mg andexanet had the study drug infusion rate decreased due to mild infusion-related reactions (diaphoresis, dyspnea, facial flushing, and facial paresthesia); the study drug infusion was decreased from 10 to 5 mL/min.
There were no serious AEs reported in the phase 2 study. The incidence of infusion reactions was 19.4% for andexanet (n = 7/36) and 11% for placebo (n = 2/18). The most frequently reported events associated with infusion reactions included facial flushing, nonproductive cough, dyspnea, and abnormal taste during infusion. All reactions were mild and classified as treatment related. One subject received symptomatic treatment with diphenhydramine. Infusion reactions were reported across the dose range without an apparent dose-dependent effect. The study drug was briefly discontinued in 1 subject (cohort 3) due to mild AEs (diaphoresis, solitary cough, dyspnea, flushing, and sensation of warmth), which began 2 minutes following treatment initiation and resolved 2 minutes after onset. Study drug infusion restarted at the original infusion rate; no symptoms returned following reinitiation of the infusion, and the entire dose was administered.
In both phase 1 and 2 studies, there were no abnormalities observed in the safety assessments for vital signs, ECGs, physical examinations, or trends in laboratory results.
No antibodies against factor X or FXa, or neutralizing antibodies to andexanet, were detected. Two subjects in the phase 2 study exhibited a low-titer nonneutralizing antibody to andexanet on days 1 (1:20 titer in 1 subject), 20 (1:20 and 1:10 titers), and 34 (1:20 and 1:40 titers) that resolved by day 48.
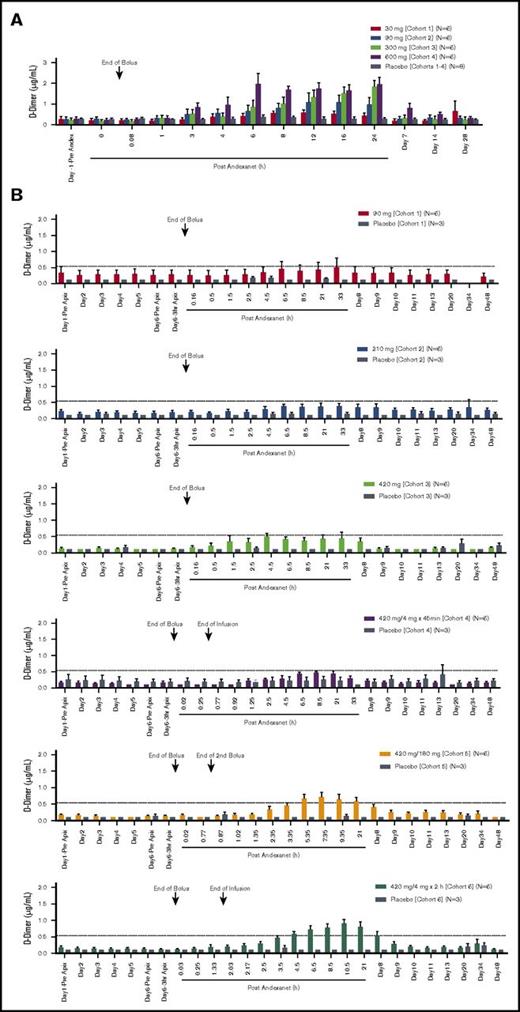
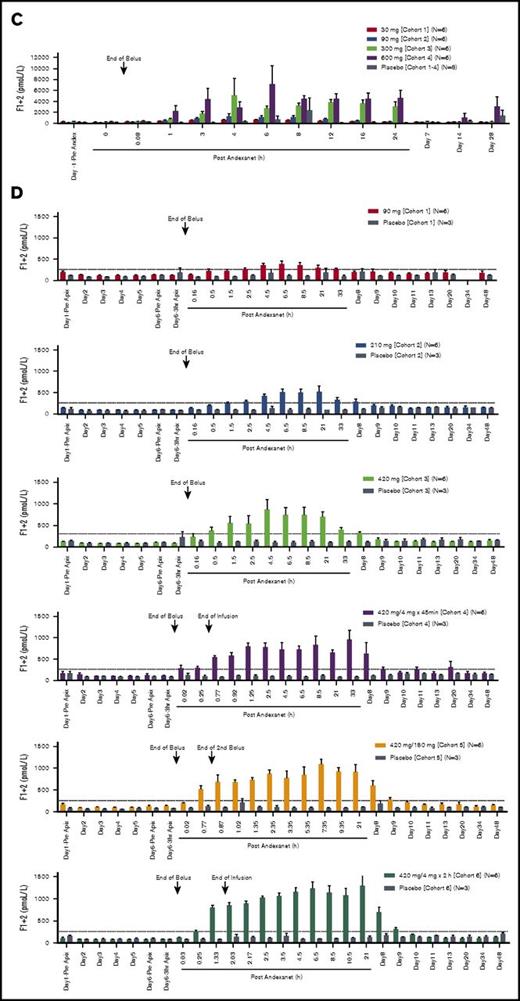
There were dose-dependent transient increases in D-dimer and prothrombin fragment 1+2 levels (F1+2) following andexanet administration (Figure 4). In the phase 1 study, the highest mean D-dimer value (1.97 µg/mL) was seen with the 600-mg dose, and the highest mean F1+2 levels (5157 pmol/L and 7130 pmol/L) occurred with the 300-mg and 600-mg doses, respectively (Figure 4A-C). D-dimer and F1+2 elevations were also noted in the phase 2 study where maximum increases in D-dimer and F1+2 occurred within 36 hours and 24 hours, respectively, after andexanet infusion (Figure 4B-D). The greatest mean D-dimer and F1+2 values (0.91 µg/mL and 1230 pmol/L, respectively) were observed in patients in cohort 6, who received the highest total dose (bolus plus infusion) of andexanet. Notably, when andexanet was administered in the presence of apixaban (phase 2 study), elevations in D-dimer and F1+2 were lower than those observed in patients treated with andexanet alone (phase 1 study) (compare Figure 4A with B and Figure 4B with C).
D-dimer and F1+2 levels for andexanet. D-dimer levels for andexanet alone (phase 1) (A) and in the presence of apixaban (phase 2) (B). F1+2 levels for andexanet alone (phase 1) (C) and in the presence of apixaban (phase 2) (D). Values are means ± standard error of the mean. Dashed lines represent the pre-anticoagulant ranges on day 1 (mean ± 2 SD) for D-dimer and F1+2, respectively.
D-dimer and F1+2 levels for andexanet. D-dimer levels for andexanet alone (phase 1) (A) and in the presence of apixaban (phase 2) (B). F1+2 levels for andexanet alone (phase 1) (C) and in the presence of apixaban (phase 2) (D). Values are means ± standard error of the mean. Dashed lines represent the pre-anticoagulant ranges on day 1 (mean ± 2 SD) for D-dimer and F1+2, respectively.
Discussion
This is the first report of the phase 1 and phase 2 safety and dose-escalation studies of andexanet, a putative FXa inhibitor reversal agent, evaluating the PK and PD properties of andexanet in healthy volunteers. The PK parameters of andexanet in the absence (phase 1 study) or in the presence (phase 2 study) of apixaban therapy were not significantly different. Andexanet dose dependently increased total apixaban and decreased unbound apixaban plasma concentrations. These effects of andexanet are believed to be due to drug redistribution resulting from high affinity binding of apixaban to andexanet in the central compartment.
The phase 2 study evaluated the effect of several andexanet doses on apixaban-induced changes in hemostatic measures in healthy volunteers. Andexanet rapidly (ie, within 2 minutes) and dose dependently reversed the effects of apixaban on anti-FXa activity and restored thrombin generation to pre-anticoagulant levels. The greatest effect of andexanet on PD markers was observed following the 420-mg bolus, which yielded >90% reduction in anti-FXa activity and normalization of thrombin generation. As andexanet was eliminated, both anti-FXa activity and thrombin generation returned gradually (within 1-2 hours) to placebo levels. Consistent with its mechanism of action, andexanet rapidly and dose dependently reduced unbound apixaban concentration. These results from the phase 2 study established that the molar ratio of andexanet to total apixaban required for reversal of the anticoagulant effects was ≥1.3:1, which is consistent with the results from the animal model studies.17
In the phase 2 study, for subjects who received a second bolus of andexanet 45 minutes after the first bolus, the second bolus reversed apixaban-induced anti-FXa activity and inhibition of thrombin generation to the same extent as the initial bolus. Importantly, in subjects who received continuous infusions after the initial bolus, the effectiveness of andexanet was sustained throughout the duration of the infusion.
Andexanet was well tolerated with the majority of AEs characterized as mild and related to reactions at administration sites. No subjects withdrew from the studies due to AEs. Levels of D-dimer and F1+2 increased transiently following andexanet administration, without evidence of thrombotic events. The mechanism for these elevations may be related to the binding of andexanet to tissue factor pathway inhibitor (TFPI; an endogenous inhibitor of FXa) in a manner analogous to its binding to FXa.24,25 The elevations in D-dimer and F1+2 were lower when andexanet was administered in the presence of apixaban (phase 2) vs when andexanet was given alone (phase 1); reduction in the binding of andexanet to TFPI may be expected in the presence of an FXa inhibitor (apixaban). Furthermore, clinical trials of concizumab, a TFPI-binding antibody, in healthy volunteers and patients with hemophilia also showed elevations in D-dimer and F1+2 without evidence of thrombotic events.26 These observations suggest that the elevations in these markers may reflect the hemostasis of endogenous coagulation and fibrinolytic activities and may not necessarily be associated with the development of thrombotic events. In support of the hypothesis that binding of andexanet to TFPI does not present a major safety concern, there was no evidence of thrombotic events attributed to andexanet in the preclinical toxicology studies in monkeys18 or in clinical phase 3 studies in healthy volunteers.19
The design and results of the phase 2 study reported here informed the design of the phase 3 studies that evaluated the ability of andexanet to reverse apixaban and rivaroxaban anticoagulation in older healthy subjects.19 In the phase 3 study with apixaban, healthy subjects received andexanet IV bolus (400 mg) or andexanet bolus + 2-hour infusion (400 mg plus a continuous infusion of 4 mg per minute). Compared with placebo, andexanet rapidly reversed anticoagulation biomarkers (anti-FXa activity and unbound FXa concentration). In the ongoing, prospective, open-label, ANNEXA-4 study in patients anticoagulated with an FXa inhibitor and presenting with acute major bleeding, andexanet was dosed as a 15- to 30-minute bolus followed by a 2-hour infusion.20 For patients who had received enoxaparin, edoxaban, or rivaroxaban, the bolus dose was 800 mg and the infusion dose was 960 mg. For patients who had received apixaban, the bolus dose of andexanet was 400 mg and the infusion dose was 480 mg. In an interim analysis from ANNEXA-4, anti-FXa activity was reduced by 89% in patients receiving rivaroxaban and by 93% in patients receiving apixaban; 79% of patients were adjudicated to have good or excellent hemostasis 12 hours following andexanet infusion.20 The interim safety results showed 18% of patients experienced thrombotic events, and 27% had resumed anticoagulation therapy within 30 days.20 In an updated analysis, the rate of thrombotic events was 12% and 40% of patients had resumed anticoagulation.21
Some limitations of these studies include sample sizes and inclusion of young, healthy study participants who may not reflect the population for whom FXa inhibitors are prescribed. The phase 2 study was designed to evaluate apixaban reversal based on anti-FXa activity and thrombin generation assays confirming the mechanism of action of andexanet in humans; the effect of andexanet on bleeding outcomes was not assessed. An ongoing ANNEXA-4 study is evaluating the clinical efficacy and safety of andexanet in patients receiving an FXa inhibitor who have acute major bleeding, including biomarkers for measuring the reversal of FXa inhibitors.20,21
There are currently no approved agents to specifically reverse the anticoagulant activity of FXa inhibitors. Therefore, plasma, prothrombin complex concentrates (PCCs), activated PCC (FEIBA), and recombinant factor VIIa, which were developed for warfarin reversal or bleeding in hemophilia patients, have been included in treatment guidelines for nonspecific reversal of anticoagulation of FXa inhibitors if a specific antidote is not available or sufficient.27 The clinical efficacy and safety of these agents have not yet been established in patients with FXa inhibitor–associated bleeding,28-31 and current treatment guidelines recommend weighing the potential prothrombotic effects of PCCs against the potential benefits.32-34 As a reversal agent with a specific mechanism of action, andexanet is a catalytically inactive FXa decoy protein that specifically binds and sequesters FXa inhibitors, enabling restoration of normal coagulation mechanisms. As reported here and in the phase 3 studies in healthy volunteers, andexanet reversed the anticoagulant activity of rivaroxaban and apixaban (as measured by anti-FXa activity, unbound anticoagulant concentration, and thrombin generation).17,19 The preliminary results of the ongoing ANNEXA-4 study in patients who experience acute, potentially life-threatening bleeding showed potential clinical efficacy of andexanet, and the clinical safety profile of andexanet will continue to be assessed after completion of the study.20,21
The full-text version of this article contains a data supplement.
Acknowledgments
Editorial assistance was provided by Iwona Bucior, from Portola Pharmaceuticals, Inc.
This study was funded by Portola Pharmaceuticals, Inc.
Authorship
Contribution: S.J.H., J.T.C., P.B.C., G.L., and M.C. shared in the duties of study concept and design; and all authors analyzed and interpreted the data and drafted and revised the manuscript for important intellectual content and approval of the submission draft.
Conflict-of-interest disclosure: G.L., J.M.L., M. Karbarz, J.C., J.T.C., and P.B.C. are current employees of Portola Pharmaceutical. V.M., A.H., U.S., M. Kitt, M.M., and S.J.H. are past employees of Portola Pharmaceuticals. D.S. has attended advisory boards for Boerhinger Ingelheim, Daiichi-Sankyo, Servier, and Portola Pharmaceuticals and has received honoraria from Bristol-Myers Squibb–Pfizer and Bayer. M.C. has attended advisory boards for Janssen, Leo Pharma, Portola Pharmaceuticals, and Asahi Kasei Pharma America and has received funding for presentations from Leo Pharma, Bayer, Celgene, Shire, and CSL Behring. M.C.’s institution has received funding for research projects from Leo Pharma.
Correspondence: Mark Crowther, Department of Medicine and Department of Pathology and Molecular Medicine, McMaster University and St. Joseph's Healthcare, 2N16 Health Sciences Centre, 50 Charlton Ave, Hamilton, ON L8N 4A6, Canada; e-mail: crowthrm@mcmaster.ca.