TO THE EDITOR:
We read with interest the article by Giri et al1 on overall survival (OS) among very elderly (age ≥80 years) patients with diffuse large B-cell lymphoma (DLBCL) diagnosed in the United States between 1983 and 2013. The authors concluded that the OS of these patients had improved significantly over the past 3 decades. Although information regarding treatment was not available in that study, the authors suggested that the introduction of rituximab may have contributed to the improved OS over time. Also, general advances in medical care, which, in turn, steadily increases the average life expectancy, were mentioned as a contributing factor. However, the OS estimates presented by Giri et al were not corrected for the expected survival (ES) of a comparable group from the general population (ie, relative survival [RS]). Therefore, their study findings leave aside whether the improvement in OS was related to improved DLBCL management.
To complement and extend their observations, we here report the results of a nationwide population-based study that assessed the contribution of primary therapy to RS among very elderly patients with DLBCL diagnosed in The Netherlands.
We selected all patients with DLBCL age ≥80 years diagnosed between 1989 and 2015, with follow-up until February 2017, from the nationwide population-based Netherlands Cancer Registry (NCR) using International Classification of Diseases for Oncology morphology codes (details provided in the supplemental Data). The choice to include patients diagnosed from 1989 was because the NCR was established in that year. Information on the dates of birth and diagnosis, vital statistics, sex, disease stage, morphology and topography, and primary therapy (ie, no therapy, chemotherapy alone, radiotherapy and/or resection, chemotherapy plus radiotherapy [CT+RT], and other/unknown therapy) was available for individual patients. Information on the use of targeted immunotherapy and the exact therapeutic regimen was registered in the NCR for patients diagnosed from 2007 and 2014, respectively.
RS is the OS in the patient cohort divided by the ES of an equivalent group from the general population, matched to the patients with respect to age, sex, and period (details provided in the supplemental Data).2 OS and RS were calculated for 3 periods (1989-2002, 2003-2007, and 2008-2015) and 3 age categories (80-84, 85-89, and ≥90 years) and measured from the time of diagnosis until death or end of follow-up, whichever occurred first. The periods were selected based on the availability of rituximab in The Netherlands and thus slightly differed from the US periods. The first, second, and third period represent the prerituximab era, the era in which rituximab was gradually introduced into daily practice (transitional period), and the era in which rituximab was considered part of standard first-line therapy (established period), respectively. Poisson regression was used to assess linear trends in RS over time and estimate the relative excess risk of death (details provided in the supplemental Data).3 P < .05 indicated statistical significance. The Privacy Review Board of the NCR approved use of anonymous data for this study.
A total of 4737 newly diagnosed patients with DLBCL age ≥80 years were included in the analysis. The majority of patients were women (58%; supplemental Table 1); however, incident rates per 100 000 person-years were higher among men than women (supplemental Figure 1). Furthermore, most patients were age 80 to 84 years (60%) and had stage 2 to 4 DLBCL (63%), whereas only 10% of patients were age ≥90 years (supplemental Table 1).
The application of CT+RT exclusively increased over time among patients with stage 1 DLBCL age 80 to 89 years (supplemental Table 2). There were no noteworthy increases in chemotherapy application over time for patients with stage 2 to 4 DLBCL across the 3 age groups (supplemental Table 3). In the most recent period (2008-2015), 96%, 91%, and 79% of the chemotherapy-treated patients in the 3 age groups received immunochemotherapy, respectively. This was independent of stage. Of note, assessment of trends in immunochemotherapy application in earlier periods was not possible. Furthermore, the application of chemotherapy decreased with increasing age (supplemental Tables 2 and 3). More specifically, detailed data of patients diagnosed during 2014 to 2015 revealed that 63%, 29%, and 14% of patients in the 3 age groups received combined treatment with rituximab and anthracycline–containing chemotherapy, respectively (supplemental Table 4).
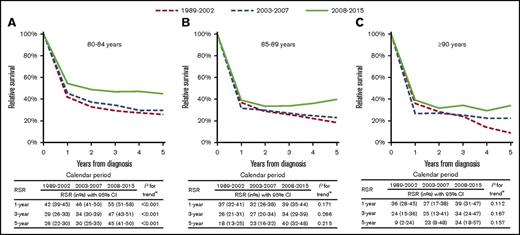
As shown in Figure 1, RS improved over time; however, statistically significant improvement was restricted to patients age 80 to 84 years and was most pronounced in the period from 2008 to 2015. In contrast, OS significantly improved over time for patients in all 3 age groups (supplemental Figure 3). The OS estimates in the period from 2008 to 2015 were comparable to the estimates from Giri et al1 in the period from 2006 to 2013 (supplemental Table 5). Five-year RS stratified by treatment group was 45%, 44%, and 36% for patients in the 3 age groups who received treatment (ie, chemotherapy alone, radiotherapy and/or resection, or CT+RT) and 4%, 3%, and 3% for patients in the 3 age groups who received no therapy, respectively (supplemental Figure 4). Furthermore, 5-year RS for patients diagnosed during 2008 to 2015 who received immunochemotherapy was markedly higher than that for those who received chemotherapy alone (69% vs 11%; supplemental Figure 5).
RS of very elderly patients with DLBCL in The Netherlands according to period of diagnosis and age at diagnosis from 1989 to 2015. RS rates (RSRs) are shown for the following age categories: 80 to 84 (A), 85 to 89 (B), and ≥90 years (C). The tables present the projected 1-, 3-, and 5-year RSRs with 95% confidence intervals (CIs) according to period of diagnosis. Additionally, the OS in the patient cohort and the expected survival of an equivalent group from the general population, matched to the patients with respect to age, sex, and period, are plotted in supplemental Figure 2 to provide readers an enhanced understanding of the dynamics of RS. *P value for likelihood ratio test assessing linear trends from the period of 1989 to 2002 to the period of 2008 to 2015.
RS of very elderly patients with DLBCL in The Netherlands according to period of diagnosis and age at diagnosis from 1989 to 2015. RS rates (RSRs) are shown for the following age categories: 80 to 84 (A), 85 to 89 (B), and ≥90 years (C). The tables present the projected 1-, 3-, and 5-year RSRs with 95% confidence intervals (CIs) according to period of diagnosis. Additionally, the OS in the patient cohort and the expected survival of an equivalent group from the general population, matched to the patients with respect to age, sex, and period, are plotted in supplemental Figure 2 to provide readers an enhanced understanding of the dynamics of RS. *P value for likelihood ratio test assessing linear trends from the period of 1989 to 2002 to the period of 2008 to 2015.
The primary multivariable model for RS, including period, sex, age, and stage, demonstrated an improvement of RS in the most recent period and a prognostic effect of sex, age, and stage (Table 1, model 1). The effect of sex and age lost statistical significance after adjustment for primary therapy (Table 1, model 2). This suggests that neither covariate has prognostic value when patients receive treatment. After information on the application of immunotherapy was added to model 2 in Table 1, the effect of period lost statistical significance (Table 1, model 3). This suggests that the application of rituximab contributed to the improved RS in the most recent period. Advanced stage remained a predictor of poor prognosis.
EMR during the first 5 years after DLBCL diagnosis
| . | Model without therapy (model 1) . | Model with therapy . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for primary therapy (model 2) . | Adjusted for primary therapy and immunotherapy (model 3) . | |||||||||
| EMR* . | 95% CI . | P† . | EMR* . | 95% CI . | P† . | EMR* . | 95% CI . | P† . | ||
| Period of diagnosis | ||||||||||
| 1989-2002 | 1.03 | 0.93-1.14 | .554 | 1.02 | 0.92-1.13 | .761 | 0.92 | 0.83-1.01 | .086 | |
| 2003-2007 | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 2008-2015 | 0.74 | 0.67-0.82 | <.001 | 0.79 | 0.71-0.88 | <.001 | 0.98 | 0.88-1.09 | .709 | |
| Sex | ||||||||||
| Male | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| Female | 1.09 | 1.00-1.18 | .046 | 1.00 | 0.92-1.08 | .926 | 1.00 | 0.92-1.08 | .929 | |
| Age at diagnosis, y | ||||||||||
| 80-84 | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 85-89 | 1.43 | 1.31-1.56 | <.001 | 1.03 | 0.94-1.12 | .512 | 1.00 | 0.92-1.09 | .962 | |
| ≥90 | 1.48 | 1.29-1.70 | <.001 | 1.00 | 0.88-1.15 | .956 | 0.98 | 0.86-1.13 | .797 | |
| Stage | ||||||||||
| 1 | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 2 | 1.55 | 1.36-1.77 | <.001 | 1.58 | 1.38-1.80 | <.001 | 1.56 | 1.37-1.78 | <.001 | |
| 3 | 2.33 | 2.04-2.66 | <.001 | 2.23 | 1.94-2.56 | <.001 | 2.21 | 1.93-2.54 | <.001 | |
| 4 | 2.81 | 2.50-3.17 | <.001 | 2.41 | 2.13-2.73 | <.001 | 2.44 | 2.16-2.77 | <.001 | |
| Unknown | 3.72 | 3.23-4.47 | <.001 | 2.00 | 1.74-2.31 | <.001 | 1.95 | 1.69-2.24 | <.001 | |
| . | Model without therapy (model 1) . | Model with therapy . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for primary therapy (model 2) . | Adjusted for primary therapy and immunotherapy (model 3) . | |||||||||
| EMR* . | 95% CI . | P† . | EMR* . | 95% CI . | P† . | EMR* . | 95% CI . | P† . | ||
| Period of diagnosis | ||||||||||
| 1989-2002 | 1.03 | 0.93-1.14 | .554 | 1.02 | 0.92-1.13 | .761 | 0.92 | 0.83-1.01 | .086 | |
| 2003-2007 | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 2008-2015 | 0.74 | 0.67-0.82 | <.001 | 0.79 | 0.71-0.88 | <.001 | 0.98 | 0.88-1.09 | .709 | |
| Sex | ||||||||||
| Male | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| Female | 1.09 | 1.00-1.18 | .046 | 1.00 | 0.92-1.08 | .926 | 1.00 | 0.92-1.08 | .929 | |
| Age at diagnosis, y | ||||||||||
| 80-84 | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 85-89 | 1.43 | 1.31-1.56 | <.001 | 1.03 | 0.94-1.12 | .512 | 1.00 | 0.92-1.09 | .962 | |
| ≥90 | 1.48 | 1.29-1.70 | <.001 | 1.00 | 0.88-1.15 | .956 | 0.98 | 0.86-1.13 | .797 | |
| Stage | ||||||||||
| 1 | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||||
| 2 | 1.55 | 1.36-1.77 | <.001 | 1.58 | 1.38-1.80 | <.001 | 1.56 | 1.37-1.78 | <.001 | |
| 3 | 2.33 | 2.04-2.66 | <.001 | 2.23 | 1.94-2.56 | <.001 | 2.21 | 1.93-2.54 | <.001 | |
| 4 | 2.81 | 2.50-3.17 | <.001 | 2.41 | 2.13-2.73 | <.001 | 2.44 | 2.16-2.77 | <.001 | |
| Unknown | 3.72 | 3.23-4.47 | <.001 | 2.00 | 1.74-2.31 | <.001 | 1.95 | 1.69-2.24 | <.001 | |
EMR, excess mortality ratio; ref, reference.
Each covariate is simultaneously adjusted for all other covariates in the table, along with 5 years of follow-up.
P values are compared with the reference category.
In contrast to the study by Giri et al,1 we calculated RS to assess trends in DLBCL survival over time. The advantage of RS is that it takes into account the effect of general changes in population survival over time.2 We have illustrated with our data that crude OS estimates can give the wrong impression about true benefits in survival of patients with cancer, especially when benefits in OS are modest. Therefore, we recommend the use of RS in the analysis of cancer registry data. Furthermore, the key strength of our study includes the use of a nationwide population-based cancer registry with comprehensive data available for individual patients. Therefore, unlike the study by Giri et al, we could directly link improvements in survival with changes in DLBCL management.
In summary, we show that significant improvement in RS was confined to patients age 80 to 84 years who were diagnosed in the period from 2008 to 2015. The multivariable analysis demonstrated that the application of rituximab accounted for the improvement. The lack of significant improvement among patients age ≥85 years might be related to the lower application of rituximab-containing chemotherapy in this age group than in patients age 80 to 84 years. Another factor might be the higher rates of chemotherapy application without rituximab among patients age ≥90 years. Taken together, despite recent improvements, the overall outcome of patients with DLBCL age ≥80 years remains unsatisfactory. Therefore, the design of prospective studies specifically tailored to the unique clinical and biological characteristics of the 80+ age group is of paramount importance to establish evidence-based treatment recommendations. In the meantime, population-based studies can support clinical decision making.
The full-text version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank the registration clerks of the Netherlands Cancer Registry (NCR) for their dedicated data collection. Established in 1989, the nationwide population-based NCR is maintained and hosted by the Netherlands Comprehensive Cancer Organisation. The authors also thank Ronald A. M. Damhuis at the Netherland Comprehensive Cancer Organisation for fruitful discussions regarding survival analyses.
Contribution: A.G.D. designed the study, analyzed the data, and wrote the manuscript; O.V. collected the data; and all authors contributed to the writing of the manuscript, interpreted the data, and read, commented on, and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Avinash G. Dinmohamed, Department of Research, Netherlands Comprehensive Cancer Organisation, Godebaltkwartier 419, 3511 GT Utrecht, The Netherlands; e-mail: a.dinmohamed@iknl.nl.