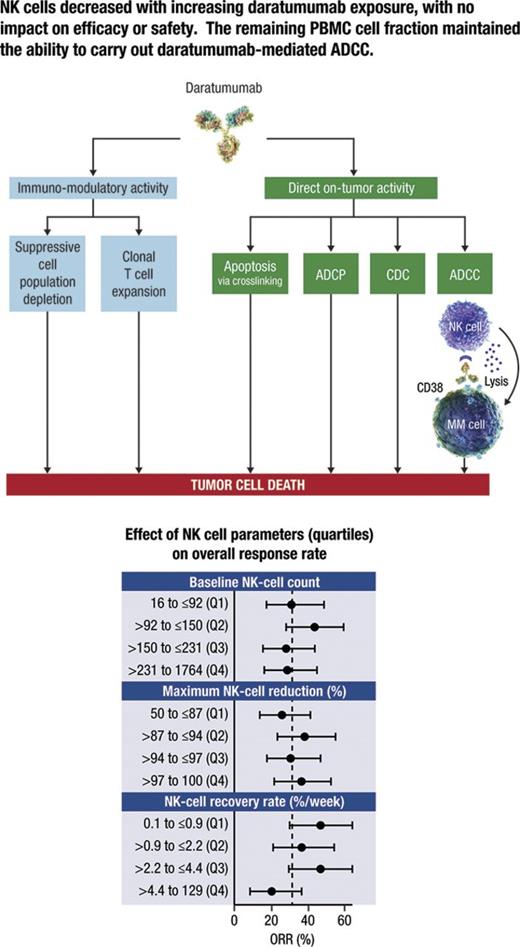
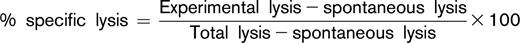Key Points
NK cells decline as daratumumab exposure increases in a maximum effect type dose-response relationship, with no efficacy and safety impact.
Remaining PBMC cell fractions maintained the ability to carry out daratumumab-mediated ex vivo ADCC.
Abstract
Daratumumab, a human CD38 imunoglobulin G 1κ monoclonal antibody, has demonstrated clinical activity and a manageable safety profile in monotherapy and combination therapy clinical trials in relapsed and/or refractory multiple myeloma. CD38 is expressed at high levels on myeloma cells and, to a lesser extent, on immune effector cells, including natural killer (NK) cells, which are important for daratumumab-mediated antibody-dependent cellular cytotoxicity (ADCC). Here, the pharmacodynamic effects of daratumumab monotherapy on NK cells, and the effect of NK cell dynamics on daratumumab efficacy and safety, were assessed. Daratumumab, like other CD38 antibodies, reduced NK-cell counts in peripheral blood mononuclear cells (PBMCs) of healthy donors in vitro. Data on NK-cell counts, clinical efficacy, and adverse events were pooled from two single-agent daratumumab studies, GEN501 and SIRIUS. In daratumumab-treated myeloma patients, total and activated NK-cell counts reduced rapidly in peripheral blood after the first dose, remained low over the course of treatment, and recovered after treatment ended. There was a clear maximum effect relationship between daratumumab dose and maximum reduction in NK cells. Similar reductions were observed in bone marrow. PBMCs from daratumumab-treated patients induced lysis by ADCC of CD38+ tumor cells in vitro, suggesting that the remaining NK cells retained cytotoxic functionality. There was no relationship between NK-cell count reduction and the efficacy or safety profile of daratumumab. Furthermore, although NK cell numbers are reduced after daratumumab treatment, they are not completely depleted and may still contribute to ADCC, clinical efficacy, and infection control.
Introduction
Daratumumab (Darzalex; Janssen Biotech, Inc.) is a human monoclonal antibody targeting CD38 that received conditional accelerated approval from the US Food and Drug Administration for the treatment of patients with multiple myeloma (MM) who have received ≥3 prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD) or who are double refractory to a PI and an IMiD.1 Daratumumab has also received conditional marketing authorization from the European Medicines Agency for the treatment of adult patients with relapsed or refractory MM whose prior therapy included a PI and an IMiD and who have demonstrated disease progression on the last therapy.2 In the phase 1 and 2 trials GEN501 and SIRIUS, daratumumab demonstrated robust clinical activity as a single agent, with overall response rates (ORRs) of 36% and 29%, respectively.3,4 In recent phase 3 trials (POLLUX and CASTOR), the addition of daratumumab to standard-of-care regimens provided a significant decrease in the risk of disease progression or death compared with the standard-of-care regimen alone (POLLUX hazard ratio [HR], 0.37; CASTOR HR, 0.39) and substantially improved the response rates in patients with ≥1 prior lines of therapy.5,6 On the basis of these results, daratumumab in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, was approved for the treatment of patients with MM who have received ≥1 prior lines of therapy.7
Daratumumab mediates the death of CD38-expressing tumor cells through a variety of immunologic mechanisms, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, and the induction of apoptosis through Fc-mediated crosslinking.8,9 Daratumumab has also been shown to decrease CD38+ immunosuppressive regulatory cells, while increasing helper and cytotoxic T cells, T cell functional responses, and T cell receptor clonality, all of which may represent additional immunomodulatory mechanisms of action for daratumumab.10 Because natural killer (NK) cells express high levels of CD38,10 we hypothesized that daratumumab may also reduce NK cell populations.8 Given the role of NK cells in ADCC, a mechanism of action of daratumumab, we wanted to determine whether the predicted reduction of this cell population had detrimental effects on clinical efficacy.
We investigated the effects of daratumumab monotherapy on CD38+ NK cells in vitro and in patients treated in the phase 1 and 2 GEN501 and SIRIUS studies to understand the potential impact of NK cells on the efficacy and safety of the drug.
Methods
In vitro analysis of CD38+ NK cells from healthy donors by combined ADCC/CDC flow cytometry assay
Peripheral blood samples were collected from multiple healthy donors, and peripheral blood mononuclear cells (PBMCs) were isolated by using standard methodology. PBMCs were treated with 0.01, 0.1, or 1 µg/mL daratumumab, biosimilar versions of isatuximab (SAR650984; humanized immunoglobulin G1 [IgG1] CD38 monoclonal antibody) and MOR202 (human IgG1 CD38 monoclonal antibody), or 1 µg/mL of isotype control with 10% human complement and incubated for 3 days. Samples were evaluated by flow cytometry for CD38 antibody-mediated cytotoxicity as a percentage of live NK (CD45+CD3–CD56+) cells and normalized to controls with no complement or antibody added.
Daratumumab clinical study design and patients
For the clinical analyses, data on patients from two concurrent clinical trials (NCT00574288 [GEN501] and NCT01985126 [SIRIUS]) were used. The study designs of both trials have previously been described in detail.3,4 Briefly, GEN501 was an open-label, phase 1/2 dose-escalation and dose-expansion study. In part 1 (dose escalation), patients received daratumumab doses from 0.005 to 24 mg/kg. In part 2, patients received infusions of either daratumumab 8 mg/kg or daratumumab 16 mg/kg on a tapering dosing schedule (once per week, then once every 2 weeks, and then once every 4 weeks thereafter until disease progression).4 SIRIUS was an open-label, phase 2 study in which patients were randomly assigned to receive infusions of either daratumumab 8 mg/kg once every 4 weeks or daratumumab 16 mg/kg (the recommended dose) once per week for 8 weeks, once every 2 weeks for 16 weeks, and once every 4 weeks thereafter.3 Patients enrolled in both studies also received low to intermediate doses of corticosteroids before and after daratumumab dosing to manage infusion-related reactions.
Patients in both studies had documented relapsed or refractory MM requiring systemic therapy and an Eastern Cooperative Oncology Group performance status of ≤2. In GEN501, patients had relapsed from or were refractory to ≥2 prior lines of therapy, including PIs, IMiDs, chemotherapy, or autologous stem cell transplantation.4 Patients enrolled in SIRIUS had progressed on their most recent line of therapy and had received ≥3 prior lines of therapy, including a PI and an IMiD, or were refractory to both classes of agents.3 Best overall clinical responses were determined by using the International Myeloma Working Group consensus recommendation for MM treatment response criteria.11 Patients were grouped into responders (ie, patients with best overall responses of partial response [PR], very good PR, complete response [CR], or stringent CR) and nonresponders (ie, patients with a best outcome of minimal response [MR], stable disease [SD], or progressive disease [PD]). ORRs included all groups of responders.3,4
The investigators and sponsors were responsible for the study design and statistical analysis plan. The investigators and their research teams collected the data. Janssen Research & Development and Genmab compiled the data for summation and analysis and confirmed the accuracy of the data. All investigators had full access to the data and analyses and were not restricted by confidentiality agreements. Ethics committees or institutional review boards at each study site approved the study protocols and statistical analysis plans. The studies were conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All the patients provided written informed consent prior to study enrollment.
Ex vivo analyses of NK cells
Peripheral blood and bone marrow samples were collected in heparinized tubes at baseline, immediately before the first infusion, and at specified time points during treatment. The majority of samples were evaluated as they arrived at a central laboratory, 24 to 48 hours after collection. PBMCs were obtained from whole blood, isolated by density-gradient centrifugation, and stored frozen until analysis. NK cells were quantified in blood samples by using a single platform measurement (TruCount, Becton Dickinson) with 50 µL of whole blood incubated with monoclonal antibodies CD3 fluorescein isothiocyanate, CD16/CD56 phycoerythrin, CD45 peridinin-chlorophyll protein, and CD19 allophycocyanin (Multitest, Becton Dickinson). After antibody staining, red blood cells were lysed with BD Pharm Lyse (Becton Dickinson). Total (CD16+CD56+) and activated (CD16+CD56dim, measured only in the SIRIUS study) NK cells were measured by real-time flow cytometry. Absolute cell numbers per microliter and the percentage of lymphocytes were both calculated, and the kinetics of total (CD16+CD56+) and activated (CD16+CD56dim) NK cells after daratumumab treatment were explored as percent changes from baseline to allow normalization between patients.
PBMCs were also evaluated for their ability to mediate daratumumab-induced ADCC in an ex vivo assay against the CD38+ Daudi tumor cell line. Daudi cells were labeled with calcein-AM and incubated with various concentrations of daratumumab at room temperature to enable opsonization. PBMCs from healthy volunteers were also added and incubated for 3 hours at 37°C as a control (data not shown). Cell death was determined by measuring the amount of calcein released into the culture medium by Daudi cells after incubation. Untreated cells and cells lysed with Triton X-100 were used to measure spontaneous and total lysis. Percent specific lysis was calculated as
Concentration-response curves were generated and the output was transformed to nonlinear regression. After the analysis, half maximal effective concentration (EC50) values were calculated and presented. A Wilcoxon rank sum test was conducted to compare values between response groups per time point.
Clinical correlative analyses
The pharmacodynamics of NK cells in daratumumab-treated patients was characterized by two summary metrics (ie, percent maximum reduction in NK cell numbers from baseline) calculated as ([nadir of NK-cell counts after treatment − baseline NK-cell count] / baseline NK-cell count) × 100, and percent recovery rate of NK cells from nadir relative to baseline as ([maximum NK-cell counts after nadir − nadir] / [baseline NK-cell count] / time interval between time at maximum NK-cell count after nadir and time at nadir) × 100. The correlation between daratumumab doses and percent maximum reduction in NK cell numbers from baseline after daratumumab treatment or percent recovery rate of NK cells from nadir relative to baseline was examined to investigate dose-response relationships. A maximum effect (Emax) model was used to explore the dose-response relationships with the nls function in R (version 3.2.2). The relationship between the dynamics of NK cells after daratumumab treatment and clinical efficacy (as measured by ORR and PFS) and safety (as measured by overall grade ≥3 adverse events [AEs] and incidence of infections including any grade, grade ≥3, or herpes zoster infection) was also studied according to quartiles of the posttreatment maximum NK reduction and recovery rate of NK cells after nadir. Generalized linear models were used to investigate the association between ORR and NK reduction or recovery, whereas Cox proportional hazards models were used to explore the association between PFS and the NK dynamic metrics. Comparisons between NK-cell counts at different time points were made by using a paired Student t test.
Results
Reduction in NK cells in vitro with CD38 antibodies
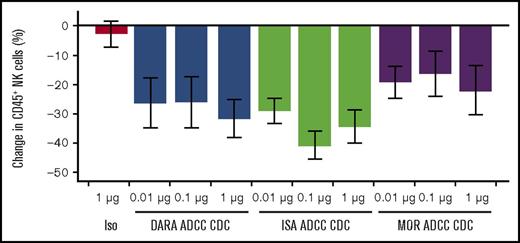
We previously confirmed that NK cells express high levels of CD38 compared with other immune cell populations.10 To assess whether daratumumab could mediate reduction of CD38+ NK-cell counts and to compare this activity to other CD38 monoclonal antibodies (mAbs), PBMCs from healthy donors were treated in vitro with varying concentrations of daratumumab and biosimilar versions of MOR202 and isatuximab CD38 mAbs in a combined ADCC/CDC assay. NK cells (CD16+CD56+), which act as effector cells in ADCC, were reduced with a trend toward greater reduction at higher concentrations of daratumumab, isatuximab and, to a lesser extent, MOR202, but not by an isotype control, demonstrating the ability of CD38 mAbs to reduce NK cell numbers (Figure 1). Anti-CD38 mAb-mediated NK-cell reduction was specific, and other immune cell types, such as T cells and B cells, were unaffected (supplemental Figure 1).
CD38 antibody-mediated reduction of NK cells in in vitro ADCC/CDC assays. Bars show percent change of NK cells in the lymphocyte (CD45+) population, averaged from 3 replicates from 3 PBMC donors. Error bars represent standard error. Samples were incubated for 3 days and then assessed by flow cytometry. Test sample values were normalized to no complement, no antibody controls. DARA, daratumumab; ISA, biosimilar SAR650984 antibody; Iso, isotype control; MOR, biosimilar MOR202 antibody.
CD38 antibody-mediated reduction of NK cells in in vitro ADCC/CDC assays. Bars show percent change of NK cells in the lymphocyte (CD45+) population, averaged from 3 replicates from 3 PBMC donors. Error bars represent standard error. Samples were incubated for 3 days and then assessed by flow cytometry. Test sample values were normalized to no complement, no antibody controls. DARA, daratumumab; ISA, biosimilar SAR650984 antibody; Iso, isotype control; MOR, biosimilar MOR202 antibody.
Clinical trial patients
Peripheral blood and bone marrow samples were available for analyses of NK cells from 148 patients treated with daratumumab 16 mg/kg (GEN501 part 2, n = 42; SIRIUS, n = 106), 48 patients treated with daratumumab 8 mg/kg (GEN501 part 2, n = 30; SIRIUS, n = 18), and 32 patients treated with daratumumab 0.005 (n = 1), 0.05 (n = 1), 0.1 (n = 6), 0.5 (n = 3), 1 (n = 6), 2 (n = 3), 4 (n = 3), 8 (n = 3), 16 (n = 3), and 24 (n = 3) mg/kg (GEN501 part 1). Baseline demographic and clinical characteristics have been previously described in detail for patients in each study; a summary of pooled clinical characteristics is provided in supplemental Tables 1 and 2.
Dynamics of NK cells in MM patients treated with daratumumab
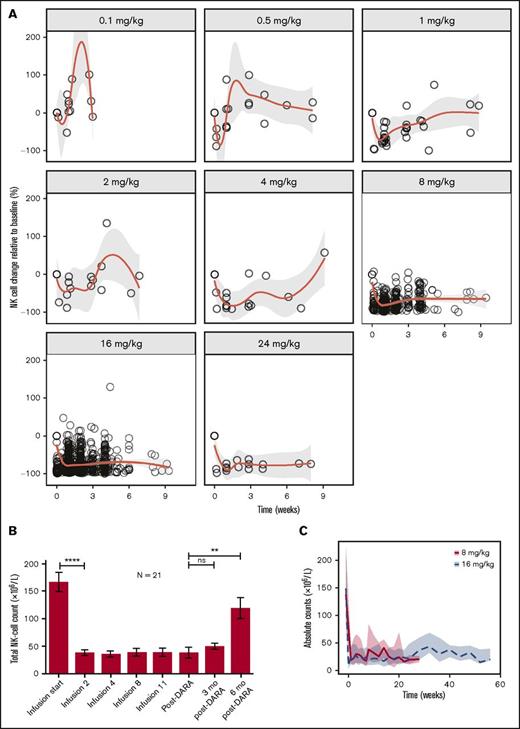
NK cells (CD16+CD56+) in peripheral blood were sensitive to daratumumab at doses ≥0.5 mg/kg. At a dose of 0.1 mg/kg, there was no apparent NK-cell reduction after the infusions (Figure 2A for NK cell changes from baseline vs time since previous dose and supplemental Figure 2 for percent change from baseline vs time since first infusion). In patients treated with daratumumab 0.5 mg/kg or higher, NK cells were rapidly reduced after the first infusion, with a degree of reduction that was dose dependent. NK cells remained low while patients were receiving daratumumab therapy and recovered rapidly (within ∼1 week) after the 0.5 mg/kg dose, but the recovery time increased with increasing dose (Figure 2A). In patients from the GEN501 study treated at 16 mg/kg (n = 21), total NK cells showed some recovery at 3 months after daratumumab treatment and significant recovery at 6 months after daratumumab (Figure 2B). As with total NK cells, activated NK cells (CD16+CD56dim) in peripheral blood were also rapidly reduced and remained low during treatment (data not shown). Total and activated NK cells in the marrow were measured at baseline and at cycle 3 day 1 (after 8 once-per-week doses of daratumumab) and showed similar reductions (supplemental Figure 3 and data not shown, respectively). Part 1 of the SIRIUS study evaluated 2 differing daratumumab doses and schedules to investigate whether a monthly treatment regimen would allow for NK-cell recovery and a greater clinical response compared with a more intense regimen with potentially less NK-cell recovery. In the 8-mg/kg (administered once every 4 weeks) cohort, NK cell levels reduced rapidly and then partially recovered after the first dose of daratumumab (Figure 2C), but recovery was less pronounced over time with repeated doses of daratumumab.
Reduction and recovery of NK cells after daratumumab treatment. (A) Rapid, dose-dependent reduction of NK cells was observed upon daratumumab treatment at all dose levels except for 0.1 mg/kg. Recovery of NK cells was slower with increasing dose. The centered curves and shaded areas represent smoothing lines and the 95% confidence intervals around the smoothing curves, respectively. (B) Longitudinal data representation of absolute NK-cell counts over time in peripheral blood. Peripheral blood was obtained from 21 patients during treatment with daratumumab (16 mg/kg) in GEN501 at the time of progression, and at 3 and 6 months after development of progressive disease. Data are presented as mean ± standard error of the mean. (C) Reduction and recovery of NK-cell counts in the peripheral blood after daratumumab treatment. Patients received daratumumab 16 mg/kg on the recommended dosing schedule or 8 mg/kg (given once every 4 weeks); NK cells showed some recovery in patients treated with daratumumab 8 mg/kg. The lines and shaded areas represent the medians of those doses per time point and their interquartile range, respectively. P values between the indicated groups were calculated by using a paired Student t test. **P < .01; ****P < .0001. ns, not significant.
Reduction and recovery of NK cells after daratumumab treatment. (A) Rapid, dose-dependent reduction of NK cells was observed upon daratumumab treatment at all dose levels except for 0.1 mg/kg. Recovery of NK cells was slower with increasing dose. The centered curves and shaded areas represent smoothing lines and the 95% confidence intervals around the smoothing curves, respectively. (B) Longitudinal data representation of absolute NK-cell counts over time in peripheral blood. Peripheral blood was obtained from 21 patients during treatment with daratumumab (16 mg/kg) in GEN501 at the time of progression, and at 3 and 6 months after development of progressive disease. Data are presented as mean ± standard error of the mean. (C) Reduction and recovery of NK-cell counts in the peripheral blood after daratumumab treatment. Patients received daratumumab 16 mg/kg on the recommended dosing schedule or 8 mg/kg (given once every 4 weeks); NK cells showed some recovery in patients treated with daratumumab 8 mg/kg. The lines and shaded areas represent the medians of those doses per time point and their interquartile range, respectively. P values between the indicated groups were calculated by using a paired Student t test. **P < .01; ****P < .0001. ns, not significant.
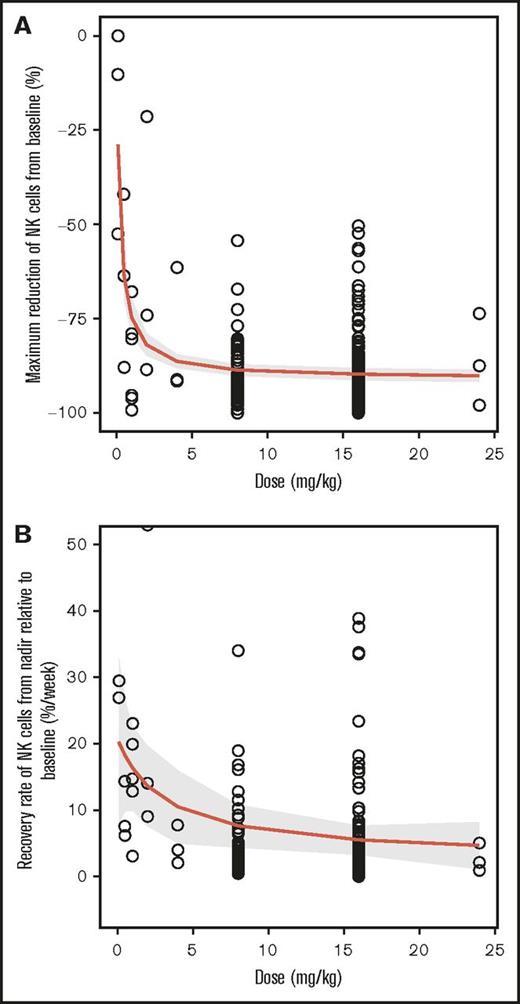
Relationship between daratumumab dose and NK cell dynamics
A clear Emax relationship was observed between the maximum reduction in NK cells in peripheral blood and the daratumumab dose (Figure 3A). The estimated median effective dose (ED50) and maximum reduction in Emax were 0.22 mg/kg and 91%, respectively. On average, ≥95% of the maximum effect could be attained when the dose was ≥4 mg/kg. The recovery rate of NK cells after reaching nadir also followed an Emax relationship with dose (Figure 3B). The estimated ED50 was approximately 3 mg/kg. The predicted recovery rates were approximately 7.6% and 5.5% per week for daratumumab 8 mg/kg and 16 mg/kg, respectively.
Emaxdose-response relationship between daratumumab exposure and maximum reduction in NK cells. (A) Maximum percent reduction of NK cells from baseline by dose and (B) recovery rate of NK cells from nadir relative to baseline. The centered curves and shaded areas represent predicted average Emax curves and the 95% confidence interval around the Emax curves, respectively.
Emaxdose-response relationship between daratumumab exposure and maximum reduction in NK cells. (A) Maximum percent reduction of NK cells from baseline by dose and (B) recovery rate of NK cells from nadir relative to baseline. The centered curves and shaded areas represent predicted average Emax curves and the 95% confidence interval around the Emax curves, respectively.
Correlation of dynamics of NK cells and clinical response
Clinical response was significantly improved with the approved 16 mg/kg dosing schedule compared with the 8 mg/kg schedule of dosing once every 4 weeks, and the former was chosen for subsequent studies.3 The clinical responses observed in all patients treated with 8 and 16 mg/kg are summarized in supplemental Table 3.
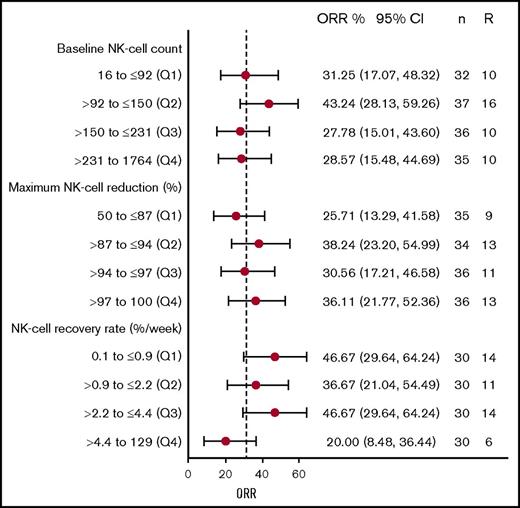
In patients treated with 16 mg/kg, no difference was observed between the ORRs of patients stratified into quartiles on the basis of baseline NK-cell counts, maximum NK-cell reduction, or recovery rate of NK cells (Figure 4). The 95% confidence intervals for ORRs in all quartile groups included the ORR (31.7%), and the confidence intervals generally overlapped each other. Furthermore, logistic regression analyses confirmed the lack of a statistically significant relationship between ORR and baseline NK-cell counts (P = .76), maximum reduction in NK cells (P = .17), and recovery rate of NK cells (P = .11).
NK cell dynamic metrics and clinical response with daratumumab treatment. ORR for patients divided into quartiles based on (top) baseline NK-cell count, (middle) maximum NK-cell reduction, and (bottom) NK-cell recovery rate. Numbers on the right show the mean and 95% confidence interval of ORR for that quartile, and the number of patients (n) and responders (R) in each group. Q, quartile.
NK cell dynamic metrics and clinical response with daratumumab treatment. ORR for patients divided into quartiles based on (top) baseline NK-cell count, (middle) maximum NK-cell reduction, and (bottom) NK-cell recovery rate. Numbers on the right show the mean and 95% confidence interval of ORR for that quartile, and the number of patients (n) and responders (R) in each group. Q, quartile.
Correlation of ex vivo ADCC and clinical response
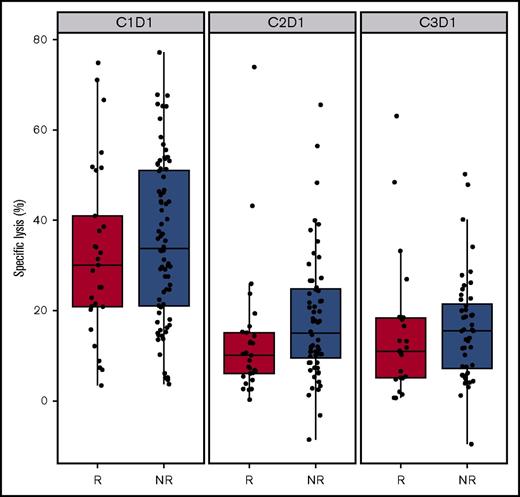
The contribution of NK cells to ADCC was measured in vitro by using PBMCs isolated from patient whole blood samples. At baseline, patients who responded to daratumumab (PR or better) had similar levels of percent ADCC in vitro lysis of Daudi cells compared with those patients who did not respond to daratumumab (MR, SD, or PD; P = .4; Figure 5). The percentage of ADCC in vitro lysis induced by PBMCs decreased with daratumumab treatment and was slightly lower for responders than for nonresponders at cycle 2 day 1 (P = .02) and was similar between groups at cycle 3 day 1 (P = .16; Figure 5). ADCC in vitro lysis was reduced after daratumumab treatment, probably as a result of the decrease in NK-cell counts, which contribute to the total PBMC population. However, in the majority of patients, PBMCs isolated during daratumumab treatment were still able to induce some level of ADCC, suggesting that the remaining NK cells retained some cytotoxic functionality. Alternatively, the observed cytotoxicity may have been mediated by granulocytes from isolated PBMCs.
Correlation of ex vivo ADCC and clinical response. Percent of in vitro ADCC lysis against Daudi cells induced by patient PBMCs in both responders and nonresponders upon treatment. C, cycle; D, day; R, responder; NR, nonresponder.
Correlation of ex vivo ADCC and clinical response. Percent of in vitro ADCC lysis against Daudi cells induced by patient PBMCs in both responders and nonresponders upon treatment. C, cycle; D, day; R, responder; NR, nonresponder.
Correlation of dynamics of NK cells and PFS
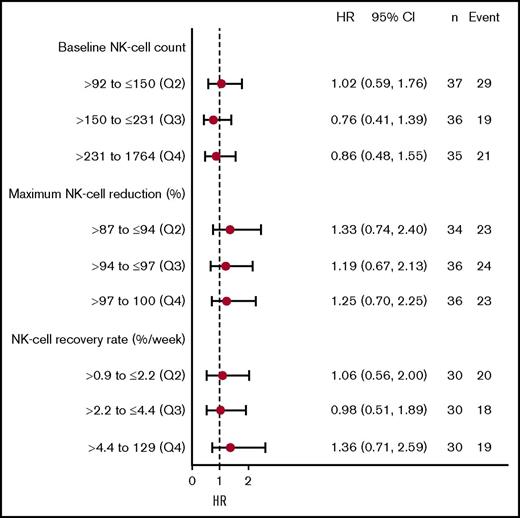
Time-to-event analyses for PFS were performed with the patients stratified according to quartiles (Q) of baseline NK-cell counts, maximum NK-cell reduction, and recovery rate of NK cells. No significant differences in PFS were observed for baseline NK-cell counts, maximum NK-cell reduction, or recovery rate of NK cells (P = .7, P = .8, and P = .7, respectively). The median PFS durations for patients in Q1, Q2, Q3, and Q4 for baseline NK-cell counts were 5.4, 4.7, 3.0, and 5.0 months, respectively; median PFS durations for maximum NK-cell reductions were 5.6, 3.8, 3.8, and 5.0 months, respectively; and NK recovery rates were 6.6, 6.5, 5.5, and 5.6 months, respectively. Cox proportional hazard models showed that HRs were consistent across different quartiles for baseline NK-cell counts, maximum NK-cell reduction, and recovery rate of NK cells, and the HRs were close to 1 (Figure 6).
NK cell dynamic metrics and PFS with daratumumab treatment. HRs according to PFS for patients divided into quartiles based on (top) baseline NK-cell count, (middle) maximum NK-cell reduction, and (bottom) NK-cell recovery rate. The numbers on the right show the mean and 95% confidence interval for the HR for that quartile and the number of patients (n) and PFS events in each group. Q1 was used as the reference group.
NK cell dynamic metrics and PFS with daratumumab treatment. HRs according to PFS for patients divided into quartiles based on (top) baseline NK-cell count, (middle) maximum NK-cell reduction, and (bottom) NK-cell recovery rate. The numbers on the right show the mean and 95% confidence interval for the HR for that quartile and the number of patients (n) and PFS events in each group. Q1 was used as the reference group.
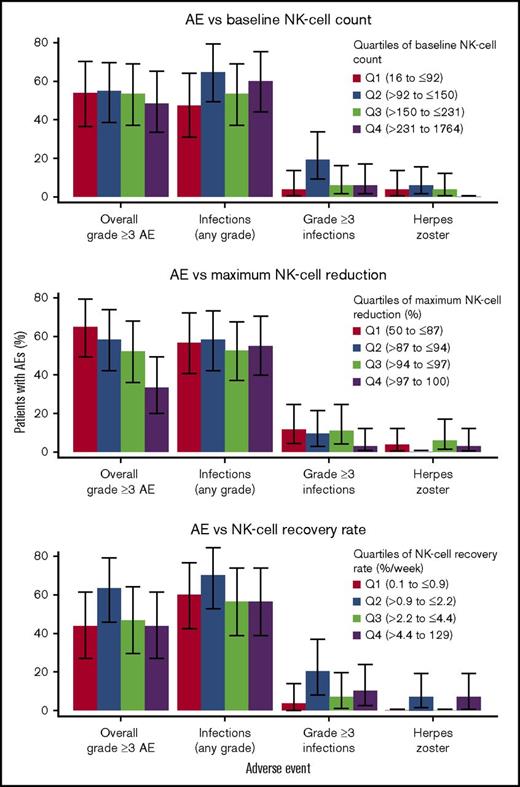
Correlation of NK cell dynamics with safety
NK cells regulate immune activities and provide protection against infections. Therefore, the incidences of infections, including herpes zoster reactivation, were evaluated. In addition, the relationship between NK-cell reduction and overall grade ≥3 AEs was also examined.
Overall, approximately 55% of patients experienced grade ≥3 AEs at 16 mg/kg (n = 151). The most common treatment-emergent AEs (TEAEs) related to infection were upper respiratory tract infection, nasopharyngitis, pneumonia, and sinusitis. In the 16-mg/kg group, 83 patients (55%) experienced a TEAE of infection. In 15 patients (∼10%), the TEAEs of infection were considered grade 3 or 4. The incidence of herpes zoster was low (∼2%).
No apparent relationship was observed between baseline NK-cell counts, maximum NK-cell reduction, or recovery rate of NK cells and the safety profile of daratumumab in relapsed or refractory MM. The incidence of overall grade ≥3 AEs, infections (any grade and grade ≥3), or herpes zoster infection was similar among patients stratified into quartiles according to these three summary metrics of NK cell dynamics (Figure 7). Interestingly, the incidence of overall grade ≥ 3AEs seems to be lower with greater reduction in NK cells.
Relationship between NK cell dynamic metrics and safety. Percent of patients with AEs divided into quartiles on the basis of (top) baseline NK-cell count, (middle) maximum NK-cell reduction, and (bottom) NK-cell recovery rate. Reduction in NK cells after treatment was not associated with the incidence of reported AEs.
Relationship between NK cell dynamic metrics and safety. Percent of patients with AEs divided into quartiles on the basis of (top) baseline NK-cell count, (middle) maximum NK-cell reduction, and (bottom) NK-cell recovery rate. Reduction in NK cells after treatment was not associated with the incidence of reported AEs.
Discussion
NK cells have a critical role in ADCC, which is one of the identified mechanisms of action of daratumumab.8 NK cells also function as key innate immune cells that regulate immune activity and provide protection against infection.12 Therefore, this study sought to determine whether a daratumumab-mediated reduction in NK cells would impact either the efficacy of daratumumab or patient safety. This analysis characterized the dynamics of NK cells during treatment with daratumumab and evaluated the potential clinical impacts of the observed daratumumab-mediated NK-cell reduction in patients with relapsed or refractory MM.
NK cells, which express high levels of CD38,8 were shown to be highly sensitive to daratumumab treatment both in vitro and in the clinical setting. Total and activated NK cells from peripheral blood and bone marrow decreased with increasing daratumumab exposure in the vast majority of patients. Low levels of NK cells were maintained during treatment at therapeutic doses, exhibiting an Emax-type dose-response relationship. NK-cell counts recovered after patients completed daratumumab treatment, with an approximately 50% recovery 3 months after treatment at the approved 16-mg/kg dosing schedule.
Although this study focused on the immunophenotypic changes induced by daratumumab treatment, the mechanism by which daratumumab mediates NK-cell reduction is interesting. Preliminary data from PBMCs of healthy donors suggest that NK cells are reduced in the presence of daratumumab, even in the absence of other effector cells or complement. These data support a hypothesis of ADCC by fratricide. However, additional experiments using patient-derived cells are needed to elucidate the mechanism of daratumumab-mediated NK-cell reduction in the clinical setting.
Daratumumab demonstrated promising single-agent activity in patients with heavily pretreated, relapsed or refractory MM in the GEN501 and SIRIUS studies, with ORRs of 36% and 29%, respectively.3,4 ADCC, a robust in vitro mechanism of action for daratumumab-mediated killing of MM cells, is heavily dependent on NK effector cells.8 However, although the number of NK cells was greatly reduced after daratumumab treatment, these cells were not completely depleted and thus may still enable ADCC and contribute to clinical efficacy. Indeed, PBMCs from patients treated with daratumumab were still able to induce in vitro daratumumab-mediated ADCC against CD38+ tumor cell lines. Other effector cells, such as granulocytes, which are not depleted by daratumumab, are also capable of mediating ADCC and may contribute to the efficacy of daratumumab. Moreover, in addition to ADCC, daratumumab has several known mechanisms of action that may also contribute to its efficacy, including CDC,8 antibody-dependent cellular phagocytosis,9 apoptosis,13 and modulation of CD38 enzymatic activity.14 Therefore, the multiple mechanisms of action of daratumumab may explain the lack of association between the pharmacodynamics of NK cells and efficacy of treatment.
Recent studies have shown that, when challenged with tumor cells, NK cells expressing high levels of programmed death-ligand 1 downregulated dendritic cell priming of CD8+ effector T cells.15 When NK cells were reduced, there was an increase in CD8+ tumor-directed effector cells. Furthermore, when primed in the absence of NK cells, this population of CD8+ effector T cells protected against tumor growth and formation of secondary tumors in mice that were rechallenged.15 Given this new regulatory function, the reduction of NK cells that occurs during daratumumab treatment may enhance the antitumor immune response. An increase in CD8+ effector memory T cells was observed in patients treated with single-agent daratumumab.10 Additional studies will be required to determine whether there is a correlation between daratumumab-mediated NK-cell reduction and CD8+ effector T cell populations and any downstream antitumor effects.
Similarly, NK cell dynamics did not correlate with increased daratumumab safety concerns. NK cells are known to play a major role in the immune clearance of virally infected cells, and patients deficient in NK cells are typically susceptible to opportunistic viral infections. However, in the current analysis, the daratumumab-mediated reduction in CD38+ NK cells was not associated with an increased incidence of overall infections (any grade or grade ≥3) or herpes zoster infection. As noted above with regard to efficacy, these findings may be the result of the presence of a small number of residual NK cells and/or compensation by other immune cells and mechanisms.
Other immunomodulatory antibodies are also in development for the treatment of relapsed or refractory MM. Two other CD38 monoclonal antibodies, isatuximab and MOR202, are currently in early clinical development and have been well tolerated in clinical trials.16,17 Elotuzumab, a humanized IgG1 mAb that binds to signaling lymphocytic activation molecule F7 on myeloma cells and mediates ADCC demonstrated NK-cell reduction at all doses ≥0.5 mg/kg, with maximum reduction at 2 days after treatment and slower recovery in the 10-mg/kg and 20-mg/kg cohorts in a phase 1 dose-escalation study.18 Elotuzumab also demonstrates immunostimulatory activity on NK cells, including upregulation of interleukin 2 and tumor necrosis factor α.19 The effects of elotuzumab on NK cells in the phase 3 ELOQUENT-2 study were not reported.20 However, unlike daratumumab, elotuzumab was associated with high rates of grade 3 and 4 lymphopenia (77% vs 49% for the control group).20
In conclusion, our findings demonstrate that NK cells, which express high levels of CD38, are reduced by daratumumab treatment in a rapid, reversible, dose- and concentration-dependent manner. This reduction in NK-cell counts was not associated with response to daratumumab or PFS. In addition, reduction of NK cells was not associated with an increase in safety concerns or AEs, including infections. These data support additional correlative in vitro and in vivo studies to clarify the contribution of NK-cell reduction to the mechanism of action, including daratumumab-mediated T-cell expansion.
Presented in abstract form at the 21st Annual Congress of the European Society of Hematology, Copenhagen, Denmark, 10 June 2016.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated in the GEN501 and SIRIUS studies and their families; the study coinvestigators, research nurses, and coordinators at each of the clinical sites; Khaja Syed for assistance with interpretation and review of the data; and Jaime Bald for operational assistance in managing patient samples and central laboratory testing.
Medical writing and editorial support were provided by Erica Chevalier-Larsen, MedErgy, and were funded by Janssen Global Services, LLC.
This work was supported by Janssen Research & Development and Genmab, and the analyses were supported by Janssen Research & Development.
Authorship
Contribution: S.L., T.P., H.M.L., and N.W.C.J.v.d.D. contributed to the accrual and treatment of patients and data acquisition, interpretation, and analysis; T.C., X.S.X., H.C.A. III, A.E.A., P.L.C., C.C., I.K., T.A., X.Y., and A.K.S. contributed to data acquisition, interpretation, and analysis; T.C., X.S.X., P.L.C., and A.K.S. wrote the first draft of the manuscript; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: T.C., X.S.X., H.C.A. III, A.E.A., C.C., I.K., T.A., X.Y., P.L.C., and A.K.S. are employees of Janssen Research & Development. T.C. and P.L.C. own stock in Johnson & Johnson. S.L., T.P., H.M.L., and N.W.C.J.v.d.D. received research support from Janssen Pharmaceuticals. S.L., T.P., and N.W.C.J.v.d.D. serve on a Janssen advisory committee.
Correspondence: A. Kate Sasser, Janssen Research & Development, LLC, 1400 McKean Rd, Spring House, PA 19002; e-mail: sasserkate@gmail.com.
References
Author notes
T.C. and X.S.X. contributed equally to this study.