Key Points
Prior malignancy negatively impacts survival in patients with MM and >1 prior malignancy reduces survival even further.
A prior malignancy diagnosis increases the risk of developing a second malignancy in patients with MM.
Abstract
In the present study, we aimed to evaluate 2 hypotheses. First, we hypothesize that prior malignancy is a proxy for genetic susceptibility that could be a risk factor for subsequent malignancy development in multiple myeloma (MM) patients. Second, we hypothesize that survival after MM is influenced by a prior malignancy. All patients diagnosed with MM from 1 January 1973 to 31 December 2010 were identified from the Swedish Cancer Register. Cox regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) where prior malignancy was compared in MM patients who developed a subsequent malignancy and MM patients who did not. In another Cox regression model, survival was compared in MM patients with and without a prior malignancy diagnosis. A total of 19 791 patients were diagnosed with MM. Patients with a prior malignancy diagnosis had a significantly increased risk of developing a subsequent malignancy compared with MM patients without (HR 1.42, 95% CI 1.23-1.65, P < .001). MM patients with a prior malignancy diagnosis had a significant 1.21-fold increased risk of death (95% CI 1.115-1.26, P < .001) compared with MM patients without. MM patients with 2 or more prior malignancy diagnoses had a 1.34-fold increased risk of death (95% CI 1.19-1.52, P < .001). In this large population-based study, we report that prior malignancy increases the risk of subsequent malignancy development in MM patients. Furthermore, we found that prior malignancy negatively impacts survival and that >1 prior malignancy reduces survival even further.
Introduction
With improved survival in patients with multiple myeloma (MM), awareness of second malignancies has increased during recent years.1-5 We have previously shown in a large population-based study that the risk of developing any second malignancy is 26% higher in MM patients compared with the general population; most importantly, they had an 11-fold increased risk of developing acute myeloid leukemia and myelodysplastic syndromes (MDS) and a twofold increased risk of developing nonmelanoma skin cancer.6 Other studies have found MM patients to have an increased risk of developing certain types of second cancers, such as melanoma, nervous system tumors, and kidney and urinary tract tumors, although mechanisms and risk factors are not well understood.7-10 Suggested risk factors for second malignancies include treatment-, disease-, environmental-, behavioral-, and host-related factors.4,11-13 Host-related factors include both genetic and nongenetic; reported nongenetic factors include age, male sex, and obesity.1,8,14 Genetic factors implicated in the development of second malignancies include polymorphisms in genes encoding drug-metabolizing enzymes and DNA repair pathways.15,16 In addition, inherited genetic susceptibility for developing MM has been supported by genome-wide association studies that have identified single-nucleotide polymorphisms localized to several genomic regions that are robustly associated with MM risk.17 Furthermore, familial studies on MM patients and their first-, second-, and third-degree relatives have shown a significant excess of cases of prostate cancer and melanoma in all types of relatives.18 This might suggest that the genetic cause of MM overlaps with the causes of other cancers.18-23 Information regarding prior malignancies and their impact on MM patients is limited. For instance, patients with prior malignancies are often excluded from clinical trials,24,25 thus making it difficult to generalize the current literature to this group. In addition, previously published results on the effect of prior malignancies have been conflicting.1,26 In the present study, we aimed to evaluate 2 hypotheses. First, we hypothesize that prior malignancy is a proxy for inherent genetic susceptibility that could be a risk factor for subsequent malignancy development in MM patients. Second, we hypothesize that survival after MM is influenced by a prior malignancy.
Methods
Central registry
All residents in Sweden have equal access to health care under a largely decentralized, taxpayer-funded system. All malignancy diagnoses are reported to the centralized nationwide Swedish Cancer Registry, which was established in 1958.27 The diagnostic accuracy and overall completeness of the Swedish Cancer Registry is high (>95%).28,29 Pathologists and physicians in Sweden are obliged by law to report each malignancy diagnosis to this register. Within the register, information on sex, date of birth, date of malignancy diagnosis, malignancy type, and date of death is registered.
Patient cohort
All patients diagnosed with MM from 1 January 1973 to 31 December 2010 were identified from the Swedish Cancer Registry. Information was gathered on sex, date of birth, date of MM diagnosis, and date of death. All cancer diagnoses prior to and after MM diagnosis were identified through cross-linkage within the Swedish Cancer Registry, and the type and date of the cancer were documented. Prior and subsequent malignancies were classified according to the International Classification of Disease-7 into the following subgroups: (1) breast cancer; (2) bone and cartilage; (3) ear, nose, and throat; (4) endocrine; (5) female reproductive; (6) gastrointestinal; (7) hematological; (8) kidney and urinary tract; (9) male reproductive; (10) melanoma; (11) nervous system; (12) respiratory tract; (13) soft tissue and mediastinal; and (14) unspecified tumors.
Approval was obtained from the Regional Ethical Review Board in Stockholm for this study. Informed consent was waived because we had no contact with study patients.
Data analysis
Risk factor analysis, assessing the effect of prior malignancies on the development of second malignancies in MM
The exposure was the binary categorical variable of a first malignancy diagnosis before MM diagnosis, and the outcome was the binary categorical variable of a primary malignancy diagnosis after MM diagnosis. Demographic characteristics were compared between groups using frequency measures and percentages as well as median values. A Cox regression model was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) where prior malignancy was compared between MM patients who developed a subsequent malignancy and those who did not, adjusted for age at MM diagnosis, date of MM diagnosis, and sex. Two-sided P < .05 was considered statistically significant. All MM patients in the study were censored either at date of death, at the time of first subsequent malignancy diagnosis, or at the end of study (31 December 2013), whichever occurred first. All malignancy diagnoses from autopsies were excluded. Malignancies in the same patient with exactly the same International Classification of Disease diagnosis code were both included if they were registered with more than a 5-year interval (recurrent disease). Time from first prior malignancy diagnosis to MM diagnosis (T1) was compared between MM patients who developed a subsequent primary malignancy and those who did not with Mann-Whitney-Wilcox test. Time from MM diagnosis to subsequent malignancy diagnosis (T2) was compared between those who had a prior malignancy diagnosis and those who did not with the Mood’s median test.
A subgroup analysis was conducted with the same Cox regression model as described above to assess the risk of developing a specific subsequent malignancy subtype in MM patients with a prior malignancy diagnosis compared with those without.
Survival analysis
Survival was estimated from the date of the MM diagnosis until death, emigration, or end of study (31 December 2013), whichever occurred first. The Kaplan-Meier method was used to estimate survival in MM patients with and without a prior malignancy diagnosis. A Cox proportional hazard model was used to calculate HRs and 95% CIs. Two-sided P < .05 was considered statistically significant. A dose-dependent relationship, analyzing the effect of increasing number of malignancies on survival, was estimated using the same method, adjusting for age at MM diagnosis, date of MM diagnosis, and sex.
All statistical analyses were done with R version 3.1.1. (R foundation for Statistical Computing, Vienna, Austria).
Results
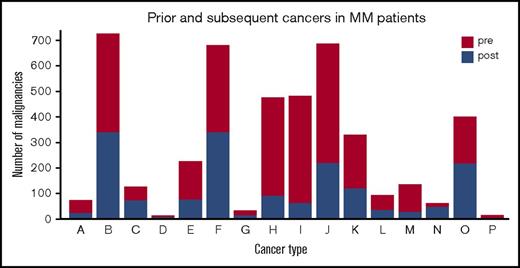
A total of 19 791 patients were diagnosed with MM in Sweden from 1 January 1973 to 31 December 2010. Of these, 2469 (12.5%) patients had 1 or more prior malignancy diagnoses at the time of MM diagnosis, and 17 322 (87.5%) patients had no prior history of malignancy. A total of 216 (8.8%) MM patients with a prior malignancy developed subsequent malignancies. The number of MM patients without a prior malignancy that developed a subsequent malignancy was 1257 (7.3%). Baseline patient characteristics of these groups are compared in Table 1. Types of both prior and subsequent malignancies are seen in Figure 1.
Patient characteristics
| . | MM with prior malignancies . | MM without prior malignancies . |
|---|---|---|
| Patients, n (%) | 2469 (12.5) | 17 322 (87.5) |
| Age at MM diagnosis, median (range), y | 75 (34-98) | 71 (19-99) |
| Male sex, n (%) | 1220 (49.4) | 9 483 (54.5) |
| Year of MM diagnosis by category, n | ||
| 1973-1980 | 197 | 3 188 |
| 1981-1990 | 463 | 4 657 |
| 1991-2000 | 720 | 4 644 |
| 2001-2010 | 1089 | 4 833 |
| Year of MM diagnosis, median (range) | 1999 (1973-2010) | 1992 (1973-2010) |
| Follow-up, median (range), y | 2.0 (0.003-29.4) | 2.6 (0.003-40.9) |
| Time to subsequent malignancy, median (range), y | 2.3 (0.02-21.5) | 3.2 (0.003-37.5) |
| Prior malignancy type, no. of patients (%) | ||
| Hematologic | 163 (6.6) | — |
| Gastrointestinal | 323 (13.1) | — |
| Male reproductive | 427 (17.3) | — |
| Female reproductive | 401 (16.2) | — |
| Breast | 329 (13.3) | — |
| Kidney and urinary tract | 186 (7.5) | — |
| Melanoma | 125 (5.1) | — |
| Nonmelanoma skin cancer | 235 (9.5) | — |
| Respiratory | 43 (1.7) | — |
| Ear, nose, and throat | 46 (1.8) | — |
| Endocrine | 93 (3.8) | — |
| Nervous system | 61 (2.4) | — |
| Bone and cartilage | 6 (0.2) | — |
| Soft tissue and mediastinal | 17 (0.7) | — |
| Unspecified tumors | 14 (0.6) | — |
| Number of prior malignancy diagnoses | ||
| 1 | 2158 | — |
| ≥2 | 311 | — |
| Patients with subsequent malignancies, n (%) | 216 (8.8) | 1 257 (7.3) |
| . | MM with prior malignancies . | MM without prior malignancies . |
|---|---|---|
| Patients, n (%) | 2469 (12.5) | 17 322 (87.5) |
| Age at MM diagnosis, median (range), y | 75 (34-98) | 71 (19-99) |
| Male sex, n (%) | 1220 (49.4) | 9 483 (54.5) |
| Year of MM diagnosis by category, n | ||
| 1973-1980 | 197 | 3 188 |
| 1981-1990 | 463 | 4 657 |
| 1991-2000 | 720 | 4 644 |
| 2001-2010 | 1089 | 4 833 |
| Year of MM diagnosis, median (range) | 1999 (1973-2010) | 1992 (1973-2010) |
| Follow-up, median (range), y | 2.0 (0.003-29.4) | 2.6 (0.003-40.9) |
| Time to subsequent malignancy, median (range), y | 2.3 (0.02-21.5) | 3.2 (0.003-37.5) |
| Prior malignancy type, no. of patients (%) | ||
| Hematologic | 163 (6.6) | — |
| Gastrointestinal | 323 (13.1) | — |
| Male reproductive | 427 (17.3) | — |
| Female reproductive | 401 (16.2) | — |
| Breast | 329 (13.3) | — |
| Kidney and urinary tract | 186 (7.5) | — |
| Melanoma | 125 (5.1) | — |
| Nonmelanoma skin cancer | 235 (9.5) | — |
| Respiratory | 43 (1.7) | — |
| Ear, nose, and throat | 46 (1.8) | — |
| Endocrine | 93 (3.8) | — |
| Nervous system | 61 (2.4) | — |
| Bone and cartilage | 6 (0.2) | — |
| Soft tissue and mediastinal | 17 (0.7) | — |
| Unspecified tumors | 14 (0.6) | — |
| Number of prior malignancy diagnoses | ||
| 1 | 2158 | — |
| ≥2 | 311 | — |
| Patients with subsequent malignancies, n (%) | 216 (8.8) | 1 257 (7.3) |
—, Not applicable; n, number of patients.
Number of prior and subsequent malignancies in MM patients according to malignancy types. Group letters: A, ear, nose, and throat; B, gastrointestinal; C, respiratory; D, bone and cartilage; E, melanoma; F, nonmelanoma skin cancer; G, soft tissue and mediastinal; H, breast malignancy; I, female reproductive; J, male reproductive; K, kidney and urinary tract; L, nervous system; M, endocrine; N, unspecified tumors; O, hematological; and P, eye tumors.
Number of prior and subsequent malignancies in MM patients according to malignancy types. Group letters: A, ear, nose, and throat; B, gastrointestinal; C, respiratory; D, bone and cartilage; E, melanoma; F, nonmelanoma skin cancer; G, soft tissue and mediastinal; H, breast malignancy; I, female reproductive; J, male reproductive; K, kidney and urinary tract; L, nervous system; M, endocrine; N, unspecified tumors; O, hematological; and P, eye tumors.
MM patients with a prior malignancy diagnosis had an increased risk of developing a subsequent malignancy after MM diagnosis compared with MM patients without a prior malignancy (HR 1.42; 95% CI 1.23-1.65, P < .001) (Table 2). In a subgroup analysis, any prior malignancy in MM patients was associated with an increased risk of developing hematological (1.59; 95% CI 1.04-2.42, P = .032), malignant melanoma (HR 2.67; 95% CI 1.43-5.00, P = .002), nonmelanoma skin cancer (HR 1.99; 1.47-2.71, P < .001), and malignancies of the respiratory tract (HR 3.24; 1.79-5.88, P < .001) (Table 2).
The risk of developing a certain subsequent malignancy subtype in MM patients with a prior malignancy diagnosis compared with those without
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Overall | 1.42 | 1.23-1.65 | <.001 |
| Hematologic (n = 193) | 1.59 | 1.04-2.42 | .032 |
| Gastrointestinal (n = 318) | 1.13 | 0.81-1.58 | .475 |
| Male reproductive (n = 204) | 0.74 | 0.44-1.26 | .276 |
| Female reproductive (n = 58) | 0.71 | 0.28-1.79 | .468 |
| Breast (n = 83) | 1.16 | 0.61-2.22 | .643 |
| Kidney and urinary tract (n = 105) | 1.40 | 0.80-2.41 | .234 |
| Melanoma (n = 58) | 2.67 | 1.43-5.00 | .002 |
| Nonmelanoma skin cancer (n = 256) | 1.99 | 1.47-2.71 | <.001 |
| Respiratory (n = 64) | 3.24 | 1.79-5.88 | <.001 |
| Oral, nasal, and pharyngeal (n = 18) | 1.30 | 0.29-5.86 | .731 |
| Endocrine (n = 24) | 0.78 | 0.18-3.37 | .736 |
| Nervous system (n = 35) | 0.86 | 0.26-2.87 | .808 |
| Bone and cartilage (n = 5) | — | — | — |
| Soft tissue and mediastinal (n = 13) | 1.91 | 0.40-9.03 | .415 |
| Unspecified tumors (n = 39) | 1.28 | 0.49-3.33 | .618 |
| . | HR . | 95% CI . | P . |
|---|---|---|---|
| Overall | 1.42 | 1.23-1.65 | <.001 |
| Hematologic (n = 193) | 1.59 | 1.04-2.42 | .032 |
| Gastrointestinal (n = 318) | 1.13 | 0.81-1.58 | .475 |
| Male reproductive (n = 204) | 0.74 | 0.44-1.26 | .276 |
| Female reproductive (n = 58) | 0.71 | 0.28-1.79 | .468 |
| Breast (n = 83) | 1.16 | 0.61-2.22 | .643 |
| Kidney and urinary tract (n = 105) | 1.40 | 0.80-2.41 | .234 |
| Melanoma (n = 58) | 2.67 | 1.43-5.00 | .002 |
| Nonmelanoma skin cancer (n = 256) | 1.99 | 1.47-2.71 | <.001 |
| Respiratory (n = 64) | 3.24 | 1.79-5.88 | <.001 |
| Oral, nasal, and pharyngeal (n = 18) | 1.30 | 0.29-5.86 | .731 |
| Endocrine (n = 24) | 0.78 | 0.18-3.37 | .736 |
| Nervous system (n = 35) | 0.86 | 0.26-2.87 | .808 |
| Bone and cartilage (n = 5) | — | — | — |
| Soft tissue and mediastinal (n = 13) | 1.91 | 0.40-9.03 | .415 |
| Unspecified tumors (n = 39) | 1.28 | 0.49-3.33 | .618 |
Two-sided P < .05 was considered statistically significant, shown in boldface.
The median time from the first prior malignancy diagnosis to MM diagnosis was 7.1 years both in the group that did not develop a subsequent malignancy and in the group that did develop a subsequent malignancy (P = .732). The median time to first subsequent malignancy diagnosis was 2.3 years (range 0.02-21.5) among patients who had a prior malignancy diagnosis compared with 3.2 years (range 0.003-37.5) in patients who did not have a prior malignancy diagnosis (P = .003).
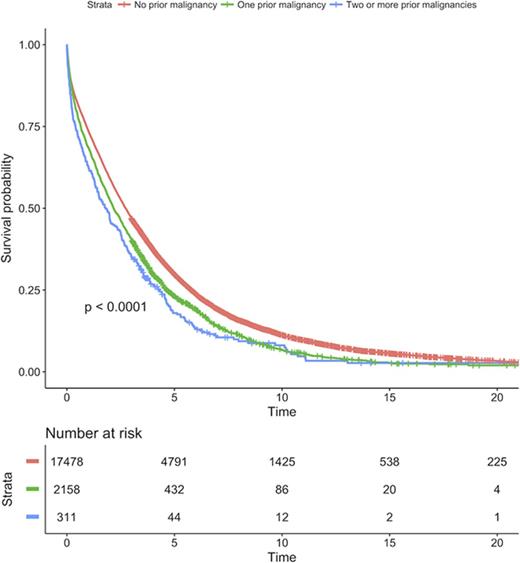
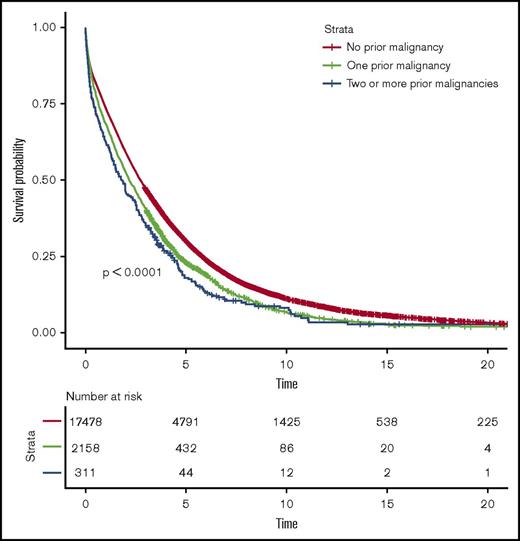
MM patients with a prior malignancy diagnosis had a statistically significant 21% increased risk of death (HR = 1.21, 95% CI 1.15-1.26, P < .001) compared with MM patients without a prior malignancy diagnosis (Figure 2). In a dose-response analysis, MM patients with ≥2 malignancy diagnoses had a 34% increased risk of death (HR = 1.34, 95% CI 1.19-1.52, P < .001) compared with MM patients without a prior malignancy diagnosis (Figure 2).
Survival in MM patients with and without prior malignancies. Survival was compared between patients with no prior malignancy, 1 prior malignancy, and 2 or more prior malignancies.
Survival in MM patients with and without prior malignancies. Survival was compared between patients with no prior malignancy, 1 prior malignancy, and 2 or more prior malignancies.
Discussion
In our large population-based study, we found that prior malignancy diagnosis increased the risk of developing hematologic malignancies, melanoma, nonmelanoma skin cancer, and respiratory tract malignancies in patients with MM. In addition, we show that prior malignancy negatively impacts survival in patients with MM and that >1 prior malignancy reduces survival even further. We confirmed prior reports of solid tumors being more common than hematologic malignancies in MM patients, both prior and subsequent to the MM diagnosis.2,26,30
We found that 12.5% of patients with MM had prior malignancy and 8.1% developed a second malignancy. These results are similar to the findings of a recent registry analysis on 744 MM patients by Engelhardt et al.1 Although another study by Hasskarl et al reported lower rates, 7% and 3%, respectively,2 we also found that 6.6% of prior malignancies and 12.5% of subsequent malignancies were hematological. This rate of subsequent hematological malignancies has been observed in hematological malignancies other than MM, such as Hodgkin lymphoma.31 The most frequently diagnosed malignancies prior to MM were gastrointestinal and female- and male-reproductive cancers. This is in line with Engelhardt et al’s study,1 although we found breast cancers to be more common compared with their study.
A prior malignancy diagnosis in MM patients was associated with a 40% increased risk of developing a subsequent malignancy, and these patients developed their subsequent malignancy almost a year sooner than those without a history of prior malignancy. There are limited published data regarding this association, and 2 recent registry studies reported conflicting results.1,26 The Connect MM registry study by Rifkin et al comprised 1430 MM patients treated with lenalidomide and reported that prior invasive malignancies increased the risk of developing subsequent malignancies.26 In contrast to this, Engelhardt et al reported in a registry study including 774 MM patients that prior malignancy did not increase the risk of a subsequent malignancy.1 These studies have a small number of patients that developed second malignancies, 49 and 59 patients, respectively. Underlying explanation for our findings, that a prior malignancy increases the risk of second malignancies in MM patients, could include genetic susceptibilities,17-22,32-35 immunosuppression,36-38 and therapy-related cancers.12,13,39
We found MM patients with a prior cancer diagnosis to have an increased risk of developing hematological malignancy, melanoma, nonmelanoma skin cancer, and respiratory malignancy compared with MM patients who did not. We have previously shown that both patients with the precursor condition monoclonal gammopathy of undetermined significance and MM have an increased risk of developing nonmelanoma skin cancer, suggesting a possible biological relationship.6 Increased incidence of melanoma in MM patients has also been reported,8,40 and it has been shown that both melanoma and nonmelanoma skin cancers are increased in other immunocompromised patient groups.36-38 Interestingly, familial studies on MM patients and their relatives have shown a significant excess of cancers such as melanoma in these families, suggesting that in some cases the genetic cause of MM might overlap with that of melanoma. In addition, studies have found the same oncogenic mutations in both MM and melanoma.19,32,33,41 We found an association between prior malignancy and the development of respiratory malignancies in MM patients. To our knowledge, MM patients have not been found to have increased risk of developing respiratory malignancies; on the contrary, data from the Surveillance, Epidemiology, and End Results database suggest reduced incidence in MM patients.40 The most common prior malignancy diagnosis in MM patients with subsequent respiratory tract cancer was female reproductive cancer (40%; more specifically, cervical cancer). The reason for this is unclear. Smoking is a risk the factor for both cervical cancer and respiratory tract malignancies,42 although it is not a risk factor for MM. The increased incidence of hematological malignancies, specifically acute myeloid leukemia/MDS, is well documented in MM patients,4,6,12,13 where alkylating agents have been considered to be 1 of the main contributing factors, although a role for non-treatment-related factors has also been reported,4-6,8 and the recent discovery of a genotype associated with the development of MDS in MM22 supports a role for susceptibility genes in this development.
Taken together, a prior cancer diagnosis increased the risk of second malignancy development in MM patients and might suggest inherent genetic susceptibility in these patients, although further research is needed to confirm this.
We found that prior malignancy negatively impacts survival in MM patients. We showed that this relationship was dose dependent because patients with 2 or more prior malignancy diagnoses had a significantly twofold increased risk of death compared with patients with 1 prior malignancy diagnosis. Interestingly, studies have found patients with other hematological malignancies, such as chronic lymphocytic leukemia, to have a reduced survival if they had a prior history of nonmelanoma skin cancer.43 The pathologic mechanism behind these findings is unknown, but the authors suggested that patients with prior history of nonmelanoma skin cancer might have more aggressive disease. Our results confirm the findings that prior or synchronous malignancies increased the risk of death in MM patients.1 Reduced survival in MM patients with prior malignancies is likely multifactorial and could include reduced dose intensity of chemo- and radiation therapy, detrimental effects of previous chemo-radiation or surgical treatments on physical condition of patients, or that MM that develops after another malignancy might be biologically different. Taken together, the impact of prior malignancies is clinically relevant for the individual patient and warrants future research and attention.
Our study has several strengths, including a large sample size, long study period, and application of high-quality population-based data from Sweden. By using the nationwide register-based design, where data are gathered prospectively, we were able to account for recall bias and ensure the generalizability of our results. Underreporting of tumors should not affect our results to any extent because the overall completeness of the Swedish Cancer Registry has been reported to be >95%, although substantial underreporting has been noted for leukemia.28 In a recent validation study, the diagnostic accuracy was ∼98% for hematopoietic lymphoproliferative malignancies in Sweden.29
Limitations include the lack of detailed clinical and treatment data for the patient population in our study, including information on cause of death, pathological stage of the prior cancers, MM, and subsequent cancers. In addition, we did not have information on genetic profiles. A possible bias in cohort selection due to left censoring cannot be excluded, but we designed the study period to allow prior malignancies to be recorded, because the Swedish Cancer Registry started in 1958 and patient enrollment started in 1973, allowing for a 15-year lead time. In addition, we stratified our data according to decade of MM diagnosis and age category. Regarding the risk factor analysis, it should be considered that because survival is significantly reduced in patients with prior malignancies, this group could possibly have had less time to develop a subsequent malignancy compared with patients without a prior malignancy. On the contrary, the patients with prior malignancy developed their subsequent malignancy significantly earlier than patients without a prior malignancy. To minimize this effect, we adjusted for age at MM diagnosis. Because patients could be diagnosed with a prior and second malignancy 1 day before and after MM diagnosis, we performed a sensitivity analysis excluding all patients diagnosed with prior or subsequent malignancy within a month of the MM diagnosis with no effect to our overall findings (data not shown). Several of the subtype analyses had a limited number of patients and should be interpreted with caution.
In this large population-based study, including ∼20 000 patients with MM, we report that prior malignancy increases the risk of subsequent malignancy development in MM patients. Furthermore, we found that prior malignancy negatively impacts survival and that >1 prior malignancy reduces survival even further. The underlying explanation for our findings could suggest a role for susceptibility genes in the development of second malignancies; other possible etiologies include immune dysfunction in these patients or side effects from treatment. Given the increase of malignancy survivors in general, our findings are of importance both for the individual patients and their families and for the treating physician.
Presented in abstract form at the 58th annual meeting of the American Society of Hematology, San Diego, CA, 3-6 December 2016.
Acknowledgments
This research was supported by grants from the Asrun Einarsdottir Foundation in Iceland (G.J.), Blodcancerfonden, the Swedish Cancer Society, the Regional Agreement on Medical Training and Clinical Research between Stockholm County Council and Karolinska Institutet, Karolinska Institutet Foundations, the University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Marie Curie Career Integration Grant (S.Y.K.), and Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748) by the National Cancer Institute, National Institutes of Health (O.L.).
G.J. is a PhD candidate at University of Iceland, and this work is submitted in partial fulfillment of the requirement for a PhD.
Authorship
Contribution: S.Y.K., G.J., and S.H.L. designed the study; G.J. and S.Y.K. obtained data; G.J. and S.H.L. performed the analyses; and G.J., S.Y.K., S.H.L., M.B., I.T., M.H., A.P., Y.S.J., and O.L. were involved in the analyses and the interpretation of the results, read, gave comments, and approved the final version of the manuscript, had full access to the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sigurdur Y. Kristinsson, Faculty of Medicine, University of Iceland, Saemundargata 2, 101 Reykjavik, Iceland; e-mail: sigyngvi@hi.is.