Key Points
Nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide carries an acceptable safety profile.
This platform may expand the donor pool for patients who lack an HLA-matched or -haploidentical donor.
Abstract
Allogeneic blood or marrow transplantation (BMT) candidates may lack HLA-matched, related haploidentical, and unrelated umbilical cord options. Barriers to partially HLA-mismatched, unrelated donor (mMUD) BMT include excess graft-versus-host disease (GVHD), graft failure, and death. We prospectively studied nonmyeloablative (NMA) mMUD BMT with high-dose posttransplantation cyclophosphamide (PTCy) for patients with hematologic malignancies. Three transplants were performed with busulfan/fludarabine conditioning, with subsequent change to fludarabine/Cy/total body irradiation (flu/Cy/TBI). Twenty mMUD transplants are reported using flu/Cy/TBI, T-cell replete bone marrow grafts, and PTCy, mycophenolate mofetil, and sirolimus or tacrolimus (1 patient) for GVHD prophylaxis. The median patient age was 56. Of these unrelated grafts, 45% had ≥2 mismatched HLA loci, 25% had ≥3 mismatched loci, and 50% had HLA-C mismatches. No graft failure or grades 3-4 acute GVHD occurred. The median times to neutrophil recovery (≥500/µL) and platelet recovery (≥20 000/µL) were 19 days and 31 days, respectively. Full-donor chimerism was achieved in 95% of evaluable patients by day 60. The 180-day probability of grades 2-4 acute GVHD (all grade 2) was 25%, and the 1-year probability of any chronic GVHD was 16% (none severe). The 2-year nonrelapse mortality probability was 6%. With 4-year median follow-up, the 1-year progression-free and overall survival probabilities were 65% and 75%, respectively. NMA, T-cell replete mMUD BMT is thus a potentially viable option for patients without other suitable donors. This trial was registered at www.clinicaltrials.gov as #NCT01203722.
Introduction
The safety and efficacy of nonmyeloablative (NMA), related HLA-haploidentical blood or marrow transplantation (haplo BMT) with high-dose posttransplantation cyclophosphamide (PTCy)1-4 and unrelated umbilical cord transplantation5 comparable to matched BMT have been proven. Unfortunately, even these mismatched sources will be unavailable for some patients, including those with cytotoxic donor-specific anti-HLA antibodies (DSAs).6 Partially HLA-mismatched unrelated donor (mMUD) BMT could be considered if a matched unrelated donor (MUD) is unavailable. However, mMUD BMT, particularly if mismatched at more than 1 locus, has historically caused excess graft-versus-host disease (GVHD), graft failure, and nonrelapse mortality (NRM).7-9 We hypothesized that PTCy might overcome this HLA barrier, as it has in other settings, without requiring T-cell depletion.10-13 We developed a trial of NMA, T-cell replete BMT with PTCy for hematologic malignancies patients lacking an HLA-matched or haplo donor, and report the initial experience with mMUD BMT.
Patients and methods
This single-arm clinical trial was conducted at Johns Hopkins University. The study received Johns Hopkins and National Marrow Donor Program (NMDP) institutional review board approval. The primary objective was to identify a transplant regimen having a ≤25% risk of grades 3-4 acute GVHD and a ≤20% risk of NRM by day 100, using mMUDs or haplo donors other than first-degree relatives or half-siblings. Results of 23 consecutive mMUD transplants are presented. Further study of the prioritized regimen continues on this trial.
Eligible patients were ages 0.5 to 75 years, lacked a family donor (at least HLA-haplo first-degree relative or half-sibling) or MUD, and had an Eastern Cooperative Oncology Group performance status of ≤2, left ventricular ejection fraction of ≥35%, forced expiratory volume in 1 second and forced vital capacity ≥40% predicted, adequate hepatic function, and no prior allografting. One patient was infected with HIV. To mitigate graft rejection, we recommended cytotoxic chemotherapy or ≥4 cycles of a hypomethylating agent before conditioning.
Unrelated donors shared ≥5/10 but <10/10 HLA alleles (HLA-A, -B, -Cw, -DRB1, -DQB1) with ≥1 allele matched for a class I gene and a class II gene. Donors having the fewest mismatched loci were prioritized for, in order of priority, major ABO compatibility, matched cytomegalovirus (CMV) IgG serostatus, minor ABO compatibility, and male donor for male recipient. Donors were ineligible if the recipient had DSAs too strong for desensitization. However, desensitization for DSAs against the unrelated donor was permitted and occurred in 1 patient using published methods.6
All patients received T-cell replete bone marrow grafts. The first 3 patients received busulfan (1 mg/kg by mouth twice daily for 4 days, with pharmacokinetic adjustment) and fludarabine (flu; 30 mg/m2 IV daily for 5 days, renally adjusted) conditioning, then PTCy, mycophenolate mofetil, and sirolimus per below. On the basis of excess graft failure in a concurrent institutional trial of busulfan/flu-conditioned related BMT, all subsequent patients received our institutional standard of flu, Cy, and TBI.13
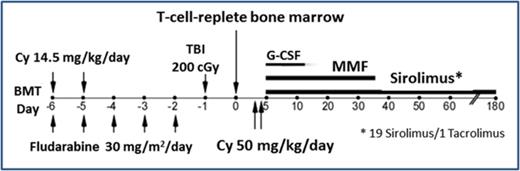
Twenty mMUD transplants with flu/Cy/TBI conditioning were performed between July 2011 and August 2015 and comprise the main analysis. The regimen is summarized in Table 1. Conditioning consisted of cyclophosphamide (14.5 mg/kg IV on days −6 and −5), flu (30 mg/m2 IV on days −6 to −2, renally adjusted), and total body irradiation (200 cGy on day −1). GVHD prophylaxis consisted of high-dose PTCy (50 mg/kg IV daily on days +3 and +4) with mesna,13 mycophenolate mofetil 15 mg/kg by mouth thrice daily (maximum 3 g/day) from day 5 to day 35, and sirolimus (19 patients) or tacrolimus (1 patient, pending early data with sirolimus as per protocol) from day 5 to day 180. Sirolimus (target trough, 5-12 ng/mL) was prioritized over tacrolimus because of potentially less posterior reversible encephalopathy syndrome14 and nephrotoxicity.15 A sirolimus loading dose (6 mg for adults) was given on day 5, then maintenance dosing (initially 2 mg daily). Filgrastim began on day 5. Prophylactic immunosuppression stopped without taper at day 180.
Schema of prioritized regimen for nonmyeloablative, HLA-mismatched unrelated BMT
| Day . | Drug and adult dosing* . |
|---|---|
| Days –6 through –2 | Fludarabine 30 mg/m2 IV qd (adjusted for renal function) |
| Days –6 and –5 | Cyclophosphamide 14.5 mg/kg IV qd |
| Day –1 | Total body irradiation, 200 cGy |
| Day 0 | Infuse T-cell replete bone marrow† |
| Begin infection prophylaxis (no voriconazole) | |
| Days 3 and 4 | Cyclophosphamide 50 mg/kg IV qd over 1-2 h with hydration‡ |
| Mesna 40 mg/kg IV qd in divided doses | |
| Day 5§ | Sirolimus loading dose: 6 mg po once |
| Begin MMF 15 mg/kg po tid (maximum 3 g/day) | |
| Begin filgrastim 5 µg/kg SC or IV qd (until ANC ≥1000/µL for 3 d) | |
| Day 6 | Begin sirolimus 2 mg po qd; adjust to maintain trough of 5-12 ng/mL |
| Day 35 | Discontinue MMF after last dose‖ |
| Day 180 | Discontinue sirolimus after last dose‖ |
| Day . | Drug and adult dosing* . |
|---|---|
| Days –6 through –2 | Fludarabine 30 mg/m2 IV qd (adjusted for renal function) |
| Days –6 and –5 | Cyclophosphamide 14.5 mg/kg IV qd |
| Day –1 | Total body irradiation, 200 cGy |
| Day 0 | Infuse T-cell replete bone marrow† |
| Begin infection prophylaxis (no voriconazole) | |
| Days 3 and 4 | Cyclophosphamide 50 mg/kg IV qd over 1-2 h with hydration‡ |
| Mesna 40 mg/kg IV qd in divided doses | |
| Day 5§ | Sirolimus loading dose: 6 mg po once |
| Begin MMF 15 mg/kg po tid (maximum 3 g/day) | |
| Begin filgrastim 5 µg/kg SC or IV qd (until ANC ≥1000/µL for 3 d) | |
| Day 6 | Begin sirolimus 2 mg po qd; adjust to maintain trough of 5-12 ng/mL |
| Day 35 | Discontinue MMF after last dose‖ |
| Day 180 | Discontinue sirolimus after last dose‖ |
ANC, absolute neutrophil count; MMF, mycophenolate mofetil; po, by mouth; qd, once daily; SC, subcutaneous; tid, three times daily.
Dose cyclophosphamide and mesna using the lesser of ideal and actual body weight. Dose fludarabine and MMF using actual body weight.
Harvest donor bone marrow with a target yield of 4 × 108 nucleated cells/kg recipient’s ideal body weight; the lowest acceptable yield is 1.5 × 108 nucleated cells/kg.
Day 3 cyclophosphamide is ideally given 60-72 h after graft infusion.
No systemic immunosuppressive agents, including corticosteroid anti-emetics, are to be given until at least 24 h after completion of all cyclophosphamide.
Optional if graft-versus-host disease is present.
Progression-free survival (PFS), overall survival (OS), and GVHD-free relapse-free survival (GRFS)16 were estimated by the Kaplan-Meier method. Median follow-up was calculated by the reverse Kaplan-Meier method. Cumulative incidences (CuI) of relapse, NRM, count recovery, and GVHD were estimated with competing-risk methods,17 as previously described.18 PFS failures and graft failure were considered competing risks for GVHD. Graft failure was defined as a ≤5% donor chimerism in peripheral blood or bone marrow by ∼day 60 without detected bone marrow disease. Donor chimerism was evaluated at ∼day 30, day 60, day 180, and 1 year.
Safety stopping boundaries were based primarily on a ≥80% posterior probability by day 100 of grades 3-4 acute GVHD being >25% or transplant-related NRM being >20%.19 The database was locked September 20, 2016.
Results
BMT with busulfan/fludarabine conditioning
The first patient (8/10 matched, with mismatches at HLA-Bw and -C), with myelodysplastic syndrome, had primary graft failure and ultimately died of relapse. The second patient (8/10 matched, with mismatches at HLA-A and -Bw) had NRM at day 29 from acute hepatic failure then multiorgan failure; engraftment was unevaluable. A third patient (9/10 matched, with mismatch at HLA-A) achieved full-donor chimerism without grades 2-4 acute GVHD or prohibitive toxicity. As was mentioned, study of this regimen was stopped, and recipients of flu/Cy/TBI conditioning comprise the remainder of the analysis.
BMT with fludarabine/cyclophosphamide/TBI conditioning
Table 2 presents baseline patient and graft characteristics. The median patient age was 56, with 7 (35%) being age ≥60. The majority (70%) had intermediate-risk disease by the refined Disease Risk Index. Reasons for mMUDs included no suitable adult haplo donor (60%) and DSAs not amenable to desensitization (30%).
Patient and transplant characteristics
| Variable . | From N of 20 . | |
|---|---|---|
| Patients | ||
| Patient age, y, median (range) | 56 | (37-66) |
| Male sex | 12 | (60%) |
| Race | ||
| White | 17 | (85%) |
| Black | 3 | (15%) |
| Reason for unrelated donor | ||
| No suitable adult family donor | 12 | (60%) |
| DSAs not amenable to desensitization | 6 | (30%) |
| Familial cancer susceptibility | 2 | (10%) |
| Diagnosis | ||
| Acute myeloid leukemia | 11 | (55%) |
| MDS or MPN | 4* | (20%) |
| Chronic myeloid leukemia in CP2 | 1 | (5%) |
| Non-Hodgkin lymphoma | 4† | (20%) |
| Refined DRI group | ||
| Low risk | 2 | (10%) |
| Intermediate risk | 14 | (70%) |
| High risk | 4 | (20%) |
| Very high risk | 0 | (0%) |
| CR at transplant | 14 | (70%) |
| Post-BMT maintenance therapy | 3‡ | (15%) |
| Unrelated grafts | ||
| HLA matches§ | ||
| 5/10 (1 locus mismatch at A, B, Cw, DRB1, DQB1) | 1 | (5%) |
| 6/10 (1 A, 1 Cw, both B loci mismatched) | 1 | (5%) |
| 7/10 (1 locus mismatch at class I, DRB1, DQB1) | 3 | (15%) |
| 8/10 | 4‖ | (20%) |
| 9/10 | 11¶ | (55%) |
| Class I + II antigen mismatches# | ||
| 3 | 2 | (10%) |
| 2 | 4 | (20%) |
| 1 | 10 | (50%) |
| 0** | 4 | (20%) |
| HLA-C mismatch | 10 | (50%) |
| Complete HLA-DP mismatch | 9 | (45%) |
| ABO incompatibility | 12 | (60%) |
| Major | 7 | (35%) |
| Minor | 4 | (20%) |
| Both major and minor | 1 | (5%) |
| CMV serostatus | ||
| CMV IgG mismatch | 8 | (40%) |
| Patient CMV seropositive | 14 | (70%) |
| Donor age, y, median (range) | 33 | (20-40) |
| Female donor/male recipient | 6 | (30%) |
| Cell dose infused, median (IQR) | ||
| Total nucleated cells × 108/kg†† | 3.2 | (2.7-4.4) |
| CD34+ cells × 106/kg | 2.8 | (2.0-4.9) |
| CD3+ cells × 107/kg | 3.4 | (2.7-5.0) |
| Variable . | From N of 20 . | |
|---|---|---|
| Patients | ||
| Patient age, y, median (range) | 56 | (37-66) |
| Male sex | 12 | (60%) |
| Race | ||
| White | 17 | (85%) |
| Black | 3 | (15%) |
| Reason for unrelated donor | ||
| No suitable adult family donor | 12 | (60%) |
| DSAs not amenable to desensitization | 6 | (30%) |
| Familial cancer susceptibility | 2 | (10%) |
| Diagnosis | ||
| Acute myeloid leukemia | 11 | (55%) |
| MDS or MPN | 4* | (20%) |
| Chronic myeloid leukemia in CP2 | 1 | (5%) |
| Non-Hodgkin lymphoma | 4† | (20%) |
| Refined DRI group | ||
| Low risk | 2 | (10%) |
| Intermediate risk | 14 | (70%) |
| High risk | 4 | (20%) |
| Very high risk | 0 | (0%) |
| CR at transplant | 14 | (70%) |
| Post-BMT maintenance therapy | 3‡ | (15%) |
| Unrelated grafts | ||
| HLA matches§ | ||
| 5/10 (1 locus mismatch at A, B, Cw, DRB1, DQB1) | 1 | (5%) |
| 6/10 (1 A, 1 Cw, both B loci mismatched) | 1 | (5%) |
| 7/10 (1 locus mismatch at class I, DRB1, DQB1) | 3 | (15%) |
| 8/10 | 4‖ | (20%) |
| 9/10 | 11¶ | (55%) |
| Class I + II antigen mismatches# | ||
| 3 | 2 | (10%) |
| 2 | 4 | (20%) |
| 1 | 10 | (50%) |
| 0** | 4 | (20%) |
| HLA-C mismatch | 10 | (50%) |
| Complete HLA-DP mismatch | 9 | (45%) |
| ABO incompatibility | 12 | (60%) |
| Major | 7 | (35%) |
| Minor | 4 | (20%) |
| Both major and minor | 1 | (5%) |
| CMV serostatus | ||
| CMV IgG mismatch | 8 | (40%) |
| Patient CMV seropositive | 14 | (70%) |
| Donor age, y, median (range) | 33 | (20-40) |
| Female donor/male recipient | 6 | (30%) |
| Cell dose infused, median (IQR) | ||
| Total nucleated cells × 108/kg†† | 3.2 | (2.7-4.4) |
| CD34+ cells × 106/kg | 2.8 | (2.0-4.9) |
| CD3+ cells × 107/kg | 3.4 | (2.7-5.0) |
CP, chronic phase; CR, complete remission; DRI, Disease Risk Index; IgG, immunoglobulin G; IQR, interquartile range; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm.
One MDS, 1 MDS/MPN, 1 primary myelofibrosis, 1 primary myelofibrosis with transformed lymphoma.
Two peripheral T-cell lymphoma, 1 follicular lymphoma, 1 low-grade lymphoma with multiple myeloma.
One ponatinib, 2 sorafenib.
Refer to supplemental Table 1 for a per-patient list of mismatched HLA alleles.
Three grafts mismatched at 1 class I and 1 class II locus, 1 graft mismatched at 2 class II loci.
Nine grafts mismatched at 1 class I locus, 1 mismatched at DRB1, 1 mismatched at DQB1.
Mismatching in either direction (composite of HLA-A, -B, -Cw, -DRB1, and -DQB1).
Allele-level mismatch without antigen-level mismatch.
Target dose, 4 × 108 nucleated cells/kg ideal body weight.
Of these unrelated grafts, 45% had ≥2 mismatched HLA loci, and 25% had ≥3 mismatched loci (three 7/10 matches, one 6/10, and one 5/10). Half were HLA-C mismatched, and 45% had complete HLA-DP mismatch. The median total number of HLA antigen mismatches was 1.
Engraftment and GVHD
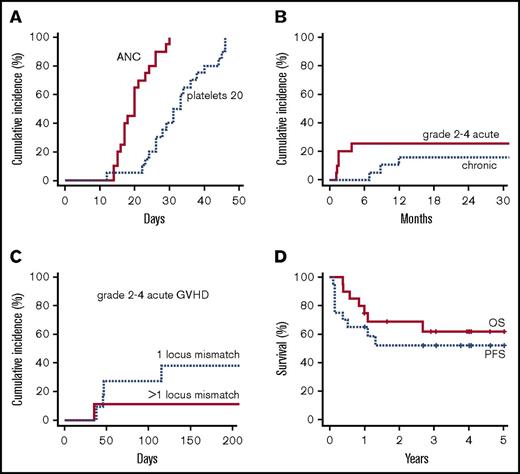
No primary or secondary graft failure occurred. All patients achieved neutrophil recovery to ≥500/µL and platelet recovery to ≥20 000/µL. The median neutrophil recovery time was 19 (range, 14-30) days; the median platelet recovery time to ≥20 000/µL was 31 (range, 12-46) days (Figure 1A). The estimated CuI of platelet recovery to ≥50 000/µL was 85% by day 60 (median, 36 days), with recovery in 17/19 evaluable patients.
Overall outcomes of nonmyeloablative, HLA-mismatched unrelated donor BMT. (A) Cumulative incidence of count recovery. (B) Cumulative incidence of grade 2-4 acute GVHD (all grade 2) and any chronic GVHD by competing-risk analysis. (C) Cumulative incidence of grade 2-4 acute GVHD by number of mismatched HLA loci. (D) Progression-free and overall survival. ANC, absolute neutrophil count.
Overall outcomes of nonmyeloablative, HLA-mismatched unrelated donor BMT. (A) Cumulative incidence of count recovery. (B) Cumulative incidence of grade 2-4 acute GVHD (all grade 2) and any chronic GVHD by competing-risk analysis. (C) Cumulative incidence of grade 2-4 acute GVHD by number of mismatched HLA loci. (D) Progression-free and overall survival. ANC, absolute neutrophil count.
Full-donor chimerism in either CD3+ or unsorted cells was achieved in 16/19 (84%) evaluable patients by day 30 and 18/19 (95%) evaluable patients by day 60. The latter included the patient (7/10 matched) who required desensitization for cytotoxic anti-HLA antibodies against the unrelated donor.6 Day 30 chimerism was not assessed in 1 patient, and day 60 chimerism was unevaluable in 1 patient because of relapse.
No grade 3-4 acute GVHD occurred. The estimated CuI of grade 2-4 GVHD (all grade 2) was 20% at day 100 (90% confidence interval [CI], 5-35) and 25% at day 180 (90% CI, 9-42) (Figure 1B). Four patients had skin-limited grade 2 GVHD; 1 patient (9/10 matched) had grade 2 skin and ungradable visceral GVHD.
The 180-day CuI of grade 2-4 acute GVHD was 38% (90% CI, 11-64) with 1 HLA-mismatched locus and 11% (90% CI, 0-29) with >1 mismatched locus (Figure 1C). Notably, 1 of 5 patients with ≥3 HLA mismatches developed grade 2 acute GVHD. The 1-year CuI of chronic GVHD was 16% (90% CI, 0-30), with 1 moderate case and no severe cases. The 1-year CuI of starting systemic immunosuppression for GVHD was 26% (90% CI, 9-43).
Nonrelapse morbidity and mortality
The estimated CuI of NRM was 0% at 1 year and 6% (90% CI, 0-17) at 2 years. One nonrelapse death occurred at day 400 from cardiovascular disease. This patient had recovered from prior critical illness (∼day 137) from grade 4 pulmonary toxicity, which was managed with steroids and discontinuation of sirolimus. Another patient had nonrelapse critical illness (sepsis at day 75) but recovered. In the absence of relapse, there were no other cases of grade 4 infection by day 180 or critical illness.
Three patients stopped sirolimus because of toxicity (the case above of grade 4 pneumonitis, a case of grade 3 pneumonitis, and a case of grade 2 hyperlipidemic pancreatitis); 2 switched to tacrolimus.
Relapse and survival
With 4.0-year median follow-up (range, 1.0-5.0), the 1-year CuI of relapse was 35% (90% CI, 17-53). The estimated PFS was 65% (90% CI, 50-85) at 1 year and 52% (90% CI, 36-76) at 2 and 3 years (Figure 1D). The estimated OS was 75% (90% CI, 61-93) at 1 year, 69% (90% CI, 53-89) at 2 years, and 62% (90% CI, 45-84) at 3 years (Figure 1D). One-year GRFS was 60% (90% CI, 44-81).
Discussion
These data demonstrate the feasibility and acceptable safety profile of T-cell replete mMUD BMT with PTCy-based GVHD prophylaxis. With the prioritized transplant regimen, engraftment was brisk, with stable donor chimerism in all patients. Rates of GVHD and prohibitive toxicity were also low.
Although some centers routinely perform 1-locus mismatched unrelated BMT, few prospective studies are published for NMA or reduced-intensity conditioned (RIC) mMUD BMT.8,20-22 A report including mMUD BMT described stable engraftment, but also excess GVHD and a 2-year NRM of 47%.8 Promising results were reported with 1 to 2 locus mismatched mMUD BMT using bortezomib-based GVHD prophylaxis.20 Acceptable safety profiles were also reported with mMUD BMT using alemtuzumab21 and antithymocyte antiglobulin.22 However, T-cell depletion has been associated with impaired disease-free survival in RIC transplantation23 and infectious morbidity.11
This study was not designed to address the number and types of permissible mismatches, and the small number and heterogeneity of patients are limitations. The data need confirmation in larger prospective trials, as in the just-opened phase 2 trial (15-MMUD) using this platform under the auspices of the NMDP. Nevertheless, the results are promising for expanding the donor pool for patients lacking a related or matched unrelated donor, including minorities, patients with rare HLA haplotypes, and patients with DSAs not amenable to desensitization. The safety results also raise the possibility of prioritizing mMUDs on the basis of non-HLA factors to improve disease outcomes, such as killer-cell immunoglobulin-like receptor haplotype24 or CCR5 delta32 mutation status for HIV-infected patients.25
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the Cell Therapy Laboratory at Johns Hopkins for graft data, Mary Leffell for evaluation of HLA typing, and Judy Baker for data management.
This work was supported by National Institutes of Health National Cancer Institute grants K23 CA124465 (Y.L.K.), P01 CA015396 (R.J.J.), and P30 CA006973.
Authorship
Contribution: Y.L.K. was involved in the study’s conception and design, conduct, data collection, and analysis and interpretation and wrote the first version of the manuscript; L.L. and R.J.J. were also involved in conception and design, data collection, analysis and interpretation, and manuscript writing; Y.L.K, R.J.J., and E.J.F. additionally evaluated HLA typing and donor prioritization; R.F.A. was involved in study conduct, data collection, analysis, and interpretation, and manuscript writing; and M.Z. and G.L.R. designed and statistically analyzed the study. The remaining authors contributed to data collection. All authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Luznik, 1650 Orleans St, CRB I, Room 2M-88, Baltimore, MD 21287; e-mail: luznile@jhmi.edu.
References
Competing Interests
Presented in abstract form at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5-9 December 2015.