Key Points
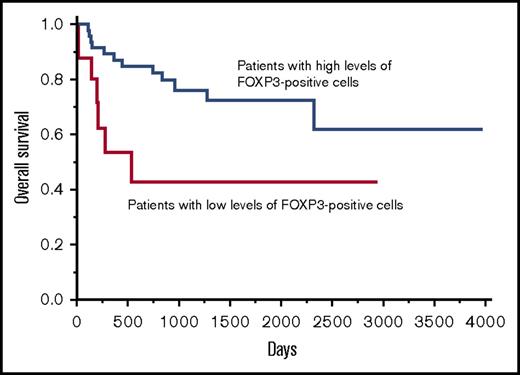
In the patients with DLBCL, NOS, a high infiltration of FOXP3-positive cells in tumor was associated with a better prognosis.
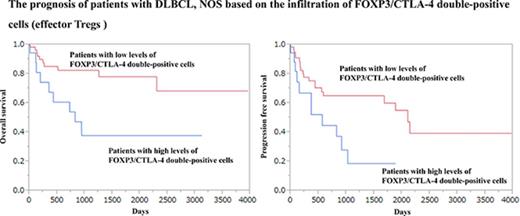
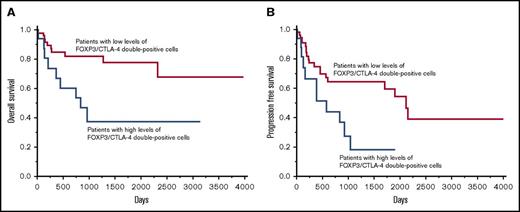
However, a high infiltration of FOXP3/CTLA-4 double-positive cells, which are eTregs, was associated with a worse prognosis.
Abstract
Regulatory T cells (Tregs) specifically express the transcription factor forkhead box P3 (FOXP3) and contribute to tumor progression. FOXP3-positive cells have been recently proven to be heterogeneous in phenotype and function, including effector Tregs (eTregs), naïve Tregs, and non-Tregs, which harbor no suppressive function. Therefore, it is crucial to investigate the “true Treg (eTreg)” population, rather than the entire FOXP3 population, with regards to their effect on tumor immunity. In particular, in diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS), FOXP3-positive cells correlated with a better prognosis. The present study sought to evaluate the relationship between the prognosis of DLBCL, NOS patients and the infiltration of true Tregs by employing dual immunostaining with FOXP3 and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4). CTLA-4 is a negative immunomodulatory known to be expressed by eTregs, but not by non-Tregs. Lymph nodes from 82 nodal DLBCL, NOS patients were stained with anti-FOXP3 and anti–CTLA-4 antibodies. A high infiltration of FOXP3-positive cells was associated with a significantly better prognosis than patients with low levels of FOXP3-positive cells for overall survival (OS) (P = .0233). In sharp contrast, a high infiltration of FOXP3/CTLA-4 double-positive cells was significantly associated with a poor prognosis than patients with low levels of FOXP3/CTLA-4 double-positive cells for OS (P = .0121) and progression-free survival (P = .0171), independent of the international prognostic index. FOXP3/CTLA-4 double-positive cells, eTregs, play an important role in DLBCL, NOS progression.
Introduction
Diffuse large B-cell lymphoma, not otherwise specified (DLBCL, NOS) is the largest category of aggressive lymphomas, which comprise a heterogeneous group of lymphomas with respect to surface marker expression, histology, and clinical features.1 The introduction of anti-CD20 monoclonal antibody (rituximab) has improved the survival of patients with DLBCL, NOS, with a 5-year overall survival (OS) rate of ∼60%; however, the remaining DLBCL, NOS patients die of disease progression.2 Therefore, it is important to determine accurate prognostic factors for patients with DLBCL, NOS for the application of extensive therapies, including stem cell transplantation. The International Prognostic Index (IPI) is most widely used for predicting outcomes in patients with DLBCL, NOS.3 However, the IPI is not sufficient to make an accurate prediction of treatment outcomes for individual patients because the IPI is based only on clinical findings, and do not reflect the biological findings of lymphoma cells or the tumor microenvironment. There have been some reports showing a relationship between biological markers on the tumor cells (eg, bcl2, CD44, and tumor necrosis factor-α expression), as well as the Ki67 labeling index and patients’ prognosis.4,5 In particular, an immunophenotypic analysis revealed that the germinal center B-cell–like (GCB) phenotype has a better prognosis than non-germinal center B-cell–like (non-GCB) phenotype, which is an independent predictor of IPI.6
The tumor microenvironment is important for the development and progression of tumors, and influences the patients’ prognosis7 ; thus, it is essential for the development of novel treatment strategies. The recent success of cancer immunotherapy has shed light on the critical role of the immune system in the development and treatment of lymphoma. In particular, regulatory T cells (Tregs) are crucial for the modulation of the immune response, especially the suppression of tumor-associated antigen-reactive lymphocytes.8
Tregs were originally characterized as cells with a CD4+CD25+ phenotype, and forkhead box P3 (FOXP3) was later identified as a specific marker for Tregs.9 Moreover, FOXP3 is a master regulator of Tregs for their generation, survival, and suppressive activity.10,11 However, because FOXP3 is induced in humans upon T-cell receptor stimulation in conventional T cells, FOXP3-positive cells have been found to have both a heterogeneous phenotype and function. In addition, it has recently been shown that CD4+FOXP3+ T cells can be dissected into 3 subpopulations: (1) effector Tregs (eTregs), which have a strong suppressive function; (2) naïve Tregs, which have the potential to differentiate into eTregs upon antigenic stimulation; and (3) non-Tregs, which are a nonsuppressive subpopulation.8,12 In addition, cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), a negative immunomodulator expressed by activated T cells is also expressed by eTregs and is essential for their suppressive function.8,13,14 Despite the immunosuppressive function of Tregs on antitumor immune responses, a high infiltration of Tregs, as defined by FOXP3 expression, is reportedly associated with a better prognosis of patients with DLBCL, NOS.15 To reconcile this discrepancy, the present study aimed at retrospectively evaluating the relationship between the infiltration of eTregs (FOXP3/CTLA-4 double-positive cells) and the prognosis of patients with DLBCL, NOS.
Materials and methods
Patients and samples
We examined 82 lymph nodes (LNs) from previously untreated patients with nodal DLBCL, NOS who were diagnosed and treated from 2002 to 2015 at Osaka Medical College Hospital, Osaka, Japan. Informed consent was obtained in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of Osaka Medical College (No. 1314).
All patients were initially treated with an R-CHOP regimen consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. Diagnosis of DLBCL, NOS was based on the World Health Organization classification system.1 Patients were classified into one of the following 4 groups by the IPI classification: (1) low risk (IPI score = 0 to 1); (2) low-intermediate risk (IPI score = 2); (3) high-intermediate risk (IPI score = 3); and (4) high risk (IPI score = 4 to 5).3
Histopathology and immunostaining
Tumor tissues were obtained from every patient by a LN biopsy and fixed in 10% buffered formalin and embedded in paraffin. Sections were cut to a thickness of 4 μm and stained with hematoxylin and eosin. Immunohistologic staining was performed using an EnVision system kit (Dako, Glostrup, Denmark), with the following primary antibodies: CD3 (PS1; Nichirei, Tokyo, Japan), CD5 (4C7; Nichirei), CD10 (56C6; Dako), CD20 (SL26; Kyowa Medix, Tokyo, Japan), B-cell lymphoma 6 (BCL6) (LN22; Novocastra, Newcastle, United Kingdom), mutated myeloma-associated antigen 1 (MUM1) (MUM1p; Dako), and Ki67 (MIB-1; Dako). A case was considered positive when at least 30% of the tumor cells were positive for CD10, BCL6, and MUM-1.6 For the DLBCL, NOS subtype, a case that was positive for CD10 or BCL6 and negative for MUM1 was considered to have a GCB phenotype, and any other DLBCL, NOS was considered to have a non-GCB phenotype according to the report by Hans et al.6 For CD5, membranous reactivity in ≥20% of the tumor cells was considered CD5-positive DLBCL, NOS according to the method reported by Yamaguchi et al.16 The Ki67 index was quantified by determining the number of cells expressing nuclear Ki67 of the total number of tumor cells within a high-power (objective ×40) microscopic field. A cutoff of ≥80% was used to define a high proliferation index.17,18 We immunostained the LNs with the EnVision system kit using primary antibodies for FOXP3 (236A/E7; Abcam, Cambridge, United Kingdom). We also performed FOXP3/CTLA-4 (H-126; Santa Cruz Biotechnology, Santa Cruz, CA) dual staining to detect FOXP3/CTLA-4 double-positive cells using the EnVision system kit and EnVision, G/2 system/AP (Dako). The number of FOXP3-positive cells or FOXP3/CTLA-4 double-positive cells in the entire lesion for each LN was counted under a light microscope by 2 pathologists, using a grid inserted into the ocular piece of the microscope. The area of the LNs was measured by an objective quantitative analysis using the WinROOF image processing software program (Mitani Corporation, Tokyo, Japan). The number of FOXP3-positive cells or FOXP3/CTLA-4 double-positive cells was expressed per square centimeter (cm2).
Statistical analysis
OS was calculated as the time between the onset of treatment and death, as well as the date of the last follow-up evaluation. Progression-free survival (PFS) was calculated from the onset of treatment until relapse, disease progression, or the last follow-up evaluation. The survival rate and curves for OS and PFS are presented using the Kaplan-Meier method.
We analyzed the effects of diverse explanatory variables on the prognosis. For each of the several categorical variables (ie, infiltration of FOXP3-positive cells, infiltration of FOXP3/CTLA-4 double-positive cells, IPI category, subtype of GCB or non-GCB phenotype, and Ki67 index), we performed a univariate Cox regression analysis to determine whether these variables had an influence on the OS and PFS of patients. Regarding the numerical variables (eg, the infiltration of FOXP3-positive cells and FOXP3/CTLA-4 double-positive cells), we converted them into binomial categorical variables by using a cutoff value, and then performed a univariate Cox analysis on these converted variables. For the method of detecting a cutoff value, receiver operating characteristic analysis is usually used for logistic regression; however, this method only uses prognosis results without the information regarding observational time before determining the results. Because the cases in the present study contain a wide range of observation times, we need to consider the times to distinguish between shorter and longer survival or censored times. However, in the Cox regression, there does not seem to be any existing approaches for this purpose. Hence, we detected a cutoff value for the Cox regression analysis by imitating the procedure of the receiver operating characteristic analysis. For numerical variables, denoted as x, we examined if there was an optimal cutoff value that allowed us to convert x into a binomial variable. To this end, for each data value c of x, we created a temporary binomial variable by using c as a dividing point (≤c or >c). In addition, we performed a univariate Cox regression analysis using this binomial variable to obtain the P value. If this P value was < .05, and at the same time the sample sizes of 2 groups of the binomial variable were not biased, we concluded that the numerical variable x had an optimal cutoff, c, using R statistical software (http://www.R-project.org).19 Bivariate Cox regression analysis was applied to test whether the infiltration of FOXP3/CTLA-4 double-positive cells was independent of the established prognostic factors, such as IPI, subtype, and Ki67 index. In addition, we examined which combination of IPI, subtype, Ki67 index, and infiltration of FOXP3/CTLA-4 double-positive cells was the best method for predicting the prognosis.
Associations between factors (eg, the infiltration of FOXP3-positive cells or FOXP3/CTLA-4 double-positive cells) and clinical or pathological variables were determined using a Pearson χ2 test and the Student t test.
P values < .05 were considered to be significant. Statistical analyses were performed using the JMP Pro 11 (SAS Institute, Inc., Cary, NC), and R statistical software (http://www.R-project.org).
Results
Clinicopathological features of the patients
The clinicopathological features of the 82 patients with DLBCL, NOS at the time of presentation are summarized in Table 1. The median age and sex ratio of the patients enrolled in this study was comparable to the world cohort of DLBCL, NOS.1 The 2- and 5-year OS rates of all patients were 75.0% and 66.7%, respectively. Moreover, the 2- and 5-year PFS rates of all patients were 60.2% and 48.9%, respectively. These survival rates were consistent with those of the world survey of DLBCL, NOS.2
Patient clinicopathological features
| Characteristics . | No. of patients (%) . |
|---|---|
| Age at diagnosis, y | |
| Median ± SE | 68.3 ± 1.5 |
| <60 | 17 (21) |
| ≥61 | 65 (79) |
| Sex (male/female) | 48 (59)/34 (41) |
| Clinical stage | |
| I | 12 (14) |
| II | 22 (27) |
| III | 17 (21) |
| VI | 31 (38) |
| IPI category | |
| Low | 23 (28) |
| Low-intermediate | 22 (27) |
| High-intermediate | 31 (38) |
| High | 6 (7) |
| Subtype | |
| GCB type | 25 (30) |
| Non-GCB type | 57 (70) |
| Ki67 index | |
| ≥80% | 12 (15) |
| <80% | 70 (85) |
| Characteristics . | No. of patients (%) . |
|---|---|
| Age at diagnosis, y | |
| Median ± SE | 68.3 ± 1.5 |
| <60 | 17 (21) |
| ≥61 | 65 (79) |
| Sex (male/female) | 48 (59)/34 (41) |
| Clinical stage | |
| I | 12 (14) |
| II | 22 (27) |
| III | 17 (21) |
| VI | 31 (38) |
| IPI category | |
| Low | 23 (28) |
| Low-intermediate | 22 (27) |
| High-intermediate | 31 (38) |
| High | 6 (7) |
| Subtype | |
| GCB type | 25 (30) |
| Non-GCB type | 57 (70) |
| Ki67 index | |
| ≥80% | 12 (15) |
| <80% | 70 (85) |
Histologic features and immunohistochemical results
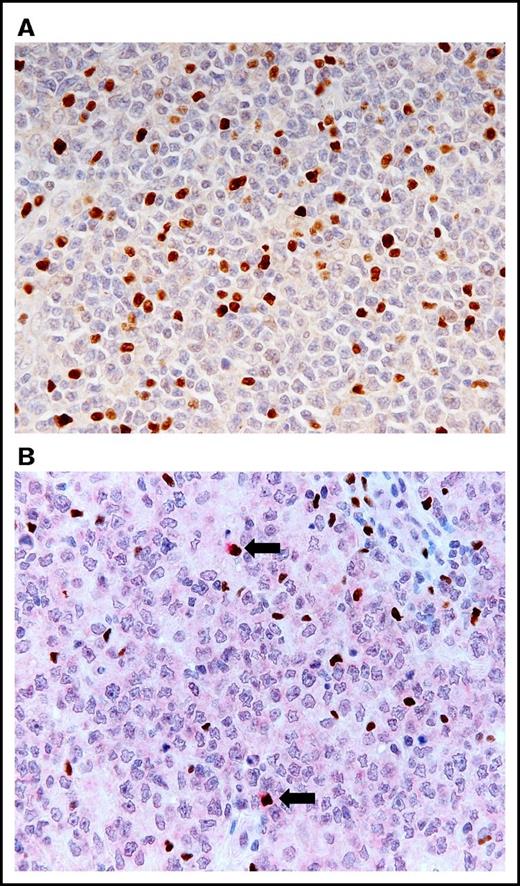
The histologic examination of the hematoxylin and eosin-stained LN specimens revealed a diffuse proliferation of large lymphoid cells and a complete effacement of the normal architecture of the LN. The large lymphoid cells stained for CD20 but not for CD3, which is representative of a B-cell phenotype. They were negative for CD5 in all cases. Among the 82 patients with DLBCL, NOS, 25 (30%) and 57 (70%) patients exhibited GCB and non-GCB phenotypes, respectively. The Ki67 index varied from 16% to 91% (mean ± standard error [SE]: 54.9 ± 2.1%); 12 cases (15%) had a Ki67 index of ≥80% and 70 cases (85%) had a Ki67 index of <80%. FOXP3-positive cells were heavily infiltrated (range: 11 × 102 to 2014 × 102/cm2; mean ± SE: 290.1 × 102 ± 44.1 × 102/cm2) (Figure 1A). FOXP3/CTLA-4 double-positive cells (defined as eTregs) were observed remarkably less than the FOXP3-positive cells (0 to 506/cm2, 21.6 ± 7.0/cm2) (Figure 1B).
Histologic findings of LNs obtained from patients with DLBCL, NOS. (A) Increased infiltration of FOXP3-positive cells in the group of high levels of FOXP3-positive cells (immunostaining for FOXP3; objective magnification ×40). (B) Infiltration of FOXP3/CTLA-4 double-positive cells (arrows) in the group of high levels of FOXP3/CTLA-4 double-positive cells. FOXP3 stains the nuclei a brown color, and CTLA-4 stains the cytoplasm a red color (double immunostaining for FOXP3 and CTLA-4; objective magnification ×40).
Histologic findings of LNs obtained from patients with DLBCL, NOS. (A) Increased infiltration of FOXP3-positive cells in the group of high levels of FOXP3-positive cells (immunostaining for FOXP3; objective magnification ×40). (B) Infiltration of FOXP3/CTLA-4 double-positive cells (arrows) in the group of high levels of FOXP3/CTLA-4 double-positive cells. FOXP3 stains the nuclei a brown color, and CTLA-4 stains the cytoplasm a red color (double immunostaining for FOXP3 and CTLA-4; objective magnification ×40).
The association of a better prognosis for DLBCL patients and the infiltration of FOXP3-positive cells
We analyzed the clinical impact of the infiltration of FOXP3-positive cells and found that an optimal cutoff value in OS was identified as 40 × 102/cm2 with a significant P value (P = .0233) in the univariate analysis of the Cox method. This value was achieved by repeating the univariate Cox regression analysis by changing the dividing point using R statistical software as described in the “Materials and methods.” The patients were divided into the following 2 groups using this cutoff value: (1) 63 (77%) patients with high levels of FOXP3-positive cells (>40 × 102/cm2) (mean ± SE: 370.5 × 102 ± 53.5 × 102 /cm2); and (2) 19 (23%) patients with low levels of FOXP3-positive cells (≤40 × 102/cm2) (mean ± SE: 23.3 × 102 ± 8.8 × 102/cm2) for the OS. The 2-year OS rate of the patients with high levels of FOXP3-positive cells and the patients with low levels of FOXP3-positive cells was 82.9% and 43.1%, respectively. Additionally, the 5-year OS rate for the patients with high levels of FOXP3-positive cells and patients with low levels of FOXP3-positive cells was 72.8% and 43.1%, respectively, indicating that the patients with high levels of FOXP3-positive cells had a significantly better OS than patients with low levels of FOXP3-positive cells (Figure 2). There were no significant differences found for the age, sex, clinical stage, IPI, subtype, and Ki67 index between the patients with high levels of FOXP3-positive cells and the patients with low levels of FOXP3-positive cells (supplemental Table 1). However, we could not determine the cutoff value that demonstrated a significant P value in the univariate analysis using the Cox method in PFS.
The OS curve of patients with DLBCL, NOS based on the infiltration of FOXP3-positive cells. Patients with high levels of FOXP3-positive cells were associated with a significantly better prognosis than patients with low levels of FOXP3-positive cells for OS.
The OS curve of patients with DLBCL, NOS based on the infiltration of FOXP3-positive cells. Patients with high levels of FOXP3-positive cells were associated with a significantly better prognosis than patients with low levels of FOXP3-positive cells for OS.
The opposite effect of the infiltration of FOXP3/CTLA-4 double-positive cells on the patients’ prognosis
The infiltration of FOXP3/CTLA-4 double-positive cells was evaluated. The optimal cutoff value in OS was identified as 17/cm2 with a significant P value (P = .0121) in the univariate analysis of the Cox method. The patients were classified into 2 groups based on the cutoff value: (1) 19 (23%) patients with high levels of FOXP3/CTLA-4 double-positive cells (>17/cm2) (82.2 ± 26.2/cm2); and (2) 63 (77%) patients with low levels of FOXP3/CTLA-4 double-positive cells (≤17/cm2) (3.4 ± 0.6/cm2). The 2-year OS rate of the patients with high levels of FOXP3/CTLA-4 double-positive cells and patients with low levels of FOXP3/CTLA-4 double-positive cells was 53.9% and 82.4%, respectively. In addition, the 5-year OS rate for patients with high levels of FOXP3/CTLA-4 double-positive cells and patients with low levels of FOXP3/CTLA-4 double-positive cells was 37.7% and 78.0%, respectively, indicating that the patients with high levels of FOXP3/CTLA-4 double-positive cells had a significantly worse OS than the patients with low levels of FOXP3/CTLA-4 double- positive cells (Figure 3A).
The OS curve and PFS curve of patients with DLBCL, NOS based on the infiltration of FOXP3/CTLA-4 double-positive cells. Patients with high levels of FOXP3/CTLA-4 double-positive cells were more significantly associated with a poor prognosis than patients with low levels of FOXP3/CTLA-4 double-positive cells for OS (A) and PFS (B).
The OS curve and PFS curve of patients with DLBCL, NOS based on the infiltration of FOXP3/CTLA-4 double-positive cells. Patients with high levels of FOXP3/CTLA-4 double-positive cells were more significantly associated with a poor prognosis than patients with low levels of FOXP3/CTLA-4 double-positive cells for OS (A) and PFS (B).
The value of the infiltrating of FOXP3/CTLA-4 double-positive cells in PFS was also evaluated. The optimal cutoff value in PFS was 18/cm2 with a significant P value (P = .0171) in the univariate analysis using the Cox method: (1) 18 (22%) patients with high levels of FOXP3/CTLA-4 double-positive cells (>18/cm2) (85.7 ± 27.4/cm2); and (2) 64 (78%) patients with low levels of FOXP3/CTLA-4 double-positive cells (≤18/cm2) (3.6 ± 0.6/cm2). The 2-year PFS rate of the patients with high levels of FOXP3/CTLA-4 double-positive cells and patients with low levels of FOXP3/CTLA-4 double-positive cells was 44.6% and 65.0%, respectively. Moreover, the 5-year PFS rate for the patients with high levels of FOXP3/CTLA-4 double-positive cells and patients with low levels of FOXP3/CTLA-4 double-positive cells was 18.6% and 60.0%, respectively (Figure 3B). There was no significant difference in the age, sex, clinical stage, IPI, subtype, and Ki67 index between the patients with high levels of FOXP3/CTLA-4 double-positive cells and patients with low levels of FOXP3/CTLA-4 double-positive cells (supplemental Tables 2 and 3).
Taken together, FOXP3/CTLA-4 double-positive cells exhibited a different prognostic value compared with FOXP3 single-positive cells, reflecting the heterogeneous populations in FOXP3 single-positive cells.
Relationship between novel prognostic factors (infiltration of FOXP3/CTLA-4 double-positive cells) and other established prognostic factors (IPI, subtype, and Ki67 index)
To examine whether the established prognostic factors such as IPI, subtype, and Ki67 index were significant prognostic factors in this study, a univariate Cox regression analysis was employed. IPI, subtype, and Ki67 index were significant prognostic factors for OS (Table 2) and PFS (Table 3). Therefore, bivariate Cox regression analysis was applied to test whether the infiltration of FOXP3/CTLA-4 double-positive cells was independent of these established prognostic factors. In addition, we examined which combination of IPI, subtype, Ki67 index, and infiltration of FOXP3/CTLA-4 double-positive cells was the best method for predicting the prognosis. Our results showed that the infiltration of FOXP3/CTLA-4 double-positive cells and established prognostic factors such as IPI, subtype, and Ki67 index were independent factors for both the OS and PFS outcome (Table 4; supplemental Tables 4-6). The statistical analyses indicated that the combination of infiltrating FOXP3/CTLA-4 double-positive cells and IPI was the most critical parameter for OS (the infiltration of FOXP3/CTLA-4 double-positive cells and IPI P < .0001, the infiltration of FOXP3/CTLA-4 double-positive cells and subtype P = .0046, the infiltration of FOXP3/CTLA-4 double-positive cells and Ki67 P = .0056, IPI and subtype P = .0002, and IPI and Ki67 P = .0003). In addition to the OS, the combination of infiltrating FOXP3/CTLA-4 double-positive cells and IPI, as well as that of IPI and Ki67 index were the most important prognostic factors for PFS (the infiltration of FOXP3/CTLA-4 double-positive cells and IPI P < .0001, the infiltration of FOXP3/CTLA-4 double-positive cells and subtype P = .0029, the infiltration of FOXP3/CTLA-4 double-positive cells and Ki67 P = .0083, IPI and subtype P = .0002, and IPI and Ki67 P < .0001).
Prognostic factors that affected the OS (univariate analysis)
| Variables . | Comparative factor/reference factor . | Univariate relative risk (95% CI) . | P . |
|---|---|---|---|
| Infiltration of FOXP3-positive cells | High/low | 0.30 (0.12-0.84) | .0233* |
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 3.33 (1.31-8.31) | .0121* |
| IPI category† | H/HI | 7.73 (1.89-28.38) | .0063* |
| H/LI | 22.56 (4.46-129.07) | .0004* | |
| H/L | 25.89 (5.00-152.12) | .0002* | |
| H-HI/L-LI | 4.10 (1.47-13.13) | .0064* | |
| Subtype | Non-GCB/GCB | 4.16 (1.19-26.25) | .0224* |
| Ki67 index | ≥80%/<80% | 2.88 (1.01-3.29) | .0493* |
| Variables . | Comparative factor/reference factor . | Univariate relative risk (95% CI) . | P . |
|---|---|---|---|
| Infiltration of FOXP3-positive cells | High/low | 0.30 (0.12-0.84) | .0233* |
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 3.33 (1.31-8.31) | .0121* |
| IPI category† | H/HI | 7.73 (1.89-28.38) | .0063* |
| H/LI | 22.56 (4.46-129.07) | .0004* | |
| H/L | 25.89 (5.00-152.12) | .0002* | |
| H-HI/L-LI | 4.10 (1.47-13.13) | .0064* | |
| Subtype | Non-GCB/GCB | 4.16 (1.19-26.25) | .0224* |
| Ki67 index | ≥80%/<80% | 2.88 (1.01-3.29) | .0493* |
CI, confidence interval; H, high; HI, high-intermediate; L, low; LI, low-intermediate.
*P < .05.
†Representative results with a significant P value are shown among all combinations of each IPI category, and among the H-HI group and the L-LI group.
Prognostic factors that affected PFS (univariate analysis)
| Variables . | Comparative factor/reference factor . | Univariate relative risk (95% CI) . | P . |
|---|---|---|---|
| Infiltration of FOXP3-positive cells | High/low | N.D. | N.D. |
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 2.64 (1.99-5.61) | .0171* |
| IPI category† | H/LI | 7.56 (1.86-27.22) | .0067* |
| H/L | 14.02 (4.05-90.67) | .0005* | |
| HI/L | 5.45 (1.94-19.37) | .0008* | |
| H-HI/L-LI | 0.0013 (1.66-8.48) | .0013* | |
| Subtype | Non-GCB/GCB | 2.94 (1.22-8.73) | .0136* |
| Ki67 index | ≥80%/<80% | 2.56 (1.07-5.53) | .0355* |
| Variables . | Comparative factor/reference factor . | Univariate relative risk (95% CI) . | P . |
|---|---|---|---|
| Infiltration of FOXP3-positive cells | High/low | N.D. | N.D. |
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 2.64 (1.99-5.61) | .0171* |
| IPI category† | H/LI | 7.56 (1.86-27.22) | .0067* |
| H/L | 14.02 (4.05-90.67) | .0005* | |
| HI/L | 5.45 (1.94-19.37) | .0008* | |
| H-HI/L-LI | 0.0013 (1.66-8.48) | .0013* | |
| Subtype | Non-GCB/GCB | 2.94 (1.22-8.73) | .0136* |
| Ki67 index | ≥80%/<80% | 2.56 (1.07-5.53) | .0355* |
N.D., no detection. All other abbreviations are explained in Table 2.
*P < .05.
†Representative results with a significant P value are shown among all combinations of each IPI category, and among the H-HI group and the L-LI group.
Prognostic factors that affect OS and PFS (bivariate analysis of infiltration of FOXP3/CTLA-4 double-positive cells and IPI categories)
| Variables . | Comparative factor/reference factor . | Multivariate relative risk (95% CI) . | P . |
|---|---|---|---|
| Prognostic factors that affected OS: | |||
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 5.79 (2.06-16.58) | .0010* |
| IPI category† | H/HI | 8.21 (1.86-32.85) | .0071* |
| H/LI | 46.28 (7.55-342.77) | <.0001* | |
| H/L | 40.95 (6.92-285.28) | <.0001* | |
| HI/LI | 5.63(1.50-27.87) | .0091* | |
| HI/L | 4.98 (1.39-23.66) | .0122* | |
| Prognostic factors that affected PFS: | |||
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 3.51 (1.50-7.97) | .0042* |
| IPI category† | H/HI | 2.71 (0.70-8.76) | .1359* |
| H/LI | 8.03 (1.90-30.70) | .0064* | |
| H/L | 18.90 (3.93-92.62) | .0005* | |
| HI/LI | 2.95 (1.21-7.82) | .0167* | |
| HI/L | 6.96 (2.42-25.13) | .0002* | |
| Variables . | Comparative factor/reference factor . | Multivariate relative risk (95% CI) . | P . |
|---|---|---|---|
| Prognostic factors that affected OS: | |||
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 5.79 (2.06-16.58) | .0010* |
| IPI category† | H/HI | 8.21 (1.86-32.85) | .0071* |
| H/LI | 46.28 (7.55-342.77) | <.0001* | |
| H/L | 40.95 (6.92-285.28) | <.0001* | |
| HI/LI | 5.63(1.50-27.87) | .0091* | |
| HI/L | 4.98 (1.39-23.66) | .0122* | |
| Prognostic factors that affected PFS: | |||
| Infiltration of FOXP3/CTLA-4 double-positive cells | High/low | 3.51 (1.50-7.97) | .0042* |
| IPI category† | H/HI | 2.71 (0.70-8.76) | .1359* |
| H/LI | 8.03 (1.90-30.70) | .0064* | |
| H/L | 18.90 (3.93-92.62) | .0005* | |
| HI/LI | 2.95 (1.21-7.82) | .0167* | |
| HI/L | 6.96 (2.42-25.13) | .0002* | |
Abbreviations are explained in Table 2.
*P < .05.
†Representative results with a significant P value are shown among all combinations.
Discussion
Treg-mediated immunosuppression contributes to the tumor progression and high Treg infiltration in tumors, and is generally associated with a poor prognosis in various types of cancers (eg, ovarian, breast, pancreatic, hepatocellular carcinoma, and melanoma).20-25 In contrast, a high infiltration of Tregs has been reportedly associated with an improved OS in colorectal cancer.26 Although FOXP3 expression has been used as a specific marker of Tregs,27 FOXP3-positive cells were revealed to include cells heterogeneous in both phenotype and function. Moreover, they can be classified into 3 subpopulations as: (1) immunosuppressive naïve Tregs; (2) eTregs; and (3) non-Tregs without immunosuppressive function.12,28 Additionally, it was recently shown that a subpopulation in colorectal cancer patients possessed a higher frequency of non-Tregs together with eTregs in the tumor tissues, whereas tumor-infiltrating FOXP3-positive T cells predominantly consisted of eTregs in melanoma with very low frequencies of non-Tregs.14
Regarding malignant lymphoma, the association of Treg infiltration with patient prognosis remains to be determined; a high infiltration of Tregs is associated with improved survival in follicular lymphoma and classical Hodgkin lymphoma.29-31 In DLBCL, the increased presence of Tregs is reported to be associated with a superior OS and PFS.15,32 In contrast, the increased presence of Tregs corresponds to the poor prognosis of patients in the IPI high-risk group of DLBCL patients.33 Moreover, a high Treg infiltration is associated with a better prognosis in the GCB type of DLBCL, but with a poor prognosis in the non-GCB type.31 Thus, the role of tumor-infiltrating Tregs in B-cell malignant lymphomas was thought to be different from those of solid nonlymphoid tumors, likely due to the direct suppression of B-cell lymphoma cells by Tregs.34
We examined the frequency of eTregs, which mainly suppress antitumor immunity,28 by identifying them as FOXP3/CTLA-4 double-positive cells in the LNs from patients with DLBCL, NOS. Our findings clearly indicate that: (1) a high infiltration of FOXP3-positive cells was associated with a better prognosis of DLBCL, NOS, in line with previous reports15,32 ; (2) there were substantially fewer FOXP3/CTLA-4 double-positive cells than FOXP3-positive cells; (3) a high infiltration of FOXP3/CTLA-4 double-positive cells was associated with a worse prognosis of DLBCL, NOS; and (4) there was no significant difference in the infiltrative degree of FOXP3-positive cells or FOXP3/CTLA-4 double-positive cells between GCB and non-GCB phenotypes, or between cases with a Ki67 index of ≥80% and cases with a Ki67 index of <80%.
In addition, we also found the potentially useful cutoff value of infiltrating FOXP3-positive cells to be a better prognostic marker, and the cutoff value of FOXP3/CTLA-4 double-positive cells was found to be a worse prognostic marker. This was determined by repeating the univariate Cox regression analysis by changing the dividing point of 2 groups according to high or low numbers by using R statistical software to avoid the influence of differential observation periods. In addition to IPI, infiltration of FOXP3/CTLA-4 double-positive cells that reflects the tumor microenvironment improves the predictive value of prognosis to a greater extent than previous prognosis prediction methods for the combination of IPI with subtype and of IPI with Ki67 in OS. Therefore, evaluating infiltration of FOXP3/CTLA-4 double-positive cells can also be used to decide the optimal course of treatment.
The main finding of our study is that the presence of eTregs is associated with a poor prognosis in DLBCL, NOS and could provide novel strategies for immunotherapy. Immunotherapies targeting eTregs via depletion or a functional alteration have recently been extensively tested in solid tumors (eg, malignant melanoma and lung cancer). For example, a decreased number of Tregs in tumor tissues following ipilimumab treatment strongly correlated with the clinical response of patients with melanoma.8,35,36 On the other hand, in DLBCL, because increased FOXP3-positive cells have been reported to be associated with a better prognosis,15,32 immunotherapies have been believed to be ineffective. However, our results showed that the increased FOXP3/CTLA-4 double-positive cells, which are eTregs, are associated with a poor prognosis of DLBDL, NOS. This result suggests that these immunotherapies would be effective in DLBCL, NOS. Indeed, there is a report of a refractory DLBCL case against chemotherapy that achieved a clinical response in the ipilimumab phase 1 study.37
To the best of our knowledge, this is the first report demonstrating that a high eTreg infiltration detected using a double-immunostaining method with anti-FOXP3 and anti–CTLA-4 antibodies is associated with a poor prognosis of patients with DLBCL, NOS. Therefore, we propose that immunotherapies targeting eTregs could be applicable to DLBCL, NOS patients with a high infiltration of FOXP3/CTLA-4 double-positive cells as an effective and novel therapeutic strategy.
This is a retrospective study with a small sample size; therefore, further prospective studies with larger sample sizes are required to confirm these results.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the Osaka Community Foundation for supporting this study.
This study was supported by a grant from the Osaka Community Foundation (S.N.).
Authorship
Contribution: S.N. designed and performed the research; S.N. and M.T. performed immunostainings; S.N., T.Y., T.A., N.H., U.N., T.M., K.I., A.T., Y.M., J.H., and M.F. collected data; S.N. and Y.N. analyzed and interpreted data, and performed statistical analysis; and S.N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shoko Nakayama, First Department of Internal Medicine, Osaka Medical College, 2-7 Daigakumachi, Takatsuki City, Osaka 569-0801, Japan; e-mail: in1304@osaka-med.ac.jp.