Key Points
A novel heterozygous ITGB3 Leu718del shows loss of synchronization between the intracytoplasmic tail of β3 with that of αIIb.
Decreased activation of αIIbβ3 accompanies enlarged platelets that contain giant granules and give a poor aggregation response.
Introduction
Rare mutations within the ITGA2B and ITGB3 genes cause macrothrombocytopenia (MTP) in addition to platelet function defects recalling Glanzmann thrombasthenia.1,2 We now report a novel phenotype in a French family with MTP in whom a single amino acid deletion (del) in the β3 cytoplasmic tail is accompanied by platelet anisocytosis, enlarged platelet α-granules, and reduced platelet aggregation.
Methods
Sporadic mucocutaneous bleeding (epistaxis, gingival bleeding, menorrhagia) occurred across 3 generations of a French family. The proband (P1) is a woman in her sixties; MTP was noted when she was 30 years old and has persisted with typical platelet counts between 100 000 and 120 000/µL. She has 3 daughters; their delivery was normal without excessive bleeding. She was successfully treated for breast cancer by surgery and radiotherapy. Only 1 daughter (P2) has thrombocytopenia (72 000-102 000 platelets/µL) and the same clinical symptoms as her mother. She had transient severe bleeding during a visit to Africa. Typical platelet volumes are 12.5 fL for P1 and 11.5 fL for P2 as measured in an ADVIA hematologic analyzer (Siemans Healthcare, Saint-Denis, France) (control range, 7-10 fL). The proband’s father, now deceased, was moderately thrombocytopenic (100 000/µL) and suffered from mucocutaneous bleeding. Other blood cell lineages were normal for affected family members. The study was performed in accordance with the Declaration of Helsinki and corresponds to the approved protocol from INSERM (RBM-04-14). DNA from P1 was analyzed by whole exome sequencing (WES) within the BRIDGE–Bleeding Platelet Disorders Consortium (Cambridge, UK).3 Mutation analysis for family members was by Sanger sequencing, and in silico models were constructed by using the PyMOL Molecular Graphics System, version 1.3, Schrödinger, LLC as detailed previously.4 Platelet function, flow cytometry, and electron microscopy with immunogold labeling were performed according to our standard protocols (more information is provided in the legends for Figures 1 and 2).5,6
Platelet studies and structural in silico modeling showing effects of the novel β3 L718del. (A) Family pedigree. Blue symbols indicate MTP, and red symbols indicate a normal platelet count and function. The heterozygous presence of the ITGB3 variant is shown (+/–). (B) Light transmission aggregometry performed in citrated platelet-rich plasma (PRP) compared typical response of platelets from the proband (P1) and her affected daughter (P2) to that of a control donor. P1 and P2 were studied on 3 and 2 occasions respectively. Aggregation with high doses of ADP, collagen (Col), or arachidonic acid (AA) was of much lower intensity and was slow. Reduced although somewhat better responses were seen with TRAP, whereas ristocetin-induced platelet agglutination was normal. (C) Flow cytometry with the monoclonal antibodies (mAbs) AP-2 and Bx-1 show αIIbβ3 and GPIb content for P1 platelets (left panel, typical histograms; mean fluorescence intensity [MFI]). Binding of the immunoglobulin M (IgM) mAb PAC-1 showed much reduced activation of αIIbβ3 for P1 after stimulation of citrated PRP with ADP and TRAP (center panel). Binding of fluorescein isothiocyanate–Fg to stimulated washed platelets from P1 confirmed a reduced activation of the integrin (right panel). (D) In silico PyMOL modeling shows how β3 Leu718del changes the synchronization of the αIIb and β3 cytoplasmic tails. Left panel: cartoon representation of the normal nonactivated αIIb (purple) and β3 (green) transmembrane and cytoplasmic tail segments highlighting amino acids engaged in their clasp. Aromatic amino acids in π interactions are shown as spheres. The salt bridge involves polar amino acids of opposite charge; the positive αIIb-R995 and the negative β3-D723 are represented as sticks. Center panel: upper view schematic representations of the αIIb and β3 transmembrane α helix showing the distribution of consecutive amino acids on the helix’s circumference. (b1) Normal distribution with a β3-D723 localizing face to αIIb-R995. (b2) Configuration with the L718del. β3-D723 is now displaced by a quarter turn away from αIIb-R995. A positively charged Arg (R) now faces αIIb-R995; in addition to the repulsive charge effect, Arg is larger than Asp (arm of 7 atoms compared with 3), and this encumbrance will also push the cytoplasmic tails apart. Right panel: Cartoon representation of αIIb and β3 transmembrane segments, with superimposed β3 α helices with and without the mutation (colored orange and white, respectively). The arrow shows the twist. All methods and details of PymMOL modeling have been previously described in Nurden et al.4
Platelet studies and structural in silico modeling showing effects of the novel β3 L718del. (A) Family pedigree. Blue symbols indicate MTP, and red symbols indicate a normal platelet count and function. The heterozygous presence of the ITGB3 variant is shown (+/–). (B) Light transmission aggregometry performed in citrated platelet-rich plasma (PRP) compared typical response of platelets from the proband (P1) and her affected daughter (P2) to that of a control donor. P1 and P2 were studied on 3 and 2 occasions respectively. Aggregation with high doses of ADP, collagen (Col), or arachidonic acid (AA) was of much lower intensity and was slow. Reduced although somewhat better responses were seen with TRAP, whereas ristocetin-induced platelet agglutination was normal. (C) Flow cytometry with the monoclonal antibodies (mAbs) AP-2 and Bx-1 show αIIbβ3 and GPIb content for P1 platelets (left panel, typical histograms; mean fluorescence intensity [MFI]). Binding of the immunoglobulin M (IgM) mAb PAC-1 showed much reduced activation of αIIbβ3 for P1 after stimulation of citrated PRP with ADP and TRAP (center panel). Binding of fluorescein isothiocyanate–Fg to stimulated washed platelets from P1 confirmed a reduced activation of the integrin (right panel). (D) In silico PyMOL modeling shows how β3 Leu718del changes the synchronization of the αIIb and β3 cytoplasmic tails. Left panel: cartoon representation of the normal nonactivated αIIb (purple) and β3 (green) transmembrane and cytoplasmic tail segments highlighting amino acids engaged in their clasp. Aromatic amino acids in π interactions are shown as spheres. The salt bridge involves polar amino acids of opposite charge; the positive αIIb-R995 and the negative β3-D723 are represented as sticks. Center panel: upper view schematic representations of the αIIb and β3 transmembrane α helix showing the distribution of consecutive amino acids on the helix’s circumference. (b1) Normal distribution with a β3-D723 localizing face to αIIb-R995. (b2) Configuration with the L718del. β3-D723 is now displaced by a quarter turn away from αIIb-R995. A positively charged Arg (R) now faces αIIb-R995; in addition to the repulsive charge effect, Arg is larger than Asp (arm of 7 atoms compared with 3), and this encumbrance will also push the cytoplasmic tails apart. Right panel: Cartoon representation of αIIb and β3 transmembrane segments, with superimposed β3 α helices with and without the mutation (colored orange and white, respectively). The arrow shows the twist. All methods and details of PymMOL modeling have been previously described in Nurden et al.4
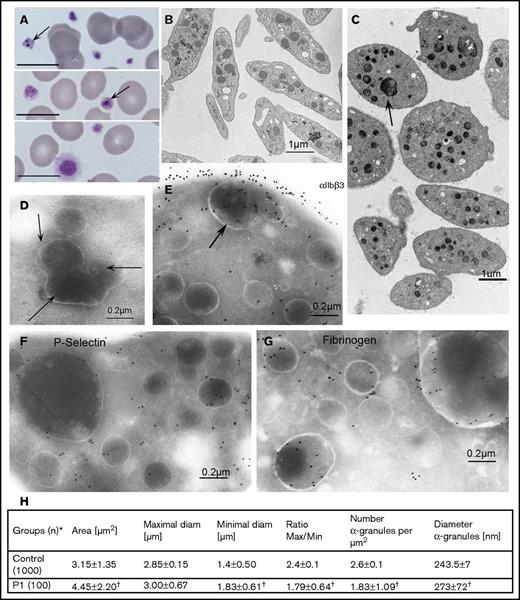
Morphologic evaluations of platelets from the proband (P1). (A) May-Grünwald-Giemsa–stained blood smears showing platelet anisotropy for P1 and abnormal granules (arrows). Scale bars represent 10 μm. (B-D) Typical electron microscopy (EM) images of platelets from (B) a control donor and (C) P1. The contrast between the discoid control platelets and the round enlarged platelets from P1 is striking; some contain many normal or 1 or more giant granules. (D-G) Selected immunogold labeling of cryosections of platelets from P1. All methods were as described in Nurden et al6 and involve incubation of sections with selected mAbs and their detection with a secondary antibody to murine IgG adsorbed on 10-nm gold particles. (D) A section without primary antibody; arrows indicate what appear to be grouped granules undergoing fusion or division. (E) αIIbβ3 (detected by using mAb AP-2) is shown on the platelet surface and within internal membrane systems, including those of an enlarged α-granule. (F) Similar labeling for P-selectin (mAb VH10). (G) Fibrinogen (Fg; with a polyclonal antibody) with gold beads often close to the α-granule membrane. For all immunogold images, scale bars represent 0.2 µm. (H) A quantitative morphometric evaluation of platelet size parameters (standard EM) and the number and diameter of α-granules of P1. *Number of platelet sections analyzed. Data are presented as mean ± standard deviation. Statistical significance was determined by Student t test for continuous variables. †A P value of < .01 was considered statistically significant.
Morphologic evaluations of platelets from the proband (P1). (A) May-Grünwald-Giemsa–stained blood smears showing platelet anisotropy for P1 and abnormal granules (arrows). Scale bars represent 10 μm. (B-D) Typical electron microscopy (EM) images of platelets from (B) a control donor and (C) P1. The contrast between the discoid control platelets and the round enlarged platelets from P1 is striking; some contain many normal or 1 or more giant granules. (D-G) Selected immunogold labeling of cryosections of platelets from P1. All methods were as described in Nurden et al6 and involve incubation of sections with selected mAbs and their detection with a secondary antibody to murine IgG adsorbed on 10-nm gold particles. (D) A section without primary antibody; arrows indicate what appear to be grouped granules undergoing fusion or division. (E) αIIbβ3 (detected by using mAb AP-2) is shown on the platelet surface and within internal membrane systems, including those of an enlarged α-granule. (F) Similar labeling for P-selectin (mAb VH10). (G) Fibrinogen (Fg; with a polyclonal antibody) with gold beads often close to the α-granule membrane. For all immunogold images, scale bars represent 0.2 µm. (H) A quantitative morphometric evaluation of platelet size parameters (standard EM) and the number and diameter of α-granules of P1. *Number of platelet sections analyzed. Data are presented as mean ± standard deviation. Statistical significance was determined by Student t test for continuous variables. †A P value of < .01 was considered statistically significant.
Results and discussion
A novel in-frame heterozygous 3-bp deletion (c.2230_2232delCTC) resulting in loss of Leu718 (L718del) (Human Genome Variation Society nomenclature for the mature protein) was retained from the WES analysis. The platelet phenotype co-segregated with the bleeding tendency across 3 generations in the family (Figure 1A), and Sanger sequencing confirmed the β3 L718del for P1 and her affected daughter (P2). No potentially causal mutations were observed in other genes known to cause MTP (supplemental Table 1). Platelet aggregation for P1 and P2 (but not her sisters) was much reduced with physiologic agonists, including adenosine 5′-diphosphate (ADP), collagen, arachidonic acid, and thrombin receptor-activating peptide (TRAP) in which a slow response was retained (Figure 1B). The father of P1 showed a similarly reduced aggregation when tested. GPIb-dependent ristocetin-induced platelet agglutination was normal. We were motivated to explore this because we had observed a similar platelet aggregation profile for an Italian patient with MTP linked to a heterozygous R995Q substitution in the αIIb cytoplasmic domain.7,8 Flow cytometry showed that platelets of P1 and P2 expressed levels of αIIbβ3 (estimated as 30 000 sites) at the lower end of the normal range9 (P1; Figure 1C). There was a major reduction in the binding of PAC-1, a marker of αIIbβ3 activation, after stimulation with ADP and TRAP, and a much lower but not absent fibrinogen-binding after ADP stimulation. Spontaneous PAC-1 binding was not observed. All results were compatible with loss of αIIbβ3 function, even when the low αΙIbβ3 density was taken into account. Interestingly, this also translated into a secretion defect with a weak platelet expression of P-selectin after stimulation with ADP and TRAP (data not shown). Dense granules were normally present as revealed by serotonin, adenosine triphosphate measurements, and the presence of CD63 (data not shown).
In silico modeling confirmed that the in-frame L718del causes the β3 intracytoplasmic domain (known to contain talin,10 kindlin-3,11 and other binding sites essential for integrin activation and signaling; supplemental Figure 1) to be out of step with the αIIb cytoplasmic domain (Figure 1D). Other membrane proximal cytoplasmic domain variants that result in MTP are αIIb R995Q/W or β3 D723H, substitutions that loosen a hypothesized salt bridge12 that holds together the two integrin subunits and results in spontaneous αIIbβ3 activation.8,13,14 Although the αIIb R995Q/W substitutions have a marked effect on αIIbβ3 function, β3 D723H (termed a nonsynonymous single nucleotide polymorphism by the authors) had no effect on platelet aggregation.14 Other mutations that affect the αIIb GFFKR sequence reported in Japanese patients with MTP and a Glanzmann thrombasthenia–like syndrome are G991C and F993del.15 Phenotypes resulting from other changes in β3 that give rise to MTP, including an extracellular β1 domain D621_E660del16 are compared in supplemental Table 1. Introduction of a cytoplasmic tail β-turn by the β3 L718P substitution17,18 found in families from Europe and Japan results in a bend and the pushing apart of the two amino acids engaged in the salt bridge (data not shown). This is highly relevant for our study because it represents the same amino acid that is deleted in our family.
Blood smears from P1 stained with May-Grünwald-Giemsa stain showed platelet anisotropy with abnormal granule structures (Figure 2A). Electron microscopy clarified the variation in platelet size with the presence of large round platelets (Figure 2B-C). However, a novel feature confirmed by electron microscopy was enlarged α-granules (Figure 2C-G) that, on occasion, seemed to regroup several α-granules of normal size, a finding reminiscent of the Paris-Trousseau syndrome,19 which results from genetic variants of FLI1, variants that WES analysis failed to show in our family. Immunogold labeling of ultrathin sections was performed (Figure 2D-G). A control without primary antibody confirmed the presence of abnormal granule structures (Figure 2D). αIIbβ3, homogeneously distributed on the platelet surface, was present in the membrane of α-granules, including the giant forms (Figure 2E). Labeling of P-selectin in the α-granule membrane (including the giant granules) suggested a normal initial granule biogenesis (Figure 2F). Fibrinogen was present in the α-granules, including the giant forms, and sometimes seemed to be in close association with the delimiting membrane, thus suggesting normal αIIbβ3 cycling (Figure 2G) (for images of control platelets, see Nurden et al6 ). A morphometric analysis (Figure 2H) confirmed platelet anisotrophy and altered α-granule concentration and size. Abnormal α-granules were seen in about 10% of sections and are novel for ITGA2B or ITGB3 defects.
Interestingly, an ITGB3 nonsense mutation resulting in R724ter with loss of much of the β3 cytoplasmic tail and a more distal S752P substitution that abrogates kindlin-3 binding (supplemental Figure 1) both failed to favor spontaneous PAC-1 binding to platelets and abrogated agonist-induced αIIbβ3 activation.6,11,20,21 Neither patient exhibited MTP, which was present in a third family with a novel ITGB3 intronic mutation and frameshift that extended the β3 cytoplasmic domain by 40 amino acids and which locked αIIbβ3 in a resting state by distancing the talin and kindlin-3 binding sites from the membrane.22,23 Genetic variants affecting αIIb and β3 cytoplasmic domains that produce MTP show that the mutations result in an altered cytoskeletal architecture and reduced proplatelet formation.2,13,24 Our ITGB3 L718del (with loss of synchronization between the intracytoplasmic tail of β3 with that of αIIb) resulted in moderate MTP, decreased activation of αIIbβ3 at the platelet surface, and abnormally large α-granules and represents a novel phenotype. Large compound granules have recently been described in platelets as part of the exocytosis mechanism,25 but in our case, the giant granules were not empty. Further studies are required to determine whether they result from a fusion process or simply lack of division. Patients such as those in our study are proving to be unique models for unraveling the biology of αIIb and β3 cytoplasmic domains whose alterations lead to diverse phenotypes.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Willem Ouwehand and the National Institutes of Health BioResource–Rare Disease and the BRIDGE–Bleeding Platelet Disorders laboratories for performing the whole exome sequencing. The authors thank the patients for their help in defining their diseases.
Authorship
Contribution: P.N. and A.T.N. supervised the study, directed experiments, and wrote the manuscript; J.-C.B. performed electron microscopy and morphometric analyses; and X.P. performed DNA sequencing and in silico PyMOL modeling.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Paquita Nurden, Plateforme Technologique d'Innovation Biomédicale-L'Institut de Rythmologie et de Modélisation Cardiaque, Hôpital Xavier Arnozan, 33600 Pessac, France; e-mail: paquita.nurden@gmail.com.

![Figure 1. Platelet studies and structural in silico modeling showing effects of the novel β3 L718del. (A) Family pedigree. Blue symbols indicate MTP, and red symbols indicate a normal platelet count and function. The heterozygous presence of the ITGB3 variant is shown (+/–). (B) Light transmission aggregometry performed in citrated platelet-rich plasma (PRP) compared typical response of platelets from the proband (P1) and her affected daughter (P2) to that of a control donor. P1 and P2 were studied on 3 and 2 occasions respectively. Aggregation with high doses of ADP, collagen (Col), or arachidonic acid (AA) was of much lower intensity and was slow. Reduced although somewhat better responses were seen with TRAP, whereas ristocetin-induced platelet agglutination was normal. (C) Flow cytometry with the monoclonal antibodies (mAbs) AP-2 and Bx-1 show αIIbβ3 and GPIb content for P1 platelets (left panel, typical histograms; mean fluorescence intensity [MFI]). Binding of the immunoglobulin M (IgM) mAb PAC-1 showed much reduced activation of αIIbβ3 for P1 after stimulation of citrated PRP with ADP and TRAP (center panel). Binding of fluorescein isothiocyanate–Fg to stimulated washed platelets from P1 confirmed a reduced activation of the integrin (right panel). (D) In silico PyMOL modeling shows how β3 Leu718del changes the synchronization of the αIIb and β3 cytoplasmic tails. Left panel: cartoon representation of the normal nonactivated αIIb (purple) and β3 (green) transmembrane and cytoplasmic tail segments highlighting amino acids engaged in their clasp. Aromatic amino acids in π interactions are shown as spheres. The salt bridge involves polar amino acids of opposite charge; the positive αIIb-R995 and the negative β3-D723 are represented as sticks. Center panel: upper view schematic representations of the αIIb and β3 transmembrane α helix showing the distribution of consecutive amino acids on the helix’s circumference. (b1) Normal distribution with a β3-D723 localizing face to αIIb-R995. (b2) Configuration with the L718del. β3-D723 is now displaced by a quarter turn away from αIIb-R995. A positively charged Arg (R) now faces αIIb-R995; in addition to the repulsive charge effect, Arg is larger than Asp (arm of 7 atoms compared with 3), and this encumbrance will also push the cytoplasmic tails apart. Right panel: Cartoon representation of αIIb and β3 transmembrane segments, with superimposed β3 α helices with and without the mutation (colored orange and white, respectively). The arrow shows the twist. All methods and details of PymMOL modeling have been previously described in Nurden et al.4](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/8/10.1182_bloodadvances.2016002808/3/m_advances002808f1.jpeg?Expires=1769112405&Signature=xDQmqjsp0toXQGA7AcvNOFIQVSOMaVTA5yxcuD5m27s5LAIhN1GGXByU8WPUxYC6igyun2Y-Zkebs5VDDMXnstrr4vAFx46BGTEMWmLZYsOvktUsdzIcFX2jLIFWZYDY7kBQMKFncyQx9qVjWEKM~iPAONkcwmPyaduvpgiGgLDYwB2mQ1PYtiTeScNrryYQ1qlCxSbmf-Bdc2y65EFZ58dEH1ne89dY0XxZpdVi8W6Xbd2CpcGwRu-bjHHFtgXSQF8lKO8Pupn9-uchnsm5vBKVk76qVI3zt9mIsamxkBQZijweRja7FMgbFhgZr~pFN~PsN2nZYxIrvVeDlFVsDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)