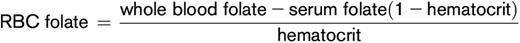Introduction
The prevalence of neural tube defects (NTDs) is estimated to be 4.1 per 1000 total births in India.
Folate deficiency can cause megaloblastic anemia, and maternal folate insufficiency is a risk factor for fetal NTDs.
World Health Organization (WHO) recommends that, at the population level, red blood cell (RBC) folate concentrations should be >400 ng/mL (906 nmol/L) in women of reproductive age (WRA) to achieve the greatest reduction of NTDs.
WHO recommends using a microbiologic assay (MA) as the most reliable choice to obtain comparable results for RBC folate concentrations across countries.
Due to the simplicity of analysis, automated clinical analysers are currently used in India to measure blood folate concentrations despite problems with data accuracy (folate underestimation).
Currently, to our knowledge, only 1 laboratory in the Southeast Asia region is using MA for RBC folate estimation.
Quality control (QC) systems for the assessment of RBC folate using MA are required to ensure high-quality data for national nutritional surveillance.
Protection from light during setup of the test and growth of L rhamnosus is crucial.
Protection from light during setup of the test and growth of L rhamnosus is crucial.
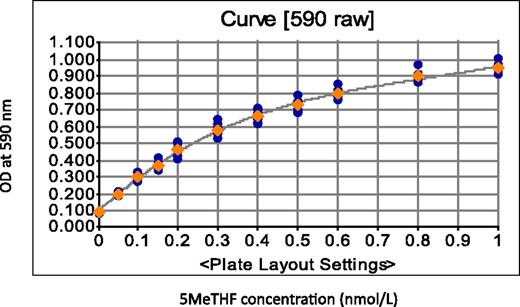
Measuring turbidity at 590 nm using Microplate Reader and Imager software.
Measuring turbidity at 590 nm using Microplate Reader and Imager software.
Results for CDC QC samples measured by PGIMER in 10 independent analytical runs
| . | . | Mean, nmol/L . | SD . | CV, % . | Range −2 SD . | Range +2 SD . | Difference (PGIMER vs CDC), % . |
|---|---|---|---|---|---|---|---|
| CDC WB low QC | CDC | 232 | |||||
| PGIMER | 249 | 22.2 | 9 | 205 | 294 | 7 | |
| CDC WB medium QC | CDC | 429 | |||||
| PGIMER | 431 | 31.9 | 7 | 367 | 495 | 1 | |
| CDC WB high QC | CDC | 723 | |||||
| PGIMER | 741 | 58.2 | 8 | 624 | 857 | 2 | |
| CDC serum low QC | CDC | 20.0 | |||||
| PGIMER | 17.5 | 1.6 | 9 | 14.4 | 20.7 | -12 | |
| CDC serum high QC | CDC | 53.4 | |||||
| PGIMER | 46.1 | 5.1 | 11 | 35.9 | 56.3 | -14 |
| . | . | Mean, nmol/L . | SD . | CV, % . | Range −2 SD . | Range +2 SD . | Difference (PGIMER vs CDC), % . |
|---|---|---|---|---|---|---|---|
| CDC WB low QC | CDC | 232 | |||||
| PGIMER | 249 | 22.2 | 9 | 205 | 294 | 7 | |
| CDC WB medium QC | CDC | 429 | |||||
| PGIMER | 431 | 31.9 | 7 | 367 | 495 | 1 | |
| CDC WB high QC | CDC | 723 | |||||
| PGIMER | 741 | 58.2 | 8 | 624 | 857 | 2 | |
| CDC serum low QC | CDC | 20.0 | |||||
| PGIMER | 17.5 | 1.6 | 9 | 14.4 | 20.7 | -12 | |
| CDC serum high QC | CDC | 53.4 | |||||
| PGIMER | 46.1 | 5.1 | 11 | 35.9 | 56.3 | -14 |
Results for PGIMER QC pools after 10 replicate runs to determine our ranges
| . | Mean, nmol/L . | SD . | CV, % . | Range −2 SD . | Range +2 SD . |
|---|---|---|---|---|---|
| PGIMER low WB folate QC | 197 | 20.4 | 10 | 156 | 238 |
| PGIMER high WB folate QC | 490 | 52.7 | 11 | 385 | 596 |
| PGIMER low serum folate QC | 16.5 | 1.0 | 6 | 14.4 | 18.5 |
| PGIMER high serum folate QC | 54.6 | 4.4 | 8 | 45.9 | 63.3 |
| . | Mean, nmol/L . | SD . | CV, % . | Range −2 SD . | Range +2 SD . |
|---|---|---|---|---|---|
| PGIMER low WB folate QC | 197 | 20.4 | 10 | 156 | 238 |
| PGIMER high WB folate QC | 490 | 52.7 | 11 | 385 | 596 |
| PGIMER low serum folate QC | 16.5 | 1.0 | 6 | 14.4 | 18.5 |
| PGIMER high serum folate QC | 54.6 | 4.4 | 8 | 45.9 | 63.3 |
Aim
To establish a proficient laboratory at the Department of Hematology, Postgraduate Institute of Medical Education and Research (PGIMER) (Chandigarh, India) under the WHO-US Centers for Disease Control and Prevention (CDC; Atlanta, GA) cooperative agreement, at which serum and RBC folate can be measured by MA to assess folate status in nonpregnant WRA from Haryana, India.
Objectives
To train laboratory personnel from PGIMER at the CDC to perform MA and to prepare for the biomarker survey.
To provide logistic and technical support to set up the MA at PGIMER with the help of the National Health Mission, the WHO, and the CDC.
To generate local indigenous quality control pools from blood samples collected in India and provide QC support.
Materials and methods
Two laboratory personnel from PGIMER received training on performing serum and RBC folate MA at the CDC for 3 weeks.
CDC supplied small quantities of key reagents needed to set up the MA at PGIMER and QC materials with low, normal, and high folate levels to verify the performance of the assay.
Blood samples from 15 volunteers in India were tested for both RBC and serum folate concentrations and were run alongside the CDC QC pool samples at PGIMER.
Formula to calculate red blood cell folate:
Results
CDC continues to provide QC support to the PGIMER laboratory through the Vitamin A Laboratory–External Quality Assurance program, an external quality assessment program for blood-based nutritional biomarkers.
Strategies for field blood sample collection, barcode labelling, and cold chain maintenance for the biomarker survey were developed.
Blood folate values were measured to screen for potential QC materials.
Two levels of bench QC pools (low and high) for whole-blood (WB) lysates and serum were prepared and aliquoted for future use.
PGIMER QC pools were characterized to assess MA performance.
Each PGIMER QC pool was analyzed in 10 runs in 4 replicates (2 dilutions) together with the CDC QC pools.
The mean, standard deviation (SD), and coefficient of variation (CV) were calculated for each QC pool, and the acceptability range for the pools was expressed as mean ± 2 SD.
Results were compared and analyzed for MA performance and validation
Within-assay CV (among 2 replicates from serum QC and 4 replicates for WB QC) was <10% and between-assay CV was 5% to 10% for both matrices, serum, and WB lysates, which is an acceptable imprecision.
The average difference between PGIMER and CDC WB results was ∼3% based on the CDC WB QC pools.
The average difference between PGIMER and CDC serum results was ∼13%, based on CDC serum QC pools; although this difference was slightly higher than expected, it was only based on 2 samples and can be considered acceptable.
Conclusions
This collaborative initiative has enabled PGIMER to set up a laboratory in Haryana, India, to perform RBC and serum folate analysis using an MA.
The prevalence of folate deficiency/insufficiency in Haryana can now be compared with other countries that are using MA.
The results of the biomarker survey on nonpregnant WRA from Haryana will help to inform decisions to implement food fortification to reduce NTD prevalence.
Acknowledgments:
This work was supported by financial and technical assistance from the CDC and the WHO for the Haryana Demonstration Project on Wheat Flour Fortification to Improve Iron, Folate, and Vitamin B12 Status.
The findings and conclusions in this presentation have not been formally disseminated by the US Centers for Disease Control and Prevention and should not be construed to represent any agency determination or policy.
Conflict-of-interest disclosure: No competing financial interests declared.
Correspondence: Reena Das, Department of Hematology, Postgraduate Institute of Medical Education and Research, Chandigarh, India; e-mail: das.reena@pgimer.edu.in.