Introduction
Treating children with cancer is challenging in developing countries such as Cambodia. The large burden of mortality and morbidity from infectious diseases and perinatal complications leaves few resources for pediatric cancer programs. Angkor Hospital for Children (AHC) is a Cambodian pediatric teaching hospital, providing free treatment to children <16 years old. In 2013, AHC started a pediatric oncology program through a collaboration with the American Society of Hematology and Health Volunteers Overseas (ASH-HVO). Physicians affiliated with ASH-HVO provide educational and technical support to the staff at AHC.
Initially the program treated retinoblastoma patients, and in 2016, AHC treated its first patient with chronic myeloid leukemia (CML). CML accounts for a minority of pediatric leukemias, but with the development of tyrosine kinase inhibitors (TKIs), such as imatinib, it has become one of the most treatable leukemias. The experience of treating children with CML at AHC illustrates how the diagnosis and treatment can be implemented successfully in a low resource setting.
Methods
Peripheral blood and bone marrow samples collected at AHC were sent for diagnostic testing at sites in the United States identified by the ASH-HVO collaborators. Multidimensional flow cytometry (MDF) was performed with the generous support of Hematologics, Inc (Seattle, WA). Samples were diluted at a 1:1 ratio in RPMI 1640 medium and sent via DHL express, with an approximate arrival time of 4 to 7 days after shipment from AHC. Filter paper provided by the laboratory of Jerry Radich (Fred Hutchinson Cancer Research Center, Seattle, WA) was used for BCR-ABL molecular testing. Dried peripheral blood spots from patients with suspected or confirmed CML were sent to the Radich laboratory, where RNA was extracted and BCR-ABL quantitative testing was performed to confirm the presence of the Philadelphia chromosome [t(9;22)(q34;q11)]. Test results from both methods had an approximate turnover time of 2 to 7 days from the time of arrival to result. Results were distributed back to the ASH-HVO and AHC physicians.
Imatinib was provided through the Max Foundation (Seattle, WA), which provides treatment to cancer treatment to patients in low- and middle-income countries.
Case description
A 12-year-old girl presented to AHC with headache, bone pain, and abdominal pain for 1 month. She had no fevers or weight loss. Physical examination revealed epigastric tenderness and splenomegaly, palpable 4 cm below the costal margin. The rest of the examination was unremarkable. Laboratory workup revealed a white blood cell count of 221.5 × 109/L, with 168.3 × 109/L neutrophils, 35.3 × 109/L blast cells, 8.9 × 109/L eosinophils, and 4.5 × 109/L monocytes. The platelet count and hemoglobin were 223 × 109/L and 107 g/L, respectively. Electrolytes, uric acid, and renal and hepatic function tests were within normal limits. Given the peripheral blood and examination findings, CML was suspected.
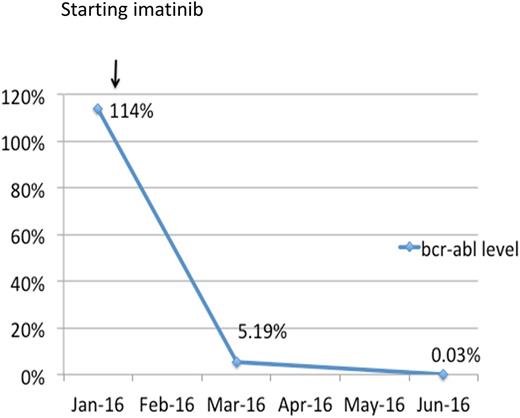
Through ASH-HVO, a peripheral blood sample from this child was sent for BCR-ABL testing to the laboratory of Jerry Radich. The patient was started on hydroxyurea pending confirmatory diagnostic testing. Approximately 2 weeks after sample collection, the molecular analysis report demonstrated the BCR-ABL fusion. A specimen sent for MDF was inadequate for definitive diagnosis due to low sample viability, but did not demonstrate any abnormal blasts. The diagnosis of CML in chronic phase was presumed. Imatinib therapy is not available at AHC. However ASH-HVO identified a source of free treatment for the patient in Cambodia. She was referred to a hospital in Phnom Penh, where she was able to obtain imatinib through the Max Foundation. After 3 months on imatinib, she had significant clinical improvement with resolution of splenomegaly and was able to return to school. The BCR-ABL level dropped to 5.19% and 0.03% after 3 and 6 months of imatinib treatment, respectively (Figure 1).
Discussion
Following recognition and diagnosis of the patient presented here with CML, an additional 3 children at AHC have been diagnosed with CML and commenced imatinib therapy. CML is a highly treatable disease, but significant barriers to appropriate diagnosis and therapy exist for children in Cambodia. The AHC and ASH-HVO collaboration used resources from local and international partnering physicians and laboratories to overcome these barriers.
CML is a myeloproliferative neoplasm that is relatively rare in children. TKI therapy has revolutionized the treatment of CML and can achieve long-lasting and durable remissions in a significant majority of patients. In Cambodia, there is no national tumor registry and expertise, diagnostics, and treatment are rarely available. In general, the diagnosis of leukemia in Cambodia is based on morphology alone. AHC has no cytogenetic or molecular diagnostic capability. Without the input from ASH-HVO, the patients presented here would have been given palliative care. In addition, the leukemia type would not have been identified and the treatable nature of the condition would not have been recognized. A similar practice of global collaboration is employed in other developing countries where patient samples are shipped to laboratories in other countries for diagnostic testing. The results allow for treatment of patients with TKI therapy in their home countries with appropriate drug access. BCR-ABL testing was initially challenging because the blood samples had degraded by the time they reached Seattle, and therefore MDF and extraction of DNA for the live cells for molecular testing was not possible. Thus, it was decided to use filter paper instead. The use of filter paper allowed for more reliable testing and monitoring of imatinib therapy by following BCR-ABL quantification.
TKI therapy for CML is prohibitively expensive and not available in Cambodia. ASH-HVO identified a center in Phnom Penh, Medical Mercy Cambodia, which received the drug from the Max Foundation and provided access to the patients at AHC. AHC now provides care and close monitoring to 4 patients with CML. Through the global collaborative efforts led by AHC and ASH-HVO, CML is the first type of leukemia to be treated at AHC, and this has helped improve oncology care for children in Cambodia.
Acknowledgments:
The authors thank Olga Sala-Torra, Jerry Radich, Mike Loken, Hematologics, Inc, and Mary Kluck.
Conflict-of-interest disclosure: P.V., American Society of Hematology (other); S.H., American Society of Hematology (consultancy); and L.K., Bristol Myers Squibb (consultancy), Kymab, Ltd (research funding), Regeneron (research funding), and Juno (research funding). The remaining authors declare no competing financial interests.
Correspondence: Prom Vireak, Angkor Hospital for Children, Siem Reap, Cambodia; e-mail: promvireak@angkorhospital.org.