Key Points
Neutrophil subsets circulating during acute inflammation are characterized by differential bacterial containment capacity.
Adequate antimicrobial containment is associated with profound phagosomal acidification yet independent of reactive oxygen species.
Abstract
Neutrophils comprise a heterogeneous population of cells essential for bacterial eradication, and defects in neutrophil function are associated with increased susceptibility to infection. In this study, neutrophils from healthy controls were shown to prevent bacterial proliferation for at least 48 hours when cocultured with methicillin-resistant Staphylococcus aureus (MRSA) in tissue-like scaffolds by establishing a bacteriostatic environment inside their phagolysosome. This intracellular bacterial containment is independent of reactive oxygen species because neutrophils that lack a functional nicotinamide adenine dinucleotide phosphate–oxidase complex displayed no defect in intracellular bacterial containment, whereas killing of the pathogen was impaired. During acute inflammation, a subset of CD16bright/CD62Ldim hypersegmented neutrophils displayed normal phagocytosis associated with a remarkably poor capacity to contain bacteria intracellularly. Conversely, CD16dim-banded neutrophils were the only neutrophil subset that adequately contained MRSA. These findings demonstrate a clear neutrophil heterogeneity in their antimicrobial capacity and the appearance of neutrophil subsets with a clear differentiation in functionality during acute inflammation. Furthermore, this study provides an evolutionary basis for the rapid release of banded neutrophils into the circulation during acute inflammation.
Introduction
Neutrophils, front-line effector cells of innate immunity, possess a wide range of functionalities from microbial killing to immune regulation.1 To protect the host against invading bacteria, neutrophils are recruited to tissues during inflammation.2 To eradicate pathogens, neutrophils carry out a complex cascade of actions: sensing of the pathogen, chemotaxis, phagocytosis, and intracellular processing. Defects in any of these functions can cause increased susceptibility to infections.3-5 Although inherited defects in neutrophil function have been linked to development of infections, the mechanisms underlying the association between acquired deficiency of neutrophils during acute systemic inflammation (eg, severe injury, ischemia/reperfusion injury, sepsis, and critical illness) and development of infections remain to be elucidated.
The antibacterial capacity of neutrophils has been studied extensively in short-term (1-3 hours) in vitro experiments by mixing neutrophils and bacteria in shaken suspensions, generally at high ratios of bacteria to neutrophils (multiplicity of infection [MOI]). Hereafter, neutrophils are lysed and the amount of colony-forming units (CFUs) is used as a direct read-out for antimicrobial capacity.6 We used an alternative approach in this study. Interactions between neutrophils and the clinically important methicillin-resistant Staphylococcus aureus (MRSA)–expressing enhanced green fluorescent protein (SA-GFP clone MW2/USA400) were studied in 3D matrices for long periods of time (48-72 hours) by using fluorescence as a read-out for the amount of bacteria present during the course of the experiment. This setup requires that neutrophils sense chemotactic gradients, migrate to and phagocytose bacteria, and subsequently use their antimicrobial armamentarium for effective prevention of bacterial outgrowth.7 By using this assay, we demonstrated that neutrophils prevent bacterial proliferation for more than 48 hours by means of intracellular containment and show that this process is nicotinamide adenine dinucleotide phosphate (NADPH)–oxidase independent because neutrophils from patients with chronic granulomatous disease (CGD) display adequate containment capacity. In addition, we show that neutrophil subsets from human volunteers, mobilized to the peripheral blood during acute inflammation after lipopolysaccharide (LPS) injection, display striking differences in antibacterial capacity that are associated with clear differences in phagosomal acidification.
Materials and methods
Donor consent and isolation of neutrophils
Our study was conducted in accordance with the Declaration of Helsinki. Blood samples were collected with approval from the ethical committees of each institution. Informed consent was provided by all patients, or by their parents, in the case of children affected by CGD.
Blood was collected in sterile collection tubes containing sodium heparin as an anticoagulant. When neutrophils were used as an entire population, cells were isolated by a 2-step procedure that was based on a method described previously.8 For isolation of subsets of neutrophils, whole blood samples were prepared as described in the supplemental Data and subsequently sorted on the MoFlo Astrios Eq Cell Sorter (Beckman Coulter Life Sciences).
Bacterial strains and bioparticle preparations
MRSA MW2 strain (clone USA400; Centers for Disease Control and Prevention, 1999) was transformed as previously described to express a robust superfolder green fluorescent protein (GFP).9 Bacteria were grown as described in supplemental Data. Fluorescence intensity, determined by using a Fluostar Optima microplate reader (BMG Labtech, Ortenberg, Germany), was used as a direct read-out for the amount of bacteria present during the experiments. Labeling of methanol-inactivated S aureus or bioparticles was performed as published previously.10
Coculture of neutrophils and bacteria
We have adapted the assay described by Li et al7 for culturing neutrophils and bacteria in tissue-like scaffolds and quantified the capacity of neutrophils to prevent bacterial proliferation by determining the time to unrestrained outgrowth (TUO) as described in the supplemental Data and supplemental Figure 2. Where indicated, lysis or inhibition of migration and phagocytosis of neutrophils was established by pipetting 10 μL of Triton X-100 solution (0.5% end concentration; Sigma-Aldrich) or 10 μL Cytochalasin D (20 μM end concentration; Sigma-Aldrich), respectively, on top of the matrices.
Data analysis and statistics
GraphPad Prism version 6.0 was used to analyze data, which are plotted as mean ± standard error of the mean (SEM) as indicated in the figure legends. The statistical analyses used are indicated in corresponding legends. Differences with P < .05 were considered statistically significant.
Additional methods
Detailed methods regarding microscopy, viability assays, chemotaxis assays, phagosomal acidification, protease activity, and neutrophil myeloperoxidase (MPO) content are provided in the supplemental Material and methods.
Results
Intracellular containment of bacteria in neutrophils from healthy controls over extended periods of time
Increasing numbers of neutrophils were cocultured with a fixed amount of SA-GFP (5 × 106 CFUs per mL) in fibrin gels to determine the antibacterial capacity of neutrophils from healthy participants (Figure 1A). SA-GFP proliferates rapidly in the absence of neutrophils as shown by a logarithmic increase in fluorescence (Figure 1A, green line) with a direct correlation between the real-time fluorescence of bacteria in the matrix and the number of CFUs (supplemental Figure 1). The TUO of SA-GFP, as defined in supplemental Figure 2, was found to be correlated with increasing numbers of neutrophils (Figure 1B). Interestingly, the highest number of neutrophils (1 × 107/mL) displayed TUOs even longer than 48 hours (Figure 1B).
Neutrophils contain bacteria inside the phagolysosome for more than 2 days. (A) Coculture of neutrophils and SA-GFP in fibrin gels. Increasing concentrations of neutrophils from healthy controls were incubated with a fixed amount of bacteria (5 × 106 CFUs per mL) (representative data; n = 20). Symbols represent the mean of duplicates. Bacterial proliferation is expressed in GFP-relative fluorescence units (RFU). (B) TUO of SA-GFP (5 × 106 CFUs per mL) cocultured in fibrin gels with 3 × 106 neutrophils per mL (n = 15), 5 × 106 neutrophils per mL (n = 22), and 10 × 106 neutrophils per mL (n = 17). (C) Propidium iodide was added to cocultures of SA-GFP (5 × 106 CFUs per mL) and 10 × 106 neutrophils per mL in fibrin gel to determine viability and containment capacity of neutrophils simultaneously. Lines represent median fluorescence intensity (n = 4). (D) Effect of addition of the phagocytosis inhibitor cytochalasin D (20 μM end concentration) on TUO during coculture of 10 × 106 polymorphonuclear (PMN) cells per mL and 5 × 106 CFUs per mL SA-GFP in fibrin gel at different (early) time points (n = 4). (E) Effect of neutrophil lysis by Triton X-100 (0.5% end concentration) on TUO during coculture of 10 × 106 PMN cells per mL and 5 × 106 CFUs per mL SA-GFP in fibrin gel at different (late) time points (n = 4). (F) Correlation between TUO and amount of bacteria present in fibrin gels. Various amounts of SA-GFP were added to fibrin gels, and TUO was determined (representative experiment; n = 5). (G) TUO after lysis of 10 × 106 PMN cells per mL at the start vs lysis at 16 hours when cocultured with 5 × 106 CFUs per mL SA-GFP in fibrin gel (n = 9). Data in panels A-B are mean + SEM. Data in panels E-G are mean ± SEM. *P < .05; **P < .01; ***P < .001 as determined by 1-way analysis of variance (ANOVA) or repeated measures (RM) 1-way ANOVA with the Greenhouse-Geisser correction followed by Tukey’s multiple comparison test or Student t test for paired samples. ns, not significant.
Neutrophils contain bacteria inside the phagolysosome for more than 2 days. (A) Coculture of neutrophils and SA-GFP in fibrin gels. Increasing concentrations of neutrophils from healthy controls were incubated with a fixed amount of bacteria (5 × 106 CFUs per mL) (representative data; n = 20). Symbols represent the mean of duplicates. Bacterial proliferation is expressed in GFP-relative fluorescence units (RFU). (B) TUO of SA-GFP (5 × 106 CFUs per mL) cocultured in fibrin gels with 3 × 106 neutrophils per mL (n = 15), 5 × 106 neutrophils per mL (n = 22), and 10 × 106 neutrophils per mL (n = 17). (C) Propidium iodide was added to cocultures of SA-GFP (5 × 106 CFUs per mL) and 10 × 106 neutrophils per mL in fibrin gel to determine viability and containment capacity of neutrophils simultaneously. Lines represent median fluorescence intensity (n = 4). (D) Effect of addition of the phagocytosis inhibitor cytochalasin D (20 μM end concentration) on TUO during coculture of 10 × 106 polymorphonuclear (PMN) cells per mL and 5 × 106 CFUs per mL SA-GFP in fibrin gel at different (early) time points (n = 4). (E) Effect of neutrophil lysis by Triton X-100 (0.5% end concentration) on TUO during coculture of 10 × 106 PMN cells per mL and 5 × 106 CFUs per mL SA-GFP in fibrin gel at different (late) time points (n = 4). (F) Correlation between TUO and amount of bacteria present in fibrin gels. Various amounts of SA-GFP were added to fibrin gels, and TUO was determined (representative experiment; n = 5). (G) TUO after lysis of 10 × 106 PMN cells per mL at the start vs lysis at 16 hours when cocultured with 5 × 106 CFUs per mL SA-GFP in fibrin gel (n = 9). Data in panels A-B are mean + SEM. Data in panels E-G are mean ± SEM. *P < .05; **P < .01; ***P < .001 as determined by 1-way analysis of variance (ANOVA) or repeated measures (RM) 1-way ANOVA with the Greenhouse-Geisser correction followed by Tukey’s multiple comparison test or Student t test for paired samples. ns, not significant.
Real-time imaging was used to explore mechanisms by which neutrophils prevent bacterial proliferation in these fibrin scaffolds (supplemental Video 1). This showed that neutrophils were able to actively move through these gels and locate, engage, and phagocytose bacteria. In line with previous findings, only a small portion of neutrophils was found to become nonviable when neutrophils were cocultured with SA at low MOIs in the first 30 hours as determined by real-time propidium iodide staining (Figure 1C, red line).11 In other words, the majority of neutrophils remained viable over extended time periods, and neutrophil lysis induced by ingested SA was not apparent under these conditions. Furthermore, at 30 hours, the initiation of death of the remaining neutrophils preceded TUO in all instances by approximately 15 hours, which indicates that viable neutrophils were necessary to limit bacterial proliferation (Figure 1C, green line). Cytochalasin D was added to the gels at different time points after the start of the experiment to determine whether neutrophils exerted their antimicrobial functions intra- or extracellularly. Cytochalasin D inhibits migration and phagocytosis and would therefore restrict antibacterial capacity to extracellular killing similar to that mediated by degranulation and/or by the formation of neutrophil extracellular traps (NETs) because NETosis and degranulation are not inhibited by cytochalasin D.12,13 The TUOs of bacterial growth were similar in the presence or absence of 1 × 107 neutrophils per mL treated with cytochalasin D immediately after the gels were formed (0 hours). This lack of extracellular killing ruled out NETosis as a relevant antimicrobial propensity of neutrophils in this setting (Figure 1D). Experiments that applied cytochalasin D at different time points indicated that neutrophils required at least 2 hours to locate and phagocytose all bacteria in the culture system; addition of the inhibitor beyond this time point did not have a significant influence on the TUO (Figure 1D).
Once a bacterium is internalized, either bacterial killing or bacteriostasis in the phagolysosome can mediate the prevention of bacterial proliferation. To distinguish between these 2 processes, Triton X-100, a neutrophil permeabilizing agent, was added at different time points at low concentrations that did not affect bacterial growth (supplemental Figure 3). Figure 1E shows that lysing neutrophils resulted in bacterial outgrowth within 10 hours at all time points, indicating that sterility was never achieved and that some bacteria remained viable in the phagolysosome of certain neutrophils. This is in line with existing evidence that SA can survive within neutrophils.14 We hypothesized that intracellular killing of bacteria would lead to increasing TUO after lysis of neutrophils during the experiment because TUO is inversely correlated with the amount of bacteria present in the assay (Figure 1F). Indeed, increased TUOs were found after lysis of neutrophils at 16 hours compared with lysis at the start of the experiment indicating that the amount of viable bacteria had declined over time inside the cultured neutrophils and thus successful killing of bacteria was achieved (Figure 1G). Apart from a delayed TUO, the growth curves were identical (supplemental Figure 4). This ruled out a possible bias of inhibited growth potential of live intracellular bacteria resulting from damage caused by phagolysosomal conditions. These experiments support the hypothesis that control of bacteria requires viable neutrophils that can contain but not necessarily kill all SA within 48 hours.
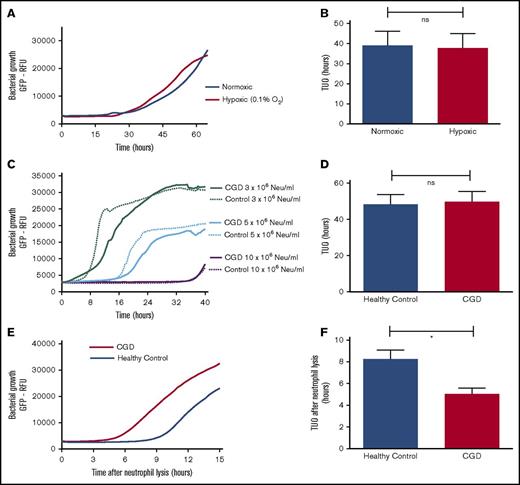
Reactive oxygen species are not essential for effective bacterial containment by neutrophils
Neutrophil-mediated killing of bacteria has previously been shown to depend on production of reactive oxygen species (ROS).15 Therefore, neutrophils from CGD patients who lack a functional NADPH oxidase and neutrophils under hypoxic conditions from healthy controls were tested to establish the role of ROS in intraphagosomal bacterial containment.16 Our experiments showed that neutrophils (from healthy controls) that were incubated under hypoxic conditions in fibrin gels (Figure 2A-B) did not exhibit impaired bacterial containment, whereas hypoxia had no effect on the growth capacity of bacteria (data not shown). Identical conclusions were drawn when comparing the coculture of SA-GFP (5 × 106 CFUs per mL) and neutrophils from either CGD patients or healthy controls in fibrin gel (Figure 2C). Neutrophils from CGD patients (1 × 107/mL) displayed a TUO of 46.7 hours, not significantly different from the 49.4 hours in cells from healthy controls (Figure 2D). However, when CGD neutrophils were lysed by Triton-X100 after 16 hours, the TUO was significantly lower in CGD cells in comparison with those from healthy controls (Figure 2E-F). This indicated that, in contrast to normal cells, the number of live intracellular bacteria did not decline over time in CGD neutrophils. These experiments demonstrated that neutrophils from CGD patients exhibited defective killing yet were able to contain bacteria intracellularly.
Intraphagosomal bacterial containment is independent of ROS. Effect of hypoxia (0.1% O2) on bactericidal capacity of 5 × 106 neutrophils (Neu) per mL from healthy participants when cocultured with 5 × 106 CFUs per mL SA-GFP in fibrin gel. (A) Growth curves after incubating neutrophils and SA-GFP in either normoxic or hypoxic conditions and (B) corresponding TUOs (n = 6). (C) Coculture of SA-GFP (5 × 106 CFUs per mL) and increasing concentrations of neutrophils from CGD patients (n = 6) and healthy controls (n = 4) in fibrin gels. (D) TUO of SA-GFP (5 × 106 CFUs per mL) cocultured in fibrin gel with 10 × 106 neutrophils per mL of healthy control neutrophils (n = 10) vs CGD patients (n = 6). (E) Mean bacterial growth measured by GFP fluorescence after lysis of 10 × 106 neutrophils per mL from healthy controls (n = 10) and CGD patients (n = 6) by Triton X-100 16 hours after starting cocultures with 5 × 106 CFUs per mL SA-GFP in fibrin gels and (F) corresponding TUOs. All data are mean + SEM. *P < .05, as determined by Student t test for unpaired samples.
Intraphagosomal bacterial containment is independent of ROS. Effect of hypoxia (0.1% O2) on bactericidal capacity of 5 × 106 neutrophils (Neu) per mL from healthy participants when cocultured with 5 × 106 CFUs per mL SA-GFP in fibrin gel. (A) Growth curves after incubating neutrophils and SA-GFP in either normoxic or hypoxic conditions and (B) corresponding TUOs (n = 6). (C) Coculture of SA-GFP (5 × 106 CFUs per mL) and increasing concentrations of neutrophils from CGD patients (n = 6) and healthy controls (n = 4) in fibrin gels. (D) TUO of SA-GFP (5 × 106 CFUs per mL) cocultured in fibrin gel with 10 × 106 neutrophils per mL of healthy control neutrophils (n = 10) vs CGD patients (n = 6). (E) Mean bacterial growth measured by GFP fluorescence after lysis of 10 × 106 neutrophils per mL from healthy controls (n = 10) and CGD patients (n = 6) by Triton X-100 16 hours after starting cocultures with 5 × 106 CFUs per mL SA-GFP in fibrin gels and (F) corresponding TUOs. All data are mean + SEM. *P < .05, as determined by Student t test for unpaired samples.
Bacterial containment is associated with acidification of the phagolysosome
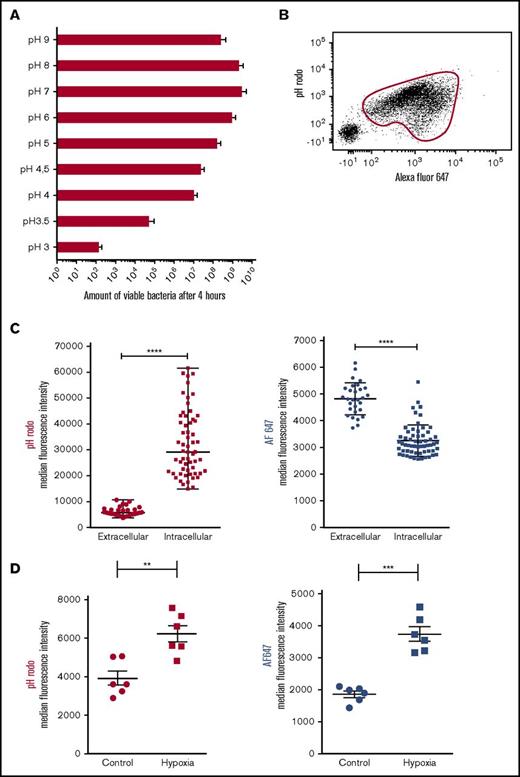
Next, nonoxidative processes were studied to unravel the biological process behind the phenotype of intraphagosomal containment of bacteria by neutrophils. Previously, several studies outlined how intraphagosomal pH shifts toward acidity after ingestion of bacteria. We tested the hypothesis that during 2 days of bacterial containment, pH plays a pivotal role in prevention of bacterial proliferation. First, to determine the effect of acidification on capacity of bacteria to proliferate, MRSA was grown in media with different pHs. This showed that the ability of bacteria to proliferate is directly correlated with the pH of its surrounding medium (Figure 3A). Next, methanol-inactivated SA (bioparticles) that were dually labeled with the pH-sensitive probe pHrodo and pH-insensitive probe Alexa Fluor647 (AF647) were fed to neutrophils from healthy controls by mixing in a shaken suspension.17 Flow cytometric analysis revealed phagocytosis (AF647 positivity) by the majority of neutrophils, and the increased pHrodo signal demonstrated profound acidification of the phagolysosome (Figure 3B). This was confirmed by fluorescence microscopy, which allows analysis of individual intraphagosomal bioparticles (supplemental Video 2). These experiments consistently showed increased pHrodo intensity of intraphagosomal bioparticles in comparison with particles found in the extracellular milieu (Figure 3C, left panel). Remarkably, the fluorescence of the pH-insensitive probe AF647 on individual bioparticles was significantly lower inside the phagolysosome compared with the extracellular milieu (Figure 3C, right panel), indicating that the probe is quenched after phagocytosis, a phenomenon that can be mimicked by adding hypochlorous acid as shown in supplemental Figure 5A. In line with these findings, AF647 signals on SA bioparticles were increased after ingestion by neutrophils treated with the NADPH inhibitor diphenyleneiodonium and the MPO inhibitor azide (supplemental Figure 5B). Therefore, it was concluded that hypochlorous acid is likely responsible for the quenching of AF647 present on the intraphagosomal bioparticles. These findings are in line with previously reported data from Schwartz et al18 on intraphagosomal quenching of GFP-labeled bacteria.
Bacteriostasis parallels phagosomal acidification. (A) Growth capacity of MRSA cultured in medium with variable pH (n = 5). Data are mean + SEM. (B) Representative data from flow cytometric analysis of isolated neutrophils after incubation for 60 minutes with dual-labeled SA bioparticles and 40% serum in shaken suspension. Phagocytosing neutrophils were gated on the basis of AF647 positivity. (C) Isolated neutrophils were incubated in suspension with dual-labeled SA bioparticles in the presence of 40% serum and were placed under the microscope. Median pHrodo (left panel) and AF647 (right panel) fluorescence intensity of individual intra- and extracellular bioparticles was determined after 60 minutes using fluorescence microscopy (representative experiment; n = 5). Horizontal lines are medians ± SEM, and data were compared by using Mann-Whitney U tests. ****P < .0001. (D) Neutrophils were incubated with dual-labeled SA bioparticles in suspension in the presence of 40% serum in either normoxic or hypoxic conditions (n = 6). pHrodo (left panel) and AF647 (right panel) fluorescence of neutrophils was determined after 120 minutes by flow cytometry. Data are mean ± SEM. **P < .01; ***P < .001 as determined by Student t test for paired samples.
Bacteriostasis parallels phagosomal acidification. (A) Growth capacity of MRSA cultured in medium with variable pH (n = 5). Data are mean + SEM. (B) Representative data from flow cytometric analysis of isolated neutrophils after incubation for 60 minutes with dual-labeled SA bioparticles and 40% serum in shaken suspension. Phagocytosing neutrophils were gated on the basis of AF647 positivity. (C) Isolated neutrophils were incubated in suspension with dual-labeled SA bioparticles in the presence of 40% serum and were placed under the microscope. Median pHrodo (left panel) and AF647 (right panel) fluorescence intensity of individual intra- and extracellular bioparticles was determined after 60 minutes using fluorescence microscopy (representative experiment; n = 5). Horizontal lines are medians ± SEM, and data were compared by using Mann-Whitney U tests. ****P < .0001. (D) Neutrophils were incubated with dual-labeled SA bioparticles in suspension in the presence of 40% serum in either normoxic or hypoxic conditions (n = 6). pHrodo (left panel) and AF647 (right panel) fluorescence of neutrophils was determined after 120 minutes by flow cytometry. Data are mean ± SEM. **P < .01; ***P < .001 as determined by Student t test for paired samples.
Next, we incubated neutrophils with dual-labeled SA bioparticles in shaken suspension under hypoxic circumstances to study the effect of ROS on intraphagosomal pH. In line with previous data, we found higher pHrodo signals under hypoxic conditions, suggesting a more acidic phagosome (Figure 3D, left panel).19,20 However, it must be taken into account that ROS-dependent quenching of pHrodo might play a role, as suggested previously.21
Concomitantly, the expression of AF647 was significantly higher under hypoxic conditions, likely a result of decreased quenching by hypochlorous acid (Figure 3D, right panel). These data confirm that, along with adequate intraphagosomal containment capacity (Figure 2A-B), adequate intraphagosomal acidification is independent of the NADPH oxidase.
Differential containment capacity of neutrophil subsets during inflammation
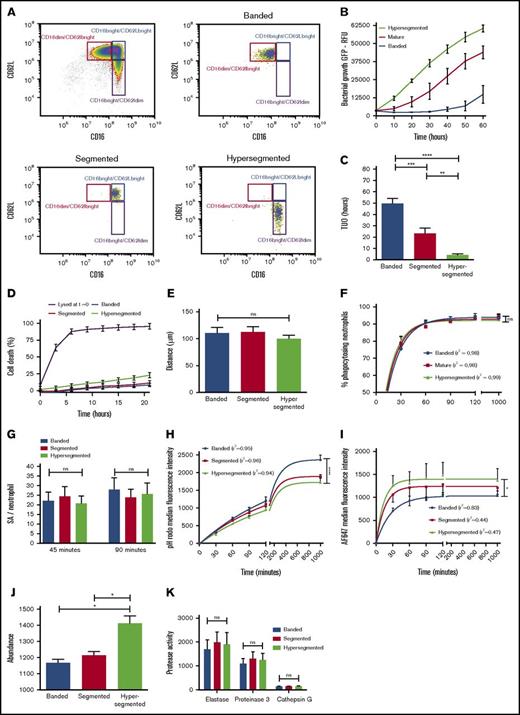
The importance of intraphagosomal containment and its putative clinical relevance was demonstrated when the antibacterial capacity of neutrophil subsets that were isolated during acute inflammation induced in healthy volunteers by challenge with LPS was assessed. Neutrophils were isolated by cell sorting from whole blood 180 minutes after intravenous injection of 2 ng/kg Escherichia coli LPS (Figure 4A). We previously showed that, apart from a difference in receptor expression, neutrophil subsets possess distinct nuclear morphology.22 When antimicrobial capacity of neutrophil subsets in fibrin gels was compared, striking differences were found (Figure 4B). The mean TUO of CD16dim/CD62Lbright neutrophils, characterized by their banded shaped nucleus, exceeded that of CD16bright/CD62Ldim-hypersegmented neutrophils by 45 hours (49.7 hours [SEM, 4.4 hours] vs 4.2 hours [SEM, 1.1 hours], respectively), whereas CD16bright/CD62Lbright-segmented neutrophils displayed an intermediate phenotype (23.3 hours [SEM, 4.7 hours]) (Figure 4C). In comparison, the mean TUO of bacteria after incubation with 5 × 106 neutrophils per mL from noninflammatory healthy controls was 32.8 hours (SEM, 4.7 hours) (Figure 1B).
Changes in intracellular containment in subsets of neutrophils is associated with increased intraphagosomal pH and decreased HOCl production. (A) Healthy volunteers provided blood samples 180 minutes after injection of LPS. Neutrophils were fluorescence-activated cell sorted based on expression of FcyRIII (CD16) and L-selectin (CD62L). (B) Mean fluorescence of coculture of sorted neutrophil subsets (5 × 106/mL) and SA-GFP (3 × 106 CFUs per mL) in fibrin gels (n = 8). (C) Corresponding TUO of experiments described in panel B (n = 8). (D) Percentage of cell death over time as determined by propidium iodide staining in fibrin gels; propidium iodide was added to the coculture of 5 × 106 neutrophils per mL in fibrin gels. (E) Migration of sorted neutrophil subsets in fibrin gels toward the bacterial peptide fMLP as studied under light microscopy. (F) Phagocytosis, as defined by neutrophil AF647 positivity, was determined over time for sorted neutrophil subsets incubated in shaken suspension with dual-labeled SA bioparticles and 40% serum using flow cytometry (n = 8). (G) Quantitative fluorescence microscopy of the number of SA bioparticles per neutrophil for sorted subsets incubated in fibrin gels after 45 and 90 minutes (n = 5). (H) Neutrophil subsets were incubated with dual-labeled SA bioparticles in shaken suspension with 40% serum (n = 8). pHrodo and (I) AF647 fluorescence were determined over time by using flow cytometry. (J) Neutrophil subsets were lysed and MPO abundance was determined (n = 3). (K) Supernatants from degranulated neutrophil subsets were incubated with protease-specific fluorescent substrates, and activity was determined by using a fluorescence plate reader (n = 4). Significance for panels C, E, G, J, and K was determined by RM 1-way ANOVA followed by Tukey’s multiple comparison test. In panels F, H, and I, data points are the mean. Lines represent the predicted value after 1-phase association nonlinear regression analyses of the data, and 1-way ANOVA was applied to the predicted plateaus. Goodness of fit was assessed by calculating the correlation coefficient (r2) and visual examination of the data. Data are mean + SEM or mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
Changes in intracellular containment in subsets of neutrophils is associated with increased intraphagosomal pH and decreased HOCl production. (A) Healthy volunteers provided blood samples 180 minutes after injection of LPS. Neutrophils were fluorescence-activated cell sorted based on expression of FcyRIII (CD16) and L-selectin (CD62L). (B) Mean fluorescence of coculture of sorted neutrophil subsets (5 × 106/mL) and SA-GFP (3 × 106 CFUs per mL) in fibrin gels (n = 8). (C) Corresponding TUO of experiments described in panel B (n = 8). (D) Percentage of cell death over time as determined by propidium iodide staining in fibrin gels; propidium iodide was added to the coculture of 5 × 106 neutrophils per mL in fibrin gels. (E) Migration of sorted neutrophil subsets in fibrin gels toward the bacterial peptide fMLP as studied under light microscopy. (F) Phagocytosis, as defined by neutrophil AF647 positivity, was determined over time for sorted neutrophil subsets incubated in shaken suspension with dual-labeled SA bioparticles and 40% serum using flow cytometry (n = 8). (G) Quantitative fluorescence microscopy of the number of SA bioparticles per neutrophil for sorted subsets incubated in fibrin gels after 45 and 90 minutes (n = 5). (H) Neutrophil subsets were incubated with dual-labeled SA bioparticles in shaken suspension with 40% serum (n = 8). pHrodo and (I) AF647 fluorescence were determined over time by using flow cytometry. (J) Neutrophil subsets were lysed and MPO abundance was determined (n = 3). (K) Supernatants from degranulated neutrophil subsets were incubated with protease-specific fluorescent substrates, and activity was determined by using a fluorescence plate reader (n = 4). Significance for panels C, E, G, J, and K was determined by RM 1-way ANOVA followed by Tukey’s multiple comparison test. In panels F, H, and I, data points are the mean. Lines represent the predicted value after 1-phase association nonlinear regression analyses of the data, and 1-way ANOVA was applied to the predicted plateaus. Goodness of fit was assessed by calculating the correlation coefficient (r2) and visual examination of the data. Data are mean + SEM or mean ± SEM. *P < .05; **P < .01; ***P < .001; ****P < .0001.
To study whether the short TUO of hypersegmented neutrophils, and to a lesser degree segmented neutrophils, was a result of a severe defect in intracellular containment, other causes for the lack of microbial control in this setting were ruled out. First, viability of neutrophils was evaluated by assessment of annexin–7-amino-actinomycin D staining. Viability was >95% immediately after cell sorting and did not differ between neutrophil subsets (supplemental Figure 6). In addition, no differences were observed when studying the kinetics of cell death when neutrophil subsets were cultured in fibrin gels (Figure 4D). Next, we studied neutrophil chemotaxis in fibrin gels, and the results demonstrated the absence of differences in cell migration between the different subsets (Figure 4E). Furthermore, flow cytometry (Figure 4F) and microscopy (Figure 4G) showed an equal percentage of phagocytosing neutrophils and an equal number of pathogens per cell, respectively. Finally, time-lapse fluorescence microscopy of neutrophil subsets in fibrin gels demonstrated that bacterial colonies arose from within the phagolysosome of hypersegmented cells instead of extracellular proliferation (supplemental Video 3).
The lack of containment of bacteria by hypersegmented and segmented neutrophils correlated with lower pHrodo values (Figure 4H) and in parallel higher AF647 values (Figure 4I) when incubated with dual-labeled bioparticles in shaken suspension. These data implied that phagosomes in these cells were characterized by higher pH values in combination with a lower concentration of hypochlorous acid indirectly determined by diminished quenching of AF647 in comparison with that of banded neutrophils. Ideally, hypochlorous acid should have been quantitatively measured directly in the phagosome to confirm these findings, but the currently available probes visualize HOCl in a more qualitative way without allowing adequate quantification of HOCl. The probes are also affected by pH, which makes reliable quantitative measurements problematic.23-25 Our previous finding of increased H2O2 production in hypersegmented neutrophils when activated with soluble stimuli is of interest because increased H2O2 combined with diminished hypochlorous acid suggests decreased MPO activity; a similar increase in H2O2 was found in MPO deficiency.22,26-28 Hypersegmented and segmented neutrophils, however, are not deficient in MPO content (Figure 4J) or proteases (Figure 4K).
Discussion
This study uses a novel approach to study long-term neutrophil-bacterial interactions in a 3D scaffold reminiscent of the in vivo environment. These conditions reveal that neutrophils have the capacity for long-term intracellular containment of live bacteria. Surprisingly, this containment is independent of ROS, because neutrophils from CGD patients show adequate intracellular containment. Conversely, failure of phagosomal acidification such as found in CD16bright/CD62Ldim hypersegmented neutrophils led to impaired intracellular containment of bacteria. CD16dim/CD62Lbright neutrophils, characterized by clear acidification, exhibited a superior containment capacity.
Many previous studies reported on neutrophils that were tested for their ability to kill bacteria in suspension, often in short-term culture assays with high MOIs in shaken suspensions and with the need for a neutrophil-lysis step to free intraphagosomal viable bacteria.29,30 These studies led to the consensus that neutrophils fail to control MRSA in vitro during these short-term assays.31,32 Our experiments do not support this hypothesis. In this study, low MOIs of GFP-expressing bacteria were combined with conditions that mimic the 3D-tissue environment. In this experimental setup, bacterial killing is dependent on multiple neutrophil functions such as pathogen sensing, migration, phagocytosis, bacteriostasis, and killing. In addition, the 3D matrix supported prolonged neutrophil survival, which allowed for long-term coculture with bacteria (Figure 1C). This relatively long in vitro survival compared with classical culture methods might be a result of the combination of a 3D-environment, the presence of serum and bacteria, and the lack of interaction with culture plastic that is known to activate neutrophils.33 We measured propidium iodide positivity of neutrophils during these culture conditions, which did not allow us to detect apoptosis or necrosis/lysis resulting from the antineutrophil functionality of SA, as described previously.34 Cell lysis and necroptosis have recently been shown in human neutrophils infected with MRSA.31 However, it was seen by using different methods of isolating neutrophils, different MRSA culture conditions, and importantly a higher MOI. Under our experimental conditions, these issues do not seem to play a role (Figure 1C). In addition, no evidence for NETosis was found in our assay because treatment with cytochalasin D did not influence bacterial outgrowth rates. Because cytochalasin D inhibits migration and phagocytosis, any bacterial killing observed would have been attributable to NETosis or degranulation. However, no differences were observed (Figure 1D), and staining with cytox green did not show evidence of NETs in our assay (results not shown). It is important to emphasize that, as with other in vitro assays, this assay was based on the entire population of neutrophils. In theory, more competent cells, especially in this long-term assay, could compensate for the presence of neutrophils with impaired antimicrobial functions. This becomes apparent in Figure 4A-C in which profound differences are seen when specific neutrophil populations are isolated and studied.
An important finding of this study is that neutrophils are able to contain live bacteria intracellularly for a prolonged period of time. This containment is an essential step between phagocytosis and killing of bacteria. However, in contrast to bacterial killing, this intraphagosomal containment was independent of ROS because neutrophils from CGD patients were able to postpone bacterial proliferation for longer than 2 days (Figure 2C-D), whereas bacterial killing was considerably disturbed (Figure 2E-F). Significant differences between CGD and normal neutrophils in TUO after lysis for 16 hours, in favor of normal neutrophils, were not present in non-lysed cells after 48 hours. We hypothesize that this is the result of increased survival in CGD and hypoxic neutrophils in comparison with normal neutrophils, as is also known from the literature.35-39 Effective intracellular bacterial containment without actually killing bacteria by CGD neutrophils might explain the marked susceptibility to disseminated infections in CGD patients.20,29,40 Migration of viable neutrophils that harbor bacteria to distant tissue sites such as bone marrow can then lead to infections at sterile sites.41 Importantly, these experiments in ROS-deficient cells demonstrated that nonoxidative bacteriostatic mechanisms played a pivotal role in intraphagosomal containment of bacteria.
The nonoxidative antimicrobial armamentarium of neutrophils is mainly situated in the granule compartment, which fuses with the phagosome upon phagocytosis of bacteria (Figure 5). These granules contain proteases and other antibacterial compounds.42 Interestingly, there was a recent study43 in patients with Papillon-Lefèvre syndrome, a rare autosomal recessive disease caused by mutations in the gene encoding lysosomal cysteine protease cathepsin C that is essential for activation of the neutrophil granule–associated serine proteases. It showed that despite a lack of functional serine proteases, these neutrophils were characterized by adequate bactericidal activity against SA. Therefore, alternative nonoxidative processes were explored to identify the mechanism underlying intraphagosomal containment by neutrophils.
Schematic representation of neutrophils oxidative (pink) and nonoxidative (green) intraphagosomal antimicrobial mechanisms after ingestion of a bacterium.
Schematic representation of neutrophils oxidative (pink) and nonoxidative (green) intraphagosomal antimicrobial mechanisms after ingestion of a bacterium.
Parallel to the release of antimicrobial proteins and/or enzymes into the phagosome, the phagosomal pH changes after ingestion of a bacterium (Figure 5). Initially, a rapid rise is observed resulting from compensatory ion movement after electron transport by the NADPH-oxidase as a result of superoxide dismutation.44-46 This alkaline environment is thought to be essential for activating proteases. This is followed by a progressive acidification upon ongoing fusion of cytoplasmic granules with the phagosome since these organelles contain the Na+/H+ antiporter V-ATPase responsible for proton pumping.19,47,48 CGD neutrophils lack initial alkalization, and acidification is induced promptly upon phagocytosis, which causes even lower pH values than those in normal neutrophils.49 This was similar to the pronounced phagosomal acidification observed when neutrophils were cultured in hypoxic conditions (Figure 3D). It is important to emphasize that all studies to date that quantify intraphagosomal pH in neutrophils have used fungal (Candida albicans) or yeast (zymosan) particles as a stimulus. In addition, reliable determination of intraphagosomal pH remains controversial because frequently used probes are quenched by several intraphagosomal agents, which biases proper quantification.50 Moreover, proper pH determination is highly dependent on adequate sealing of the phagolysosome, a process which is easily disrupted in vitro.47 In vitro, killing of SA by granule constituents is more efficient at lower pH, and simply lowering the pH of the culture medium severely impacts growth (Figure 3A).51 Therefore, a lower intraphagosomal pH would mediate containment and facilitate killing. Failure of acidification in other professional phagocytes (macrophages) results in impaired killing of SA.52,53 Decreased acidification and severely impaired bacterial containment were seen in the hypersegmented (CD16bright/CD62Ldim) and to a lesser extent segmented (CD16bright/CD62Lbright) neutrophil subpopulation isolated from normal volunteers during experimental systemic inflammation. These hypersegmented neutrophils did not show any difference in migration or phagocytosis (Figure 4). In addition, intracellular growth of bacteria (supplemental Video 3) strongly suggests an intraphagosomal containment defect. We hypothesize that this defect is a result of impaired fusion of granules which would reduce phagosomal acidification. This is supported by the finding of reduced quenching of AF647, which is HOCl dependent (supplemental Figure 5). HOCl is a product of hydrogen peroxide, MPO, and chloride. Superoxide production is not impaired in hypersegmented neutrophils, and there is no MPO deficiency (Figure 4J).22 Therefore, reduced MPO delivery to the phagosome (granule fusion) could explain the reduced AF647 quenching and reduced acidification. The testing of this hypothesis awaits the development of specific tools applicable to manipulation of the phagolysosomes of human neutrophils.
Impaired acidification in phagosomes has been correlated with poor killing, a situation that leads to severe infections. Neutrophils isolated from LAMP-2 knockout mice fail to fuse their granules with the phagosome, which results in less acidic phagosomes, and these mice are highly susceptible to periodontitis.54 Interestingly, neutrophils that lack phagosomal acidification have been described before in a murine model of sepsis in which death was correlated with improper phagosomal acidification by neutrophils.55 Although an acidic environment is thought to facilitate SA killing, other mechanisms might play a role in impaired containment in hypersegmented neutrophils. For instance, less phagosomal MPO due to decreased granule fusion might affect concentrations of oxidants, elastase, and other proteases resulting in disturbed killing and containment or damage to the neutrophil from within.56,57
Our data have important consequences for the understanding of neutrophil functions in vivo. Functional compartmentalization of neutrophils during acute inflammation was poorly understood. Now it has become clear that occurrence of banded neutrophils during acute inflammation is not a mere compensation response of the bone marrow mobilizing young and less functional neutrophils. In fact, these cells, although they are 2 days younger than the other phenotypes, exhibit superior bacterial containment.58 This provides an evolutionary basis for the well-known left shift during infection and/or inflammation. Even more intriguing is the lack of bacterial containment by L-selectin low-hypersegmented neutrophils mobilized in large amounts during acute inflammation (Figure 4B-C).22 The lack of containment is not simply caused by ageing of the cells because these cells follow the same blood kinetics as normal mature neutrophils; in fact, proteomic analysis of these cells supports the hypothesis that these cells belong to a separate lineage with immune regulatory rather than antibacterial functions.58
In conclusion, this study applied an assay that allowed investigation of the neutrophil-pathogen interactions for a prolonged period of time in a 3D scaffold. It revealed that intraphagosomal containment of live bacteria is an essential determinant of the neutrophil antibacterial function. Furthermore, marked differences in this process were observed in neutrophils circulating during acute inflammation. These differences allow the neutrophil compartment to engage in different functions required by a successful innate immune response. It shows that novel antineutrophil therapies should focus on the correct neutrophil phenotype with the required cellular function.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Dorien Kiers and Roger van Groenendael for performing the endotoxin challenges in human volunteers; Jan van der Linden, Deon Kanters, Erinke van Grinsven, Corneli van Aalst, Lucie Hustin, and Joelle Klazen for performing experiments; Gerrit Spierenburg, Koos Gaiser, and Pien van der Burght for help with operating the fluorescence-activated cell sorting machines; Anton Tool and Roel Gazendam for the gift of reagents; Daphne Stapels, Evelien Berends, Kok van Kessel, and Jos van Strijp for their guidance in microbiological experiments; and Corlinda ten Brink and George Posthuma from the Cell Microscopy Center Utrecht for their expertise and assistance.
Supported by research grants from the Dutch Lung Foundation (NAF 3.2.10.052 [L.K.] and 5.2.14.058JO [J.P.]).
Authorship
Contribution: P.H.C.L. and J.P designed and performed experiments, collected data, and wrote the manuscript with contributions from N.V., M.H., T.T., M.K., S.H.M.R., T.W.K., and P.P. as appropriate; L.P.H.L. and L.K. designed and coordinated the study; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leo Koenderman, Department of Respiratory Medicine, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: l.koenderman@umcutrecht.nl.
References
Author notes
P.H.C.L. and J.P. contributed equally to this work.
L.P.H.L. and L.K. contributed equally to this work.