Key Points
This trial evaluates addition of the histone deacetylase inhibitor valproate to standard R-CHOP therapy in DLBCL.
Addition of valproate to R-CHOP is a promising strategy in DLBCL, but auditory side effects warrant monitoring.
Abstract
The aims of the present study were to establish the maximally tolerated dose (MTD) of the histone deacetylase inhibitor valproate together with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients with diffuse large B-cell lymphoma (DLBCL). A phase 1 dose escalation study of valproate together with R-CHOP followed by a dose expansion study using the established MTD of valproate was performed. MTD of valproate together with R-CHOP was established at 60 mg/kg per day, as higher doses resulted in auditory adverse events (AEs). In the study population, 2-year progression-free survival was 84.7% (95% confidence interval [CI], 73.2%-98%). The 2-year overall survival (OS) was 96.8% (n = 31; 95% CI, 90.8%-100%). These data were compared with 2 risk-factor matched populations of R-CHOP–treated patients from the Swedish Lymphoma Registry (cohort A, n = 330 and B, n = 165). As compared with the matched cohorts, we observed a statistically significant (P = .034 and 0.028, respectively) beneficial effect of the addition of valproate to R-CHOP on the OS in the studied population. In conclusion, addition of valproate to R-CHOP is a feasible strategy in first-line treatment of DLBCL. The proposed phase 2 dose is 60 mg/kg per day together with prednisone. Auditory AEs were unexpected and warrant close monitoring. Our findings suggest that drugs that target histone deacetylation may add benefit and are tolerable when combined with standard R-CHOP in DLBCL. The phase 1 trial was registered at www.clinicaltrials.gov as #NCT01622439.
Introduction
Although 85% to 90% of primary cases of diffuse large B-cell lymphoma (DLBCL) reach a complete remission with standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment, ∼40% of patients relapse and are then in a considerably worse situation. Therefore, large efforts to improve standard R-CHOP treatment are presently being made but have so far failed.1
Interestingly, recent sequencing studies have shown that a majority of primary cases of DLBCL harbor heterozygous mutations in enzymes regulating posttranslational histone modifications, such as histone acetylation and methylation. For example, the histone acetyltransferases CREBBP and EP300 have inactivating mutations in 38% of cases, and the histone methyl transferases KMTD2 and EZH2 are mutated in 28% and 10% of DLBCL cases, respectively.2,3 Histone deacetylases (HDACs) remove acetyl groups from chromatin, resulting in a condensed, or closed, chromatin. During recent years, HDAC inhibitors (HDACi’s) were shown to have effect on specific tumor types as single agent drugs, and hematological malignancies seem to be particularly sensitive to HDACi’s. Accordingly, vorinostat, romidepsin, and belinostat have been approved by the US Food and Drug Administration, for the treatment of T-cell lymphoma. Panobinostat was approved by the US Food and Drug Administration and European Medicines Agency in 2015 for third-line treatment of multiple myeloma. Other HDACi’s, such as mocetinostat, are at present in clinical trials. The clinically most well-characterized HDACi is the anticonvulsant valproate, which has been used in the treatment of epilepsy since the 1970s.
Valproate is a GABA agonist with a long history of clinical use for treatment of epilepsy and mood disorders.4 In 2001, it was identified having inhibitory activity of class I and IIa HDAC enzymes. Interestingly, valproate pretreatment has been shown to increase epirubicin-induced DNA damage without exacerbating toxicity in mouse models, suggesting that pretreatment with valproate may sensitize to treatment with anthracyclins.5,6 Consequently, we have previously shown that 48-hour pretreatment with valproate sensitizes DLBCL cell lines to CHOP treatment, and that pretreatment with a combination of prednisone and valproate sensitizes further to CHOP.7 Moreover, valproate has been shown to increase rituximab-induced complement-dependent cell death in vitro,8 and our previously published translational data from the VALFRID (valproate in combination with rituximab and CHOP as first-line therapy in DLBCL) study, indicate that valproate increases CD20 expression in DLBCL in vivo, suggesting synergistic effects together with rituximab.9
To investigate the in vivo effects of pretreatment with a combination of valproate and prednisone before R-CHOP, the VALFRID trial was initiated. The VALFRID trial is a clinical phase 1 study with a dose expansion at the maximally tolerated dose (MTD). The primary end point was to investigate safety and tolerability of valproate together with R-CHOP and to get preliminary data on efficacy.
To evaluate overall survival (OS) data, the outcome figures from the VALFRID trial were compared with closely matched cohorts treated with standard R-CHOP therapy, from the Swedish Lymphoma Registry.
Material and methods
Eligibility
Patients aged 18 to 80 years with histologically confirmed DLBCL according to the World Health Organization (WHO) Classification and clinical stages II to IV were eligible. WHO performance status 0 to 2, and adequate organ function (international normalized ratio, bilirubin, ASAT, ALAT, pancreatic amylase, p-creatinine within normal limits, neutrophils ≥1.0 ×109/L, platelets ≥60 × 109) were required for study inclusion. Exclusion criteria were HIV positivity and seropositivity for hepatitis C virus, hepatitis B surface antigen, anti–hepatitis B core total antibodies, or other active infection, including a history of hepatitis or porphyria. Patients with psychiatric or neurological diseases or a hearing impairment >grade 2 were excluded from the study. Prior exposure to anthracyclins was an exclusion criterion, but corticosteroids for alleviation of lymphoma symptoms were allowed.
Informed consent was obtained from patients in accordance with Good Clinical Practice and national and institutional guidelines governing registered clinical trials (www.clinicaltrials.gov, #NCT01622439). The study was approved by the regional ethical committee in Lund, Sweden (EPN 2011-786).
Study treatment
During the dose escalation phase of the study, oral sodium valproate (Ergenyl, Orfiril) was given 3 times daily on days 1 to 3 (in a 3 + 3 design) at 30, 60, 80, 100, and 120 mg/kg per day (supplemental Table 1). Cohorts were limited to 3 patients in the absence of dose-limiting toxicities (DLTs). In the presence of 1 DLT during the first treatment cycle, cohorts were expanded to 6 patients (3 + 3 design), and progression to the following dose level was permitted only in the absence of further DLTs.
R-CHO (rituximab IV 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2, maximum 2 mg) was given on day 3, and prednisone (50 mg/m2) was given once daily on days 1 to 5 (supplemental Table 2). The treatment was repeated every 3 weeks for 6 cycles. Granulocyte colony-stimulating factor was given according to clinical routine, at the discretion of the treating physician.
In the dose expansion phase, oral sodium valproate (Ergenyl, Orfiril) was given 3 times daily on days 1 to 3 at 60 mg/kg per day together with R-CHOP as described previously.
Treatment assessment
Baseline safety and toxicity evaluations included patient history, physical examination, bone marrow examination, audiogram and complete blood counts with differential count, hepatic and renal function, lactate dehydrogenase (LDH), and s-electrophoresis assessment. Audiograms were repeated after cycle 3 and cycle 6. A fine-needle aspiration of an affected lymph node on day 1 and day 3 of cycle 1 was optional, and the results have been published previously.9
Differential blood counts were collected on days 3, 6, 10, 14, 18, and 21. Hepatic, pancreatic, and renal functions were evaluated on days 6, 14, and 21. LDH and β-2 microglobulin were assessed on day 21 in each cycle.
Toxicity was graded by Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, and treatment response was evaluated by computed tomography (CT) and positron emission tomography–CT, and in cases with initial bone marrow involvement, by repeated bone marrow biopsies according to the Cheson 2007 criteria.10 Patients were clinically evaluated every 3 months up to 24 months after end of treatment.
Pharmacokinetics
The total plasma-valproate (p-valproate) level was analyzed before the first morning dose on days 1, 2, and 3, and in the morning on day 4, 6, and 12 in cycles 1, 3, and 6 by standard methods at local hospitals.
Statistical analyses
Data were tabulated and described by summary statistics. Survival curves were calculated by the Kaplan-Meier product limit method. The associated pointwise 95% confidence intervals (CIs) were also calculated. Time to progression-free survival (PFS) was the minimum time of the time points related to death or relapse. In the calculations of censoring, it was assumed a 2-year complete follow-up for both death and relapse for all cases that withdrew early, and censoring was performed at the 2-year follow-up time (plus 10 days). The 1- and 2-year OSs were estimated from the survival curves with the associated 95% CIs.
To assess the comparable treatment effect, a matched cohort design was used. The Swedish Lymphoma Registry covers ∼95% of all adult (age ≥18 years) lymphoma patients in Sweden compared with the compulsory Swedish Cancer Registry, with further details described previously.11 Matching controls were obtained from the Swedish Lymphoma Registry, and matching was performed with regard to sex, age (within the range of 1 year), LDH elevation (yes/no), and the number of extranodal manifestations. A matching algorithm from the R-package (Matchit) was used to obtain 10 and 5 controls per case for cohorts A and B, respectively.12 Descriptive statistics were calculated to see the average comparability between the treated patients and the controls. In the analysis, a log-rank test was used stratified by each treated patient and the corresponding matched controls. Censoring was at 2 years after diagnosis.
Results
Patient characteristics
The study opened on 8 June 2012, and the last patient was enrolled on 11 November 2015. Twenty-five patients were included at Lund University Hospital, 1 patient at Umeå University Hospital, and 7 patients at Uppsala University Hospital.
The phase 1 dose escalation part of the study enrolled 17 patients with primary DLBCL. One patient was excluded because of a history of hepatitis B. Thus, 16 patients received at least 1 cycle of therapy in the dose escalation part of the study. One patient withdrew consent to participate after 1 cycle.
The phase 2 dose expansion study enrolled 22 patients, including 3 patients treated at the MTD from the phase 1 part of the study. Two patients chose to withdraw consent after study inclusion.
In total, 33 patients started treatment in the study (supplemental Figure 1). The mean age was 66.3 years. WHO performance status was 0 to 1 in 97% of cases. Forty percent of the patients had stage IV disease. Thirty-seven percent of patients had International Prognostic Index (IPI) 2. Table 1 summarizes patient characteristics.
Patient characteristics
| Dose, mg/kg per day . | 30 . | 60 . | 80 . | 100-120 . | Overall . |
|---|---|---|---|---|---|
| n | 3 | 20 | 3 | 7 | 33 |
| Male/female | 0/3 | 11/9 | 2/1 | 4/3 | 17/16 |
| Age, mean (SD), y | 67.7 (6.0) | 67.5 (11.1) | 66.3 (6.5) | 62.6 (9.3) | 66.3 (9.9) |
| LDH elevated, n (%) | 2 | 7 | 2 | 3 | 14 (42) |
| WHO, n (%) | |||||
| 0 | 2 | 13 | 2 | 5 | 22 (71) |
| 1 | 1 | 4 | 1 | 2 | 8 (26) |
| 2 | 0 | 1 | 0 | 0 | 1 (3) |
| Ann Arbor, n | |||||
| 2 | 0 | 7 | 2 | 2 | 11 (44) |
| 3 | 1 | 2 | 1 | 0 | 4 (16) |
| 4 | 1 | 6 | 0 | 3 | 10 (40) |
| No ex nodal manifest, n (%) | |||||
| 0 | 1 | 14 | 3 | 3 | 21 (64) |
| 1 | 1 | 4 | 0 | 4 | 9 (27) |
| 2 | 1 | 1 | 0 | 0 | 2 (6) |
| 3 | 0 | 1 | 0 | 0 | 1 (3) |
| IPI, n (%) | |||||
| 0 | 0 | 2 | 0 | 1 | 3 (11) |
| 1 | 0 | 3 | 2 | 2 | 7 (26) |
| 2 | 2 | 4 | 0 | 4 | 10 (37) |
| 3 | 0 | 3 | 1 | 0 | 4 (15) |
| 4 | 1 | 2 | 0 | 0 | 3 (11) |
| B-symptoms, n (%) | 0 | 2 | 1 | 0 | 3 (13) |
| Bulky disease, n (%) | 0 | 1 | 0 | 0 | 1 (5) |
| Dose, mg/kg per day . | 30 . | 60 . | 80 . | 100-120 . | Overall . |
|---|---|---|---|---|---|
| n | 3 | 20 | 3 | 7 | 33 |
| Male/female | 0/3 | 11/9 | 2/1 | 4/3 | 17/16 |
| Age, mean (SD), y | 67.7 (6.0) | 67.5 (11.1) | 66.3 (6.5) | 62.6 (9.3) | 66.3 (9.9) |
| LDH elevated, n (%) | 2 | 7 | 2 | 3 | 14 (42) |
| WHO, n (%) | |||||
| 0 | 2 | 13 | 2 | 5 | 22 (71) |
| 1 | 1 | 4 | 1 | 2 | 8 (26) |
| 2 | 0 | 1 | 0 | 0 | 1 (3) |
| Ann Arbor, n | |||||
| 2 | 0 | 7 | 2 | 2 | 11 (44) |
| 3 | 1 | 2 | 1 | 0 | 4 (16) |
| 4 | 1 | 6 | 0 | 3 | 10 (40) |
| No ex nodal manifest, n (%) | |||||
| 0 | 1 | 14 | 3 | 3 | 21 (64) |
| 1 | 1 | 4 | 0 | 4 | 9 (27) |
| 2 | 1 | 1 | 0 | 0 | 2 (6) |
| 3 | 0 | 1 | 0 | 0 | 1 (3) |
| IPI, n (%) | |||||
| 0 | 0 | 2 | 0 | 1 | 3 (11) |
| 1 | 0 | 3 | 2 | 2 | 7 (26) |
| 2 | 2 | 4 | 0 | 4 | 10 (37) |
| 3 | 0 | 3 | 1 | 0 | 4 (15) |
| 4 | 1 | 2 | 0 | 0 | 3 (11) |
| B-symptoms, n (%) | 0 | 2 | 1 | 0 | 3 (13) |
| Bulky disease, n (%) | 0 | 1 | 0 | 0 | 1 (5) |
SD, standard deviation.
DLTs
Valproate doses were subsequently escalated to 120 mg/kg per day, which defined the maximal administered dose together with R-CHOP. Auditory side effects such as tinnitus or a hearing impairment (defined as 1 level according to the CTCAE v4.03) were the most important adverse events (AEs). At a dose of 80 mg/kg, 2 out of 3 patients experienced tinnitus (grade 1) during the latter part (cycles 3-6) of the treatment course. In one of these, tinnitus grade 1 remained 6 months after end of treatment. One patient had an asymptomatic slight impairment (10-20 dB) of her audiogram. At a dose of 100 mg/kg, 1 out of 6 patients developed hearing impairment and tinnitus, both grade 1, after 3 cycles, which worsened to grade 3 after 4 cycles, leading to omission of valproate (Table 2). The grade 3 hearing impairment remained 2 years after end of treatment. One patient suffered acute confusion and hallucinations grade 1 leading to termination of valproate treatment (Table 3). One patient received valproate at 120 mg/kg together with R-CHOP but had problems with somnolence and confusion leading to omission of valproate after 2 cycles. Hence, DLT was observed at doses of valproate together with R-CHOP at 80 mg/kg or higher, and therefore, the MTD was defined as 60 mg/kg per day.
DLT: tinnitus and hearing impairment
| Valproate dose, mg/kg per day . | N . | Tinnitus . | Hearing impairment by audiogram . | ||
|---|---|---|---|---|---|
| Grade 1 . | Grade 3 . | Grade 1 . | Grade 3 . | ||
| 30 | 3 | — | — | — | — |
| 60 | 20 | 4 | — | 1 | — |
| 80 | 3 | 2 | — | 1 | — |
| 100 | 6 | 1 | 1 | — | 1 |
| 120 | 1 | NA | NA | NA | NA |
| Valproate dose, mg/kg per day . | N . | Tinnitus . | Hearing impairment by audiogram . | ||
|---|---|---|---|---|---|
| Grade 1 . | Grade 3 . | Grade 1 . | Grade 3 . | ||
| 30 | 3 | — | — | — | — |
| 60 | 20 | 4 | — | 1 | — |
| 80 | 3 | 2 | — | 1 | — |
| 100 | 6 | 1 | 1 | — | 1 |
| 120 | 1 | NA | NA | NA | NA |
Grading according to CTCAE v4.03.
NA, not applicable.
DLT: neuropsychiatric toxicity
| Valproate dose, mg/kg per day . | N . | Hallucinations grade 1 . | Confusion . | Somnolence grade 1-2 . | |
|---|---|---|---|---|---|
| Grade 1-2 . | Grade 3 . | ||||
| 30 | 3 | — | — | 0 | — |
| 60 | 20 | — | 2 | 0 | 1 |
| 80 | 3 | — | — | 0 | — |
| 100 | 6 | 5* | 6 | 1 | 6 |
| 120 | 1 | — | 1 | 1 | 2 |
| Valproate dose, mg/kg per day . | N . | Hallucinations grade 1 . | Confusion . | Somnolence grade 1-2 . | |
|---|---|---|---|---|---|
| Grade 1-2 . | Grade 3 . | ||||
| 30 | 3 | — | — | 0 | — |
| 60 | 20 | — | 2 | 0 | 1 |
| 80 | 3 | — | — | 0 | — |
| 100 | 6 | 5* | 6 | 1 | 6 |
| 120 | 1 | — | 1 | 1 | 2 |
Grading according to CTCAE v4.03.
All hallucinations were observed in the same patient.
Non-DLTs
In the entire study (ie, the dose escalation and the dose expansion parts combined), 27 events of nonlimiting grade 3 or 4 neutropenia were registered (Table 4).
Nonlimiting toxicity grade 3-4
| Valproate dose, mg/kg per day . | N . | Anemia . | Neutropenia . | Thrombocytopenia . | Infection . | Neuropathy . |
|---|---|---|---|---|---|---|
| 30 | 3 | 1 | 2 | 1 | 1 | 0 |
| 60 | 20 | 2 | 15 | 5 | 4 | 1 |
| 80 | 3 | 0 | 5 | 2 | 3 | 1 |
| 100 | 6 | 0 | 4 | 2 | 0 | 1 |
| 120 | 1 | 0 | 1 | 1 | 1 | 0 |
| Valproate dose, mg/kg per day . | N . | Anemia . | Neutropenia . | Thrombocytopenia . | Infection . | Neuropathy . |
|---|---|---|---|---|---|---|
| 30 | 3 | 1 | 2 | 1 | 1 | 0 |
| 60 | 20 | 2 | 15 | 5 | 4 | 1 |
| 80 | 3 | 0 | 5 | 2 | 3 | 1 |
| 100 | 6 | 0 | 4 | 2 | 0 | 1 |
| 120 | 1 | 0 | 1 | 1 | 1 | 0 |
Number of events in all cycles is shown.
The degree and frequency of neutropenia were not significantly higher with increasing doses of valproate. Eleven events of grade 3 to 4 thrombocytopenia, and 3 events of treatment-related grade 3 to 4 anemia were observed. Three events of neuropathy grade 3 were detected.
In the dose expansion part of the trial, audiogram after cycle 3 showed hearing impairment of 15 to 25 dB at 250 Hz in 1 patient, which resolved on repeated audiogram after 1 week. The patient had no symptoms of hearing impairment but ceased valproate treatment. Four patients reported mild and temporary tinnitus on 1 occasion during valproate treatment (Table 2). Two patients described transient confusion grade 1 on 1 occasion. One patient chose to withdraw from the study because of nausea and constipation after 2 cycles.
Antitumor efficacy of valproate together with R-CHOP
Sixteen patients were treated in the dose escalation phase of the study, including 3 patients treated with the MTD of valproate at 60 mg/kg per day. In the dose expansion phase, an additional 17 patients were treated with the MTD of 60 mg/kg per day of valproate together with R-CHOP. Twenty-seven out of 30 evaluable patients reached a partial remission/complete remission as judged by positron emission tomography–CT after treatment completion (data not shown).
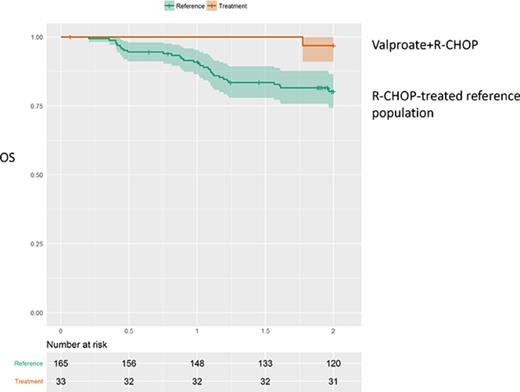
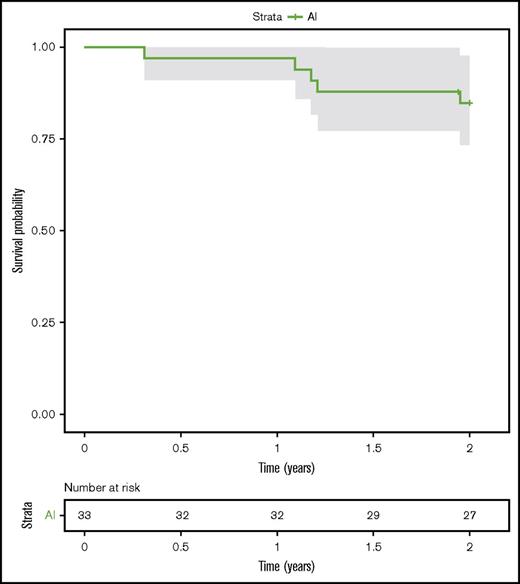
One-year PFS was 97.0% (95% CI, 91.3%-100%; n = 32) and 2-year PFS was 84.7% (95% CI, 73.3%-98.0%; n = 27) (Figure 1). One-year OS was 100% (n = 32) and 2-year OS was 96.8% (95% CI, 90.8%-100%; n = 31).
One patient in the dose expansion phase had a CD5+ DLBCL and was primarily resistant to treatment. An additional 4 patients relapsed with progressive disease. Of these, 1 patient has deceased.
Antitumor efficacy of valproate together with R-CHOP as compared with matched patient cohorts from the Swedish Lymphoma Registry
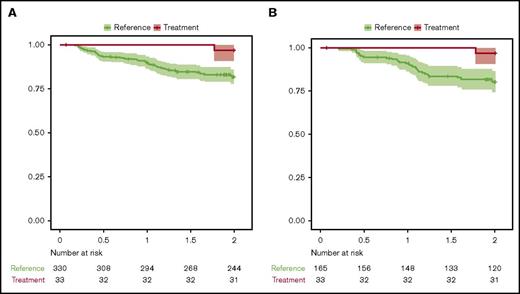
An attempt to describe the survival characteristics following treatment with valproate together with R-CHOP in the entire study was made by comparing survival data from matched, population-based cohorts. These cohorts were obtained from the Swedish Lymphoma Registry. Each patient in the trial was compared with matched individuals from the registry, based on the variables age (within the range of 1 year), sex, WHO performance status, Ann Arbor stage, LDH elevation (yes/no), and the number of extranodal manifestations. The first reference population consisted of 330 patients with DLBCL treated with R-CHOP in 2000 to 2015 (cohort A). Cohort A had a 1-year OS of 89.6% (95% CI, 86.5%-92.8%) and a 2-year OS of 81.7% (95% CI, 77.9%-85.8%). In addition, a second reference population of 165 patients treated with R-CHOP between 2010 and 2015, matched for the same risk-factors as discussed previously, was designed (cohort B). Cohort B had a 1-year OS of 90.9% (95% CI, 86.6%-95.4%) and a 2-year OS of 80.2% (95% CI, 74.3%-86.6%).
The 1-year and 2-year OS of the VALFRID study was compared with the matched populations in cohorts A and B (Figure 2). Summary by group of each variable in Figure 2 is shown in supplemental Table 3. The results suggest an additive effect of combining valproate with R-CHOP (P = .034 when compared with cohort A, and P = .028 when compared with cohort B), suggesting that valproate may add benefit when combined with standard R-CHOP in DLBCL.
OS (Kaplan-Meier) from the VALFRID study as compared with matched (age, stage, WHO performance status, LDH, extranodal manifestations) reference populations from the Swedish Lymphoma Registry. See supplemental Table 3 for summary by group of each variable used. (A) Cohort A: R-CHOP-treated patients between 2000 and 2015. P = .034. (B) Cohort B: R-CHOP-treated patients between 2010 and 2015. P = .028. The 95% CI is shown.
OS (Kaplan-Meier) from the VALFRID study as compared with matched (age, stage, WHO performance status, LDH, extranodal manifestations) reference populations from the Swedish Lymphoma Registry. See supplemental Table 3 for summary by group of each variable used. (A) Cohort A: R-CHOP-treated patients between 2000 and 2015. P = .034. (B) Cohort B: R-CHOP-treated patients between 2010 and 2015. P = .028. The 95% CI is shown.
Pharmacokinetic profile of valproate
Levels of total p-valproate were monitored on days 1, 2, 3, 4, 6, and 12 in cycles 1, 3, and 6 (Table 5).
Mean p-valproate values day 3
| Valproate dose, mg/kg per day . | p-Valproate mean, µM . | SD . |
|---|---|---|
| 30 | 569 | 164 |
| 60 | 849 | 298 |
| 80 | 860 | 193 |
| 100 | 938 | 249 |
| Valproate dose, mg/kg per day . | p-Valproate mean, µM . | SD . |
|---|---|---|
| 30 | 569 | 164 |
| 60 | 849 | 298 |
| 80 | 860 | 193 |
| 100 | 938 | 249 |
Mean values ± SD of cycles 1, 3, and 6 are shown.
As shown, levels of p-valproate on day 3 correlated to the dose of valproate. No significant differences in p-valproate levels were observed between cycles 1, 3, and 6 (data not shown).
Levels of p-valproate have been associated with a dose-dependent and reversible depletion of leukocyte and neutrophil count within 48 hours of valproate administration.13 However, no such effects could be observed, perhaps depending on the concomitant use of prednisone (data not shown). The effects of valproate on histone acetylation of peripheral blood mononuclear cells in this study has been previously reported.9
Discussion
Our findings suggest that addition of valproate to standard R-CHOP treatment could add benefit, in terms of OS, but that caution should be taken regarding auditory AEs, with neurovestibular symptoms to be carefully monitored.
The OS figures in the study were compared with 2 cohorts of R-CHOP-treated patients from the Swedish Lymphoma Registry, matched on age, stage, WHO performance status, LDH, and extranodal manifestations. As compared with the matched cohorts, we observed a statistically significant (P = .034 and 0.028, respectively) beneficial effect of the addition of valproate to R-CHOP on the OS in the studied population. To create a broad statistical basis in the comparator cohort, the study population was first compared with a cohort of 330 matched patients treated during 2000 to 2015 (cohort A). However, because the study population was treated during 2012 to 2015, the most relevant comparator cohort is probably cohort B, consisting of 165 patients treated between 2010 and 2015. Irrespective of comparator cohort, the favorable effect of addition of valproate to R-CHOP remained. Nevertheless, although the detailed data and the broad coverage of the Swedish Lymphoma Registry are powerful tools in this and other clinical trials, they cannot replace prospective randomized studies. However, importantly, patients who achieve event-free survival-24 (EFS24) have been shown to have an OS equivalent to the general population, and EFS24 has been suggested as a robust end point in DLBCL treated with immunochemotherapy.14 Therefore, the 2-year PFS in the present study of 84.7% is encouraging and supports the favorable effects of addition of valproate to R-CHOP in first-line treatment of DLBCL.
The results from the dose escalation study suggest that addition of high doses of valproate together with R-CHOP results in increased auditory AEs, where 3 cases of tinnitus and 2 cases of hearing impairment were serious AEs. The MTD of valproate together with R-CHOP was therefore established at 60 mg/kg per day, which was lower than expected. Valproate has previously been investigated by Munster and colleagues in a phase 1/2 trial in patients with breast cancer/solid malignancies, where pretreatment with valproate was followed by epirubicin/5-fluorouracil-epirubicin-cyclophosphamide.13 In this study, no pharmacodynamic, pharmacokinetic, or clinical interactions between valproate and epirubicin could be detected, and objective responses were seen in 9 out of 14 (64%) evaluable patients. The MTD was established as 140 mg/kg per day of valproate for 3 days, with grade 3 neurovestibular symptoms (somnolence, confusion, hallucinations, hearing loss, and dizziness) as DLTs. The discrepancy between the combination of valproate with R-CHOP as compared with 5-fluorouracil-epirubicin-cyclophosphamide might relate to vincristine in the R-CHOP regimen. It is possible that the combination of valproate and vincristine results in increased auditory AEs such as tinnitus and hearing loss.
Nevertheless, plasma levels of valproate in the VALFRID trial were above levels recommended during continuous valproate treatment. In continuous valproate treatment in antiepileptic patients, plasma levels between 350 and 700 µM are desired. Plasma levels at the MTD of 60 mg/kg per day for 3 days in the VALFRID study ranged between 600 and 1200 µM.
In our study, we did not experience pronounced problems with somnolence or confusion, as described by Munster et al. However, the doses used by us were considerably lower. In addition, in our study valproate was combined with prednisone from the R-CHOP regimen, possibly counteracting somnolence.
Valproate is a broad HDACi, inhibiting HDACs 1, 2, 3, 6, and 8.4 Comparable to the present investigation, the broad HDACi vorinostat, which inhibits HDACs 1, 2, 3, and 6, was recently tested together with R-CHOP in first-line treatment of 62 DLBCL patients.15 Ninety-two percent of patients had advanced-stage disease (stage III/IV). With a median follow-up of 3 years, estimated 2-year PFS was 73%, and estimated 2-year OS 86%, which as compared with historical controls was considered insufficient to warrant further investigation. Moreover, a pronounced increase in febrile neutropenia and sepsis was seen after the addition of vorinostat. Hence, although vorinostat and valproate are somewhat comparable regarding pattern of HDAC inhibition, their side effects may differ, and perhaps also their antilymphoma activity in combination with R-CHOP. Indeed, pretreatment with valproate in breast cancer cell lines and xenograft models has been shown to potentiate epirubicin-induced cell death, a fact that has been explained by valproate-induced chromatin decondensation, increasing exposure of topoisomerase II to epirubicin.5,6 It is possible that valproate treatment sensitizes to doxorubicin of the R-CHOP, increasing lymphoma cell death. Additionally, valproate has been shown to increase rituximab-induced complement-dependent cell death, and our previously published data from the VALFRID study show that valproate increases CD20 expression in the present population at study.9 This suggests additional beneficial effects of the combination of valproate with R-CHOP. Moreover, recent sequencing studies have shown that a majority of primary cases of DLBCL possess heterozygous mutations in enzymes regulating posttranslational histone modifications, such as CREBBP, EP300, KMTD2, and EZH2, and we are at present analyzing these and other genetic alterations in this study.2,3
In conclusion, our findings suggest that drugs that target histone deacetylation may add benefit when combined with standard R-CHOP in DLBCL. Although caution regarding auditory AEs is mandatory, our data indicate that valproate at 60 mg/kg per day together with R-CHOP should be tested against standard R-CHOP in a randomized trial, to support the potential benefit of the novel combination noted in this study.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank research nurses Anette Ahlin Gullers and Sandra Lovén for excellent assistance and Stefan Peterson (StatMind, Lund) for statistical analysis and data management. The authors are profoundly grateful to the patients and their families.
Respiratorius AB sponsored the trial.
Authorship
Contribution: K.D. and M.J. designed and undertook the clinical trial and wrote the manuscript; and H.H., K.P., and T.R. enrolled patients into the trial and reviewed the manuscript.
Conflict-of-interest disclosure: K.D. is a shareholder and board member of Respiratorius AB., K.D., and M.J. have received honoraria from Roche. The remaining authors declare no competing financial interests.
Correspondence: Kristina Drott, Department of Oncology, Skåne University Hospital, Lasarettsg 23 A, SE-221 85 Lund, Sweden; e-mail: kristina.drott@med.lu.se.