Key Points
Graft-versus-host effects may lead to HIV-1 reactivation and cell death of infected pre-HCT CD4+ T cells.
Natural killer cell activation correlates with in vitro HIV-1 transcriptional activity in the setting of HCT.
Introduction
Despite the success of antiretroviral therapy (ART) to suppress plasma HIV RNA, infected cells persist and constitute a long-lived HIV reservoir.1 Allogeneic hematopoietic cell transplantation (HCT) is 1 of the few interventions that leads to substantial decreases in the burden of HIV-infected cells in individuals with established viral reservoirs.2-8 HIV DNA decreases rapidly following HCT in individuals who remain on ART, but a detailed understanding of the immune mechanisms leading to reduction in viral reservoir size is lacking. Studies investigating these mechanisms have the potential to provide insight into the development of HIV curative strategies.
Early beneficial graft-versus-host responses, such as those that permit donor cells to target residual host tumor or hematopoietic cells after HCT, are mediated, in part, by the innate immune system. Natural killer (NK) cells reconstitute rapidly following HCT and have been shown to be alloreactive against HLA-matched recipient cells.9-12 An increase in the proportion of total and activated NK cells and increased frequency CD56−CD16+ NK cells, a subset that has been associated with improved cytotoxic function, were observed following HCT in 1 report of an HIV-infected individual.8 This individual experienced prolonged ART-free remission (288 days) of a similar duration to what we previously reported on the “Boston participant B.”5,8 Although beneficial graft-versus-host effects are involved in nonspecific immune targeting of cells capable of harboring HIV, it is possible that there is selective targeting of HIV-infected, and transcriptionally active, cells. To better understand the relationship between HIV-1 infection, viral reactivation, and lymphocyte activity before and after HCT, we analyzed NK-cell phenotypes and responses in 3 HIV-infected allogeneic HCT recipients. Given the rarity of HCT in HIV-infected individuals, we also designed and implemented an ex vivo assay to determine the relationship between HCT donor-derived NK and effector T-cell responses with laboratory-infected pretransplantation recipient CD4+ T cells.
Methods
First, we implemented multicolor flow cytometric assays to characterize lymphocyte phenotypes in longitudinal samples obtained from 3 HIV-infected allogeneic HCT recipients. The Harvard Cancer Center’s Institutional Review Board approved the study and written informed consent was obtained from participants. Next, we designed and implemented a flow cytometry–based assay for the study of posttransplantation NK- and T-cell activity against laboratory-infected pre-HCT recipient CD4+ T cells. Cells were obtained from uninfected HCT recipients with graft-versus-host-disease but no tumor relapse. Pre-HCT CD4+ T cells were isolated and purified from banked peripheral blood mononuclear cells (PBMCs), activated, and infected with an iGFP-gag HIV viral strain,13,14 based on prior methods of establishing viral latency.15 Pre-HCT CD4+ T cells were then stained with proliferation dye and coincubated with PBMCs obtained from the same individuals 9 to 12 months after HCT following development of donor cell chimerism. The HIV construct included an enhanced green fluorescent protein (eGFP) insert in an open reading frame of gag to minimize perturbation of regulatory genes (eg, nef). Infected pre-HCT CD4+ T cells were expanded using αCD3/αCD28 antibodies followed by resting in the presence of ART (raltegravir/efavirenz) over 6 weeks. We achieved initial infection rates of >70%, with residual eGFP production in <5% following resting. Of note, restimulation of these resting, infected cells using αCD3/αCD28 antibodies led to a desired fivefold to 10-fold increase in the frequency of eGFP-expressing cells. Control experiments were performed by infecting post-HCT engrafted CD4+ T cells infected with HIV-GFP (donor-donor controls) and incorporated unmodified total PBMCs for each time point (internal controls). Lymphocyte activation (CD38+/HLA-DR+), proliferation (Cell-Trace Violet), viral reactivation (GFP expression), and cell viability were measured over 2 weeks.
Results and discussion
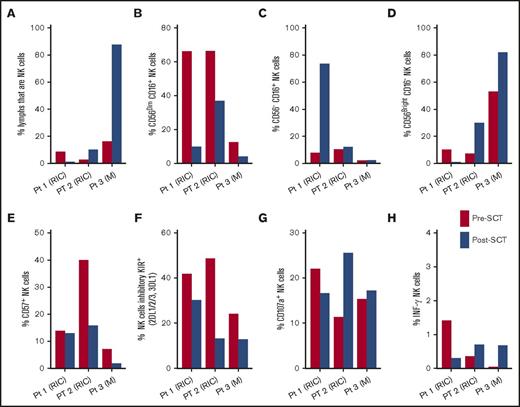
Two HIV-infected HCT participants (Pts 1 and 2) received reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma, respectively, and a third (Pt 3) received myeloablative conditioning for acute myeloid leukemia (AML); all had evidence of cytomegalovirus (CMV) infection prior to stem cell transplantation (SCT). Pts 1 and 2 experienced a 3.2 log10 and 4 log10 reduction in CD4+ T-cell–associated HIV-1 DNA 5.8 and 6.4 months following HCT. Pt 3 experienced a 1.1 log reduction in HIV-1 DNA 1 month following transplantation. In contrast, all participant samples demonstrated evidence of HIV reactivation shortly following SCT (Pt 1 experienced new low-level viremic episodes despite continued ART following SCT and Pt 2 and Pt 3 experienced transient 1.7- to 13-fold increases in cell-associated RNA levels 40 to 50 days following SCT, respectively). All individuals passed away from tumor relapse or transplant-related morbidity following the last collection time points. We observed increased frequencies of total NK cells, a decrease in the percentage of more differentiated CD56dimCD16+ NK cells, a decreased frequency of CD57+ NK cells, and decreased frequencies of NK cells expressing inhibitory killer-cell immunoglobulin-like receptors (KIRs) (Figure 1). Two individuals, 1 who received myeloablative and 1 who received reduced-intensity conditioning regimens, demonstrated increased percentage of less mature CD56bright NK cells. An increased frequency of NK cells expressing CD107a (surrogate marker for cytotoxic degranulation), and interferon-γ (INF-γ) in response to ex vivo stimulation by major histocompatibility complex (MHC)-deficient 722.221 target cells were also observed in 2 participants. Although a rejuvenated pool of alloreactive T cells is commonly observed in HIV-uninfected HCT recipients, our findings have potential relevance in HIV-infected individuals. For example, an increased frequency of mature CD56dimCD16+ NK cells have been observed in the setting of chronic HIV infection,16-18 which may represent hyporesponsive NK cells.19,20 Replacement with a rejuvenated, alloreactive NK cell pool may allow more efficient HIV-infected cell targeting. Of note, 1 recently reported HIV-infected HCT recipient experienced an increase in the frequency of CD56−CD16+ NK cells which have been associated with improved cytolytic function.8 However, only 1 of our participants experienced an increased proportion of this NK-cell subset following HSCT. Furthermore, 2 transplant recipients experienced increasing frequency of less mature CD56bright NK cells. Overall, rapid replenishment of functional NK cells following SCT has been associated with improved clinical outcomes in HIV-uninfected individuals, but increases in the immature subsets with suboptimal responses may persist for months following reduced-intensity conditioning SCT.21-23 Further studies including larger numbers of HIV-infected transplant recipients is clearly indicated to allow for controlling of factors that may influence NK cell reconstitution (eg, CMV reactivation, KIR mismatch, etc).
NK phenotype and response before and after allogeneic HSCT in 3 HIV-infected individuals on suppressive ART. (A) The percentage of lymphocytes that are NK cells, (B) percentage of CD56dimCD16+ NK cells, (C) percentage of CD56−CD16+ NK cells, (D) percentage of CD56brightCD16− NK cells, (E) percentage of CD57+ NK cells, and (F) percentage of NK cells expressing inhibitory KIRs are shown. The percentage of cells expressing a surrogate marker for cytotoxic degranulation (CD107a) and INF-γ in response to MHC-deficient target cells are shown in panels G and H, respectively. Participants (Pt) 1, 2, and 3 experienced decrease in CD4+ T cell-associated HIV-1 DNA levels 5.8, 6.4, and 1 month following HSCT, respectively. Post-HSCT chimerisms for Participants 1, 2, and 3 were 94% donor leukocyte, 89% donor leukocyte (24% Donor T-cell), and 87% donor leukocyte, respectively. M, myeloablative conditioning; RIC, reduced-intensity HSCT conditioning.
NK phenotype and response before and after allogeneic HSCT in 3 HIV-infected individuals on suppressive ART. (A) The percentage of lymphocytes that are NK cells, (B) percentage of CD56dimCD16+ NK cells, (C) percentage of CD56−CD16+ NK cells, (D) percentage of CD56brightCD16− NK cells, (E) percentage of CD57+ NK cells, and (F) percentage of NK cells expressing inhibitory KIRs are shown. The percentage of cells expressing a surrogate marker for cytotoxic degranulation (CD107a) and INF-γ in response to MHC-deficient target cells are shown in panels G and H, respectively. Participants (Pt) 1, 2, and 3 experienced decrease in CD4+ T cell-associated HIV-1 DNA levels 5.8, 6.4, and 1 month following HSCT, respectively. Post-HSCT chimerisms for Participants 1, 2, and 3 were 94% donor leukocyte, 89% donor leukocyte (24% Donor T-cell), and 87% donor leukocyte, respectively. M, myeloablative conditioning; RIC, reduced-intensity HSCT conditioning.
In order to further study the impact of donor-derived effector lymphocytes on the HIV reservoir, we implemented an ex vivo HIV assay on the pre- and post-HCT samples obtained from 30 HIV-uninfected allogeneic HCT recipients. Overall, higher CD4+ T cell death was observed in the pre-HCT CD4/post-HCT PBMC experiments by day 14 of coculture than in donor-donor control experiments (36% vs 21%). In addition, the percent of eGFP-expressing HIV-reactivated, proliferating CD4+ T cells markedly increased in several pre-HCT CD4/post-HCT PBMC experiments. Little HIV reactivation was observed in control experiments (Figure 2). Interestingly, we observed a significant positive correlation between the percentage of reactivated HIV-infected CD4+ T cells and the percentage of activated CD56dimCD16+ NK and activated CD3+CD56+ cells in pre-HCT CD4/post-HCT assays. However, no significant correlations between CD8+ T-cell activation and HIV-reactivation were identified. In contrast, only CD8+ T-cell activation correlated with HIV reactivation in post-HCT CD4/post-HCT PBMC control experiments (Figure 2). The reason for the increased CD8+ T-cell activation in the autologous control experiments is unclear, as both donor and recipient derived T cells had not been exposed prior to ex vivo infection. Further studies using either CD8 and NK depletion or positive selection prior to incubation are certainly warranted.
Results from a novel ex vivo assay of the effects of NK and CD8+T-cell responses on HIV laboratory-infected pre-HCT recipient and post-HCT donor-origin CD4+T cells. (A) A schema of our assay to determine the effects of allogeneic HSCT on HIV reactivation and effector cell activation and responses is shown. Pre- and post-SCT PBMC were obtained from 30 HIV-uninfected individuals, followed by establishment of latent HIV infection ex vivo in recipient or engrafted donor CD4+ T cells. Post-HSCT donor-derived PBMC were then coincubated with HIV-infected recipient pre-SCT (allogeneic experiments) and donor-derived post-SCT CD4+ T cells (autologous control experiments). (B) An increase in the percent of HIV-reactivated (GFP expressing) proliferating pre-HCT recipient CD4+ T cells was observed in several participant samples following 7 days of coculture (N = 30). Outlier values are shown as individual points on the box plots otherwise representing median and quartile values. Correlations between activated (CD38+/HLA-DR+) donor CD8+ T, NK, and CD3+CD56+ cells and HIV-iGFP–expressing laboratory infected CD4+ T cells on day 7 are shown in panels C (experiments using infected recipient CD4+ T cells and PBMCs following donor cell engraftment) and D (control experiments using infected post-HCT engrafted CD4+ T cells of donor origin), respectively. Significant correlations between NK and CD3+CD56+ cells were identified only in the pre-HCT CD4/post-HCT PBMC experiments, whereas only activated CD8+ T cells correlated with HIV-iGFP expression in the post-HCT CD4/post-HCT PBMC control experiments. Dashed lines represent linear regression and P values were obtained using non-parametric Spearman rank correlation analyses.
Results from a novel ex vivo assay of the effects of NK and CD8+T-cell responses on HIV laboratory-infected pre-HCT recipient and post-HCT donor-origin CD4+T cells. (A) A schema of our assay to determine the effects of allogeneic HSCT on HIV reactivation and effector cell activation and responses is shown. Pre- and post-SCT PBMC were obtained from 30 HIV-uninfected individuals, followed by establishment of latent HIV infection ex vivo in recipient or engrafted donor CD4+ T cells. Post-HSCT donor-derived PBMC were then coincubated with HIV-infected recipient pre-SCT (allogeneic experiments) and donor-derived post-SCT CD4+ T cells (autologous control experiments). (B) An increase in the percent of HIV-reactivated (GFP expressing) proliferating pre-HCT recipient CD4+ T cells was observed in several participant samples following 7 days of coculture (N = 30). Outlier values are shown as individual points on the box plots otherwise representing median and quartile values. Correlations between activated (CD38+/HLA-DR+) donor CD8+ T, NK, and CD3+CD56+ cells and HIV-iGFP–expressing laboratory infected CD4+ T cells on day 7 are shown in panels C (experiments using infected recipient CD4+ T cells and PBMCs following donor cell engraftment) and D (control experiments using infected post-HCT engrafted CD4+ T cells of donor origin), respectively. Significant correlations between NK and CD3+CD56+ cells were identified only in the pre-HCT CD4/post-HCT PBMC experiments, whereas only activated CD8+ T cells correlated with HIV-iGFP expression in the post-HCT CD4/post-HCT PBMC control experiments. Dashed lines represent linear regression and P values were obtained using non-parametric Spearman rank correlation analyses.
These experiments suggest an important relationship between NK cells, CD3+CD56+ lymphocytes (which can include NKT cells), and HIV persistence and activation following HCT. While it is possible that minor antigen mismatch played a role in non HIV-specific NK-cell recognition of allogeneic CD4+ T cells, the observed significant correlations between NK-cell activation with HIV protein expression in HLA-matched donor-recipient ex vivo experiments are intriguing. It has been postulated that HLA-dependent recognition of an activating KIR leads to NK-cell activation.24,25 However, HIV nef protein has been shown to down regulate HLA-B, which may lead to subsequent activation of NK cells.26,27 Given these observations, further study of the potential for licensed and uninhibited/activated NK cells to selectively reactivate and target HIV infected cells are warranted. Our initial ex vivo experiments were limited in that they did not involve samples selected for specific donor or recipient HLA types, KIR expression patterns, or clinical graft-versus-host disease severity. It is possible that the finding that HIV reactivation and corresponding NK-cell activation in some, but not all, participant samples was a result of these factors. Nonetheless, our data provide the rationale to further pursue the importance of NK-cell–based therapies to help purge HIV reservoirs and to more fully elucidate the innate mechanisms of HIV-infected cell clearance following HCT.
Acknowledgments
This work was supported by federal funds from the National Institutes of Health, National Institute of Allergy and Infectious Diseases grants K23AI098480 (T.J.H.) and P30 AI06035 (to the Harvard CFAR Program in Therapeutics), and by The Foundation for AIDS Research (amfAR) ARCHE award.
Authorship
Contribution: T.J.H. conceived the study, obtained funding, designed, performed and analyzed experiments, and helped write the manuscript; L.E.H. designed, performed, and analyzed experiments and wrote the manuscript; C.K., K.H., C.R.S., C.T., E.A.G., C.D.P., and S.J. designed, performed, and analyzed experiments and helped edit the manuscript; D.R.K., A.P., and F.M.M. obtained and analyzed clinical data and helped edit the manuscript; and J.R. aided in overall study design, helped obtain funding, provided clinical samples, and helped edit the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy J. Henrich, Division of Experimental Medicine, University of California San Francisco, 1001 Potrero Ave, San Francisco, CA 94110; e-mail: timothy.henrich@ucsf.edu.