Key Points
CAR-T–cell therapy normally requires the patient’s own healthy T cells.
An allogeneic CAR-T bridging therapy could rescue lymphopenic patients.
Introduction
Chimeric antigen receptor (CAR) gene therapy is a breakthrough technology in treating refractory hematologic malignancies.1-3 Successes for treating B-cell acute lymphoblastic leukemia (B-ALL) with CD19 CAR-T cells range from 67% to 90% in multiple clinical trials.4-6 We have developed a new generation of CAR-T–cell technology based on CD28 and CD27 T-cell costimulatory signals and included an inducible caspase 9 suicide gene design.7-10 Although all CAR-T studies use autologous CAR-T cells, infusion of allogeneic CAR-T cells is an attractive approach.11 Allogeneic CAR-T cells may be more robust, abrogate necessity for leukapheresing pretreated autologous T cells, and likely mediate graft-versus-leukemic effects; however, risk of graft-versus-host disease (GVHD) may limit their utility. Here, we report a novel salvage haploidentical allo–CAR-T regimen for a child with B-ALL who failed standard treatments. The patient received CD19 CAR-T cells derived from her mother (allo–CAR-T), followed by infusion of her own CAR-T cells (auto–CAR-T), which resulted in complete remission (CR) with manageable mild cytokine release syndrome (CRS).
Case description
A child suffering from relapsed and refractory B-ALL who became highly lymphopenic was successfully rescued with a sequential haploidentical allo– and auto–CAR-T-cell therapy treatment. This treatment is based on a rapid 5- to 7-day CAR-T–cell preparation protocol, which produces therapeutic CD19 CAR-T cells (4SCAR19) engineered with an inducible caspase 9 safety switch. The first round of allogeneic 4SCAR19 T cells derived from her mother was given on days 0, 4, and 7 and the patient achieved partial response in 30 days with a full immune recovery. This was then followed by an infusion of a second round of autologous 4SCAR19 cells on day 56 and she achieved CR on day 77. GVHD or CRS was not observed throughout the treatment. This novel approach establishes that 4SCAR19 may be safely administered to treat severely lymphodepleted patients, and that the combination of haploidentical allogeneic and autologous 4SCAR19 may rescue chemorefractory and high tumor burden leukemia patients.
Methods
Patient enrollment
The current study was approved by the Institutional Review Board of Geno-immune Medical Institute of Shenzhen, China (GIMI-IRB-16001) and registered at www.clinicaltrials.gov (#NCT03050190, #NCT03125577). All patients provided informed consent according to institutional guidelines and the Declaration of Helsinki.
CD19 CAR-T–cell preparation
Lentiviral vectors (LVs) were generated based on the NHP/TYF LV system as previously described.12,13 A new generation CAR, containing CD19-specific single-chain variable fragment fused with CD28-CD27-CD3z signaling domains and an inducible caspase 9 motif, was chemically synthesized and cloned into pTYF transducing vector behind a human EF1α promoter.7-9 The final LV-CAR construct was extensively verified by functional analyses. For the preparation of clinical-grade CAR-T cells, a standard operation protocol has been established in compliance with good manufacture and laboratory practice following the regulatory guideline for cell and gene therapy products. The lentivector gene transfer efficiencies were 42.1% and 34.3% for the allo–CAR-Ts and auto–CAR-Ts prepared in this report, respectively.
CAR detection by quantitative PCR
The CAR copy number in blood was determined by quantitative real-time polymerase chain reaction (PCR; based on both SYBR and Taqman probe methods) as previously described.14 Genomic DNA was harvested from blood cells using a Promega genomic DNA purification kit (Promega Corp., Madison, WI). The quantitative PCR data were collected using a MX3000P (Stratagene; Agilent Technologies, Santa Clara, CA).
Cytokine analysis based on cytokine bead array (CBA)
We used the BD CBA Human Soluble Protein Flex Set System to determine plasma concentrations of cytokines including interleukin-2 (IL-2), IL-6, IL-8, IL-10, tumor necrosis factor α (TNF-α), and interferon γ. Using flow cytometry, the CBA system captures soluble analytes with beads of known size and fluorescence.
Results and discussion
In mid-September 2011, a 3-year-old female patient presented with pallor, limb pain, fever, and cough. Bone marrow (BM) cytogenetics showed 56, XX, +X, dup (1) (q23q32), + 4, +6, +9, +14, +19, +21, +mar1, +mar2, +mar3, with leukemic blasts showing CD34+, CD10−, CD19+, and CD38+. She received vindesine, daunorubicin, cyclophosphamide, prednisone induction chemotherapy and achieved CR. This was followed by consolidation and maintenance therapies for 3 years. Toward the end of maintenance therapy, BM showed minimal residual disease (MRD) of 0.02%. She received 3 courses of reinduction chemotherapy with multiple administrations of intrathecal chemotherapy (1β-d-Arabinofuranosylcytosine [cytarabine, Ara-C] + methotrexate + dexamethasone) for central nervous system prophylaxis.
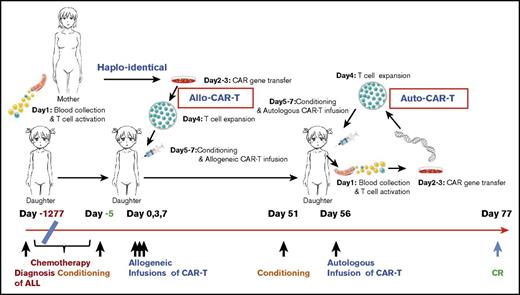
By March 2015, all chemotherapies failed, and she developed pancytopenia with 27% leukemic blasts in the BM with hypocellularity. This was complicated by pulmonary infections and gastrointestinal bleeding, and her condition deteriorated because of disease progression. In the absence of autologous T cells, haploidentical allogeneic CAR-T strategy was considered (Figure 1).
The time line and key events of the allogeneic/autologous CAR-T treatment regimen. The patient was diagnosed with ALL on day −1277 (−42.5 months) and received extensive chemotherapy but developed refractory relapses with lung infections. Maternal source CD19 CAR-T cells were prepared based on a 5- to 7-day CAR-T–cell preparation protocol as illustrated on the top. Allo–CAR-T cells were infused on days 0, 3, and 7, after conditioning chemotherapy. The patient achieved a partial response (PR) in 50 days and received an auto–CAR-T infusion on day 56 after conditioning chemotherapy. CR was achieved on day 77.
The time line and key events of the allogeneic/autologous CAR-T treatment regimen. The patient was diagnosed with ALL on day −1277 (−42.5 months) and received extensive chemotherapy but developed refractory relapses with lung infections. Maternal source CD19 CAR-T cells were prepared based on a 5- to 7-day CAR-T–cell preparation protocol as illustrated on the top. Allo–CAR-T cells were infused on days 0, 3, and 7, after conditioning chemotherapy. The patient achieved a partial response (PR) in 50 days and received an auto–CAR-T infusion on day 56 after conditioning chemotherapy. CR was achieved on day 77.
The patient received cyclophosphamide 400 mg/m2 and fludarabine 30 mg/m2 conditioning regimen on day −5 to −3 prior to CAR-T infusion. Allogeneic CAR-T cells from her mother were infused at 0.54 × 106, 0.5 × 106, and 0.9 × 106 per kg of patient’s body weight on days 0, 3, and 7, respectively. The patient developed fever at 38.5°C with scattered maculopapular rashes and pruritus on her back and neck on day 1. Based on prior CAR-T experiences,7-9 she was given 12.5 mg etanercept (anti-TNF-α antibody), and the patient defervesced. Her temperature fluctuated over several days with new onset of facial rashes, and repetitive doses of etanercept were given to control fever (Figure 2A).
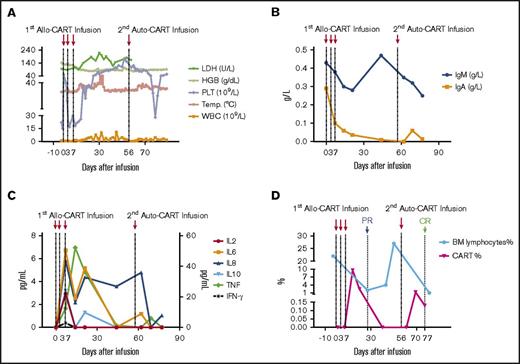
Biochemical and immunological kinetics after allo–/auto–CAR-T-cell infusions. (A) Kinetics of hematologic correlates and body temperature. After allo–CAR-T-cell infusions, the body temperature (Temp.) and white blood cell (WBC) count fluctuated continuously, and the platelet count increased gradually. The hemoglobin (HGB) remained steady, and lactate dehydrogenase (LDH) went up correlated with inflammatory response and tumor reduction. (B) The kinetics of IgA and IgM. (C) The kinetics of inflammatory cytokines. The plasma levels of various cytokines were determined by CBA bead array. (D) The kinetics of BM lymphocytes (BM%) and CAR-T cells (CART%). After allo–CAR-T and auto–CAR-T infusions, BM lymphocytes rapidly dropped as did leukemic cell burden, which elicited PR and CR, respectively. The reduction of leukemic cells correlated with CAR-T–cell expansion.
Biochemical and immunological kinetics after allo–/auto–CAR-T-cell infusions. (A) Kinetics of hematologic correlates and body temperature. After allo–CAR-T-cell infusions, the body temperature (Temp.) and white blood cell (WBC) count fluctuated continuously, and the platelet count increased gradually. The hemoglobin (HGB) remained steady, and lactate dehydrogenase (LDH) went up correlated with inflammatory response and tumor reduction. (B) The kinetics of IgA and IgM. (C) The kinetics of inflammatory cytokines. The plasma levels of various cytokines were determined by CBA bead array. (D) The kinetics of BM lymphocytes (BM%) and CAR-T cells (CART%). After allo–CAR-T and auto–CAR-T infusions, BM lymphocytes rapidly dropped as did leukemic cell burden, which elicited PR and CR, respectively. The reduction of leukemic cells correlated with CAR-T–cell expansion.
Because of the patient’s prolonged fevers, broad-spectrum antibiotics and antifungal therapy were initiated. The decrease in immunoglobulin A (IgA) and IgM correlated with B cell aplasia suggestive of CAR-T functionality (Figure 2B). CRS was mild, and no serious biochemical abnormalities were observed (Figure 2A,C). She achieved PR on day 27 with BM hypercellularity of <2% leukemic blasts and MRD of 0.42% by flow cytometry (Figure 2D). On day 50, complete blood count (CBC) returned to normal, and BM evaluation indicated immature lymphocytes at 27% and MRD of 6.71% blasts.
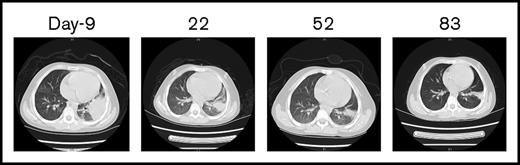
The CBC recovery was accompanied by disappearance of allo–CAR-T cells and increased MRD. Therefore, a second CAR-T course from her own source was initiated. At time of apheresis, the collected cells were 100% of her own based on genotyping, with an absolute lymphocyte count of 0.97 × 109/L and 45% CD3 T cells. Cyclophosphamide 400 mg/m2 and fludarabine 30 mg/m2 conditioning regimen was given on day 51, and autologous CAR-T cells (0.16 x 106/kg) were infused on day 56, which was well tolerated. CAR-T–cell expansion was detected on day 64, and the patient received etanercept for an associated fever. Her temperature returned to normal by day 66. On day 78, 23 days after the auto–CAR-T infusion, BM examination indicated CR by histology and flow cytometry (Figure 2D). Chromosomal analysis showed complete normal phenotype (46, XX) without abnormal clones. Computed tomography (CT) scans of lungs revealed complete resolution from previous infections by day 83 (Figure 3). Because of the lack of matched unrelated donor, and a negative test result for donor specific antibody, the patient subsequently underwent haploidentical hematopoietic stem cell transplantation from the same donor 2 months after the auto–CAR-T infusion.
CT scans of the chest before and after allo–/auto–CAR-T infusions. Before CAR-T treatment, CT scans revealed severe infections (day −9), which gradually resolved after allo– and auto–CAR-T infusions (days 22, 52, and 83).
CT scans of the chest before and after allo–/auto–CAR-T infusions. Before CAR-T treatment, CT scans revealed severe infections (day −9), which gradually resolved after allo– and auto–CAR-T infusions (days 22, 52, and 83).
Given the severe lymphopenic condition, and success with infusion of haploidentical allogeneic CAR-T cells based on a 5- to 7-day protocol, CAR-Ts with partially matched major histocompatibility complex may have the potential to act as universal effector cells to treat patients. Such allo–CAR-T cells could be manufactured “off the shelf” with much less difficulty and greatly reduced costs.15,16
Despite these advantages, serious concerns remain. Allo–CAR-T may induce severe CRS or GVHD.17 Citing safety issues from allo–CAR-T infusion because of severe adverse responses, the US Food and Drug Administration terminated 2 phase 1 “off the shelf” CAR-T (UCART123) trials sponsored by Cellectis. Rigorous donor selection or selection of virus-specific allogeneic T cells may reduce these side effects.18 Although no GVHD has been reported using the select allo–CAR-T approaches (#NCT01475058, #NCT01430390, and #NCT01195480), the efficacy of allo–CAR-Ts may be suboptimal because of limited T-cell life spans after overexpansion and reduced cytolytic CAR-T functions. Unlike the majority allo–CAR-T studies, the allo–CAR-T cells in this study were engineered from the bulk T cells of the patient’s mother, manufactured within 5 to 7 days without selection and sequentially infused in a low number of cells. In theory, bulk donor T cells are more likely to initiate GVHD than selected T cells; however, no GVHD has been observed in this case following the allo–CAR-T infusions.
GVHD is initiated through activation of donor T cells mediated by host antigen-presenting cells.19 Here, the lack of GVHD may be attributed to the highly immunodeficient state and the preconditioning regimen, which resulted in marked reduction of host immune cells. Furthermore, continuous therapeutic and prophylactic use of TNF-α antagonist (etanercept) during CAR-T expansion may have suppressed GVHD (L.-J.C. and J.-P.Z., unpublished observation). Alternatively, long-term chemotherapy may have depleted host antigen-presenting cells and mitigated the development of GVHD.19,20
In summary, an unselected CAR-T strategy with sequential allo– and auto–CAR-T infusions successfully mediated remission of a relapsed/refractory B-ALL case. This sequential strategy resulted in CR without severe adverse effects. After CR, the patient received haploidentical hematopoietic stem cell transplantation from the same allo–CAR-T donor without severe GVHD and has remained in CR for >2 years and 9 months.
Acknowledgments
The authors thank Elias Sayour for critical reading and revision of the manuscript.
This work was supported by research funds from Science and Technology Planning Technical Research Project of Shenzhen (KQTD20140630143254906, JSGG20160229170423146, JCYJ20160229170523065, and JCYJ20170413154349187) and University of Electronic Science and Technology of China (ZYGX2016Z009).
Authorship
Contribution: L.-J.C. designed the studies and finalized the manuscript; J.-P.Z. designed the treatment strategy and performed the patient treatment and clinical data collection; R.Z., S.-T.T., and Y.-C.L. performed vector and cell preparations, laboratory analysis, and manuscript drafting; X.C., D.-P.L., and P.C. participated in discussion and manuscript preparation; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lung-Ji Chang, Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, 1200 Newell Dr, ARB R1-252, Gainesville, FL 32610-0266; e-mail: lchang@mgm.ufl.edu.
References
Author notes
J.-P.Z. and R.Z. contributed equally to this study.