Key Points
Co-HP binds to CLEC-2 at N120 and K211, novel binding sites for podoplanin, and inhibits their interaction.
Co-HP prevents hematogenous tumor metastasis and arterial/venous thrombosis in mice, without a significant increase in bleeding time.
Abstract
The platelet activation receptor C-type lectin-like receptor 2 (CLEC-2) interacts with podoplanin on the surface of certain types of tumor cells, and this interaction facilitates tumor metastasis. CLEC-2 is also involved in thrombus formation and its stabilization. Because CLEC-2–depleted mice are protected from experimental lung metastasis and thrombus formation and do not show increased bleeding time, CLEC-2 may serve as a good target for antimetastatic or antithrombotic drugs. We screened 6770 compounds for their capability to inhibit CLEC-2–podoplanin binding using an enzyme-linked immunosorbent assay. In the first screening round, 63 compounds were identified and further evaluated by flow cytometry using CLEC-2–expressing cells. We identified protoporphyrin IX (H2-PP) as the most potent inhibitor and modified its hematoporphyrin moiety to be complexed with cobalt (cobalt hematoporphyrin [Co-HP]), which resulted in an inhibitory potency much stronger than that of H2-PP. Surface plasmon resonance analysis and molecular docking study showed that Co-HP binds directly to CLEC-2 at N120, N210, and K211, previously unknown podoplanin-binding sites; this binding was confirmed by analysis of CLEC-2 mutants with alterations in N120 and/or K211. Co-HP at a concentration of 1.53 μM inhibited platelet aggregation mediated through CLEC-2, but not that mediated through other receptors. IV administration of Co-HP to mice significantly inhibited hematogenous metastasis of podoplanin-expressing B16F10 cells to the lung as well as in vivo arterial and venous thrombosis, without a significant increase in tail-bleeding time. Thus, Co-HP may be a promising molecule for antimetastatic and antiplatelet treatment that does not cause bleeding tendency.
Introduction
Tumor metastasis starts with the detachment of tumor cells from the primary site. This step is followed by invasion/migration of tumor cells through the host’s bloodstream, adhesion to capillaries in a distant organ, and extravasation into and proliferation within the tissues. Most tumor cells cannot survive in the bloodstream because of shear stress and immune cell activity, and <0.01% of tumor cells in circulation result in metastases.1 Tumor cell–platelet interactions facilitate hematogenous metastasis by increasing the survival of tumor cells by covering the cells with platelets and attaching tumor cells to the vessel wall by forming platelet aggregates.1
One of the tumor cell–related molecules to activate platelets is the membrane sialoglycoprotein, podoplanin. Podoplanin is expressed on the surface of certain types of tumor cells, including melanoma, squamous cell carcinoma, and brain tumor cells,2-4 and its increased expression is associated with tumor metastasis/progression.4 We have identified C-type lectin-like receptor 2 (CLEC-2) on the platelet surface as a podoplanin receptor.5,6 IV injection of podoplanin-positive tumor cells into mice results in lung metastasis. This is greatly impaired in mice in which CLEC-2 is depleted from platelets or in the presence of antipodoplanin-blocking antibodies.7,8 Thus, reagents for blocking CLEC-2/podoplanin binding may be good candidates as antimetastatic drugs.
Platelets play a role in pathological thrombosis, which is one of the leading causes of death in industrialized countries besides cancer. In vivo thrombus formation starts when platelets interact with exposed collagen fibers at sites of vascular injury. Platelet adhesion/aggregation on collagen fibers and subsequent stable clot formation are integrated processes involving several platelet agonists including adenosine 5′-diphosphate, thromboxane A2, and thrombin.9 An important role of CLEC-2 in arterial and venous thrombosis has been proposed in several reports.10-14 We, and others, have reported that FeCl3-induced thrombus formation in vivo is significantly decreased in CLEC-2–depleted or CLEC-2–deficient chimeric mice.10-13 Payne et al reported that mice with CLEC-2 deficiency are protected against deep vein thrombosis (DVT).14 Although podoplanin is not expressed in the normal vessel wall, its presence has been reported in certain pathological states, and the interaction between CLEC-2 and unidentified ligands may be responsible for thrombus stabilization. Despite reduced thrombus stability in the absence of CLEC-2, previous studies showed that bleeding time is not significantly increased.10,12,13
In humans, CLEC-2 is almost exclusively expressed in platelets/megakaryocytes among hematopoietic cells and at lower levels in liver Kupffer cells.15-18 In contrast, podoplanin is expressed in normal cells including lymphatic endothelial cells, kidney podocytes, and type I lung alveolar cells.19 Thus, selective expression of CLEC-2 in platelets/megakaryocytes and the lack of bleeding tendency in the absence of CLEC-2 suggest that CLEC-2 may be a suitable target of antimetastatic and/or antiplatelet drugs.
Here, we identified a small molecule, cobalt hematoporphyrin (Co-HP) as a CLEC-2 inhibitor of podoplanin binding by screening 6770 compounds and optimization. Co-HP bound to N120, N210, and K211 within CLEC-2. By generating a CLEC-2 mutant, we identified N120 and K211 as previously unknown binding sites for podoplanin; this interaction was competitively inhibited by Co-HP. Co-HP greatly and significantly inhibited the lung metastasis of podoplanin-positive tumor cells in mice and in vivo arterial and venous thrombus formation. Thus, Co-HP is a promising compound for anti–CLEC-2 drugs, which may contribute to the inhibition of both hematogenous tumor metastasis and arterial/venous thrombosis.
Materials and methods
Enzyme-linked immunosorbent assay
Immulon 2 flat-bottom 96-well plates (Thermo Fisher Scientific, Waltham, MA) were coated with 10 μg/mL recombinant extracellular domain of human CLEC-2–rabbit Fc2 fusion protein (hCLEC-2–rFc2), generated as described previously,6 for 8 to 24 hours at 4°C. The plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T; pH 7.2) and blocked with Super Block (ScyTek Laboratories, Inc, West Logan, UT) for 5 minutes. Test compounds (10 μg/mL [11-35 μM] unless otherwise noted) from the library developed by RIKEN, 30 nM rhodocytin,20 or vehicle were added to each well. After washing, the recombinant extracellular domain of the human podoplanin–human Fc2 fusion protein labeled with biotin (hPod-hFc2-Biotin, 10 μg/mL) was added and incubated for 1 hour at room temperature. After washing with PBS-T, streptavidin-horseradish peroxidase (Vector Laboratories, Inc, Burlingame, CA) was added and incubated for 1 hour at room temperature. Plates were developed by adding 3,3′,5,5′-tetramethylbenzidine (Dojindo Laboratories, Kumamoto, Japan) for 3 to 5 minutes and absorbance was read at 450 nm in a microplate reader after the reaction was stopped by adding 0.5 M HCl.
Flow cytometry
CLEC-2 was expressed under a Tet repressor protein in the T-REx-293 cell line.15 CLEC-2 expression was induced by adding 1 μg/mL doxycycline to the medium 24 to 48 hours before experimentation. Cells suspended in PBS (5 × 106/mL) were incubated with 20 μg/mL (22-70 μM, unless otherwise noted) of hit compounds, 30 nM rhodocytin, or vehicle for 30 minutes on ice. hPod-hFc2-biotin or hPod-hFc2 (5 μg/mL each) were added to the cells and incubated for 30 minutes on ice. After washing, the binding of hPod-hFc2-biotin/hPod-hFc2 was detected by adding allophycocyanin-conjugated streptavidin and anti-human immunoglobulin G–Alexa Fluor 488 (Thermo Fisher Scientific), respectively, using the Accuri C6 flow cytometer (BD Biosciences, San Jose, CA).21 We chose an appropriate filter, FL1 or FL4, in which small molecules did not emit autofluorescence as described in supplemental Materials and methods.
Synthesis of hematoporphyrin or protoporphyrin derivatives
Details are described in supplemental Materials and methods. Hematoporphyrin or protoporphyrin disodium salt was dissolved in acetic acid. Cobalt acetate, copper acetate, or palladium acetate were added to the porphyrin solutions and mixed with or without heating at 80 to 90°C. After cooling, precipitates were collected via filtration.
Mutant CLEC-2
The complementary DNA of the extracellular domain of hCLEC-2 in pFUSE-hFc2 (IL2ss) was constructed as described previously.6 Site-directed mutagenesis was performed by polymerase chain reaction using primers designed with mismatching nucleotides at the center (Life Technologies, Gaithersburg, MD). DNA sequencing was performed by Macrogen Japan (Tokyo, Japan). The recombinant DNA was inserted into COS7 cells by electroporation,6 and the recombinant protein was generated by the transfected cells. Binding assay for the purified wild-type or mutant hCLEC-2–hFc2 was performed as described in “Flow cytometry,” using human podoplanin-transfected CHO cells (hPod-CHO).6
Platelet aggregation
Human or murine washed platelets were prepared using acid citrate dextrose and prostaglandin I2 and resuspended in calcium-free Tyrode buffer.10 This study was approved by the Ethical Committee at the University of Yamanashi, and written informed consent was provided according to the Declaration of Helsinki. All experimental protocols in this study were approved by the University of Yamanashi Committee on Ethics of Animal Experimentation. Washed platelets were preincubated with Co-HP or 1% dimethyl sulfoxide (DMSO) for 10 minutes at 37°C. Where indicated, platelet-rich plasma (PRP) was generated as described in supplemental Materials and methods. Platelets were stimulated with indicated collagen (collagen reagent HORM; Takeda Pharmaceutical Co, Ltd, Osaka, Japan), human α-thrombin (Haematologic Technologies, Inc, Essex Junction, VT), selective proteinase-activated receptor 4-specific agonist peptide (PAR4-AP; Sigma-Aldrich, St. Louis, MO), rhodocytin, or hPod-CHO. Platelet aggregation was evaluated by light transmission aggregometry using HEMA TRACER 712 (MC Medical, Tokyo, Japan).
Surface plasmon resonance spectroscopy
The CLEC-2–Co-HP interaction was analyzed using BIAcore X (BIAcore AB, Uppsala, Sweden). Coupling of hCLEC-2–rFc2 or recombinant human integrin α2β1 (R&D Systems, Minneapolis, MN), blocking, and regeneration of the CM5 chip were performed as described previously.5 Co-HP, anti–CLEC-2 (goat polyclonal; R&D Systems), or anti-integrin α2 antibody (P1E6; Merck Millipore, Billerica, MA) in Hepes-buffered saline with EDTA and surfactant (HBS-EP; BIAcore) were perfused over a control, immobilized CLEC-2, or α2β1 surface at 10 μL/min at 25°C, and resonance changes were recorded. The response of the control surfaces was subtracted from those of the immobilized ligand surfaces.
Protein-ligand docking analysis
The 3-dimensional structure of the extracellular region of CLEC-2 was downloaded from the RCSB protein data bank (www.rcsb.org; PDB ID: 2C6U). Co-HP 3-dimensional structures were drawn using ChemDraw software. Protein-ligand docking analysis was performed using AutoDock 4.2.1 with the Lamarckian genetic algorithm. Of the 10 best confirmations obtained, the confirmation with the lowest binding energy was considered as the final confirmation, which was then analyzed in PyMOL.22,23
Experimental lung metastasis
Eight-week-old male C57BL/6 mice were administered 100 μL of Co-HP (200 μg/mL) or control (1% DMSO) via injection through the retro orbital sinus while under sevoflurane anesthesia every other day. B16F10–green fluorescent protein (GFP) cells (5 × 105) or Lewis lung carcinoma (LLC)-GFP cells (2.5 × 106) were IV injected via the tail vein 30 minutes after injection of the first inhibitor. The lungs were excised 14 days after tumor injection, followed by weighing.
Cell proliferation assay
B16F10 cells (4 × 104) were incubated in 6-well plates for 24 hours at 37°C in the presence of 0.3% DMSO or the indicated concentrations of Co-HP. The cells were then detached by trypsin and counted.
Ferric chloride injury model
A strip of filter paper saturated with 10% FeCl3 (Merck) was applied to the adventitial surface of the exposed femoral artery for 3 minutes.10,21 Femoral blood flow was monitored using a Doppler blood flow velocimeter (Advance Co, Ltd, Tokyo, Japan) for 30 minutes. The time until the blood flow was stopped by thrombi-induced occlusion was monitored.
DVT model
A partial flow restriction model of DVT was prepared as described in supplemental Materials and methods. After IV injection of Co-HP/DMSO/PBS (estimated final concentration of 10 μg/mL) or 1% DMSO/PBS through the tail vein, the mice were anesthetized using sevoflurane. The inferior vena cava (IVC) was ligated over a 30-gauge needle and then the needle was removed to cause ∼90% stenosis in IVC. Forty-eight hours after the incisions were closed, the mice were euthanized and the thrombi were collected for analysis.
Tail-bleeding assay
The body temperature of mice anesthetized with sevoflurane was maintained at 37°C on a heat pack. Two millimeters of the tail tip was amputated using a scalpel 10 minutes after the injection of Co-HP (100 μL of 200 μg/mL). The tail was then immersed in PBS at 37°C. The initial time of hemostasis was defined as the bleeding time.
Results
Development of HTS and identification of CLEC-2 inhibitors
A high-throughput screen (HTS) for small molecule inhibitors of the CLEC-2–podoplanin interaction was designed based on a modified sandwich enzyme-linked immunosorbent assay (ELISA; supplemental Figure 1A) to detect binding between immobilized hCLEC-2–rFc2 and biotin-hPod-rFc2 in the presence or absence of the small molecules.
In total, 6770 compounds in the chemical library developed by RIKEN were screened. Because a CLEC-2–binding molecule from snake venom, rhodocytin, inhibits podoplanin binding,6 it was used as a positive control. Small molecules (10 μg/mL [11-35 μM]) that inhibited biotin-hPod-rFc2 binding by more than or comparably to rhodocytin (30 nM) were selected as hit compounds. The initial screening resulted in the selection of 65 potential inhibitors (hit rate of 0.96%) (supplemental Figure 2).
The ability of the hit compounds to inhibit binding between hPod-hFc2 and CLEC-2–expressing T-REx-293 cells was investigated by flow cytometry (supplemental Figure 1B). Among the 65 hit compounds (20 μg/mL [22-70 μM]), protoporphyrin IX (H2-PP) (Figure 1A) showed the strongest inhibitory effect on podoplanin binding to the cells (data not shown). However, we found that 20 μg/mL (32.96 μM) H2-PP did not completely inhibit podoplanin binding to CLEC-2–expressing cells (Figure 1Ai,D). Even 100 μg/mL (165 μM) H2-PP did not completely inhibit podoplanin–CLEC-2 binding (data not shown), whereas 30 nM rhodocytin completely inhibited the binding (Figure 1Aii,D).
Inhibitory effects of porphyrins and rhodocytin on podoplanin binding to CLEC-2–expressing T-REx-293 cells. (A) Chemical formula of H2-PP. Results of flow cytometric assays for detecting the inhibitory effects of H2-PP (i) and rhodocytin (ii) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red line, DMSO + hpod-hFc2; blue line, H2-PP (i) or rhodocytin (ii) + hpod-hFc2. (B) The chemical formulae of Co-protoporphyrin IX (Co-PP) (i), Cu-protoporphyrin IX (Cu-PP) (ii), and Pd-protoporphyrin IX (Pd-PP) (iii). Results of flow cytometric assays for detecting the inhibitory effects of Co-PP (i), Cu-PP (ii), and Pd-PP (iii) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red, DMSO + hpod-hFc2; blue, 20 μg/mL [28.1-33.0 μM] compound + hpod-hFc2. (C) The chemical formulae of hematoporphyrin (H2-HP) (i), Co- hematoporphyrin (Co-HP) (ii), Cu-hematoporphyrin (Cu-HP) (iii), and Pd-hematoporphyrin (Pd-HP). Results of the flow cytometric assays for the detection of the inhibitory effects of H2-HP (i), Co-HP (ii), Cu-HP (iii), and Pd-HP (iv) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red, DMSO + hpod-hFc2; blue, 20 μg/mL [28.4-33.4 μM] compound + hpod-hFc2. (D) Binding of hpod-hFc2 to CLEC-2–expressing T-REx-293 cells in the presence of indicated chemicals or rhodocytin. Results are expressed as the relative mean fluorescence intensity (MFI) ± standard deviation (SD) (n = 4, panels A-C) compared with MFI of control hFc2 binding.
Inhibitory effects of porphyrins and rhodocytin on podoplanin binding to CLEC-2–expressing T-REx-293 cells. (A) Chemical formula of H2-PP. Results of flow cytometric assays for detecting the inhibitory effects of H2-PP (i) and rhodocytin (ii) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red line, DMSO + hpod-hFc2; blue line, H2-PP (i) or rhodocytin (ii) + hpod-hFc2. (B) The chemical formulae of Co-protoporphyrin IX (Co-PP) (i), Cu-protoporphyrin IX (Cu-PP) (ii), and Pd-protoporphyrin IX (Pd-PP) (iii). Results of flow cytometric assays for detecting the inhibitory effects of Co-PP (i), Cu-PP (ii), and Pd-PP (iii) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red, DMSO + hpod-hFc2; blue, 20 μg/mL [28.1-33.0 μM] compound + hpod-hFc2. (C) The chemical formulae of hematoporphyrin (H2-HP) (i), Co- hematoporphyrin (Co-HP) (ii), Cu-hematoporphyrin (Cu-HP) (iii), and Pd-hematoporphyrin (Pd-HP). Results of the flow cytometric assays for the detection of the inhibitory effects of H2-HP (i), Co-HP (ii), Cu-HP (iii), and Pd-HP (iv) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red, DMSO + hpod-hFc2; blue, 20 μg/mL [28.4-33.4 μM] compound + hpod-hFc2. (D) Binding of hpod-hFc2 to CLEC-2–expressing T-REx-293 cells in the presence of indicated chemicals or rhodocytin. Results are expressed as the relative mean fluorescence intensity (MFI) ± standard deviation (SD) (n = 4, panels A-C) compared with MFI of control hFc2 binding.
Optimization of H2-PP results in Co-HP showed the strongest effect as a CLEC-2 inhibitor
To generate a more potent inhibitor, we optimized H2-PP. H2-PP is a member of the porphyrins family, which are heterocyclic macrocycle organic compounds composed of 4 modified pyrrole subunits. Because the nitrogen molecule in the center of the pyrrole ring can form stable complexes with metals including magnesium and iron, we generated complexes of H2-PP with Co, Cu, Pd (Figure 1B,D), Zn, and Ni (data not shown) and investigated their inhibitory effects on CLEC-2–podoplanin binding. None of these metal complexes inhibited podoplanin binding to CLEC-2–expressing cells as well as H2-PP.
We then changed the vinyl groups at C2 and C4 of H2-PP to oxyethyl groups to generate hematoporphyrin (H2-HP). Similarly, H2-HP did not completely inhibit the binding (Figure 1Ci,D). Complexes of H2-HP with Cu, Pd (Figure 1Ciii-iv,D), Zn, and Ni (data not shown) did not increase its inhibitory effect on CLEC-2–podoplanin binding. However, the complex of H2-HP with Co (Co-HP) (Figure 1Cii,D) resulted in complete inhibition of CLEC-2–podoplanin binding, as was observed for rhodocytin (Figure 1Aii,D). We investigated 8 other porphyrins, but no compound showed a stronger inhibitory effect than Co-HP (data not shown). Thus, we further characterized Co-HP as a CLEC-2 inhibitor.
Characterization of Co-HP
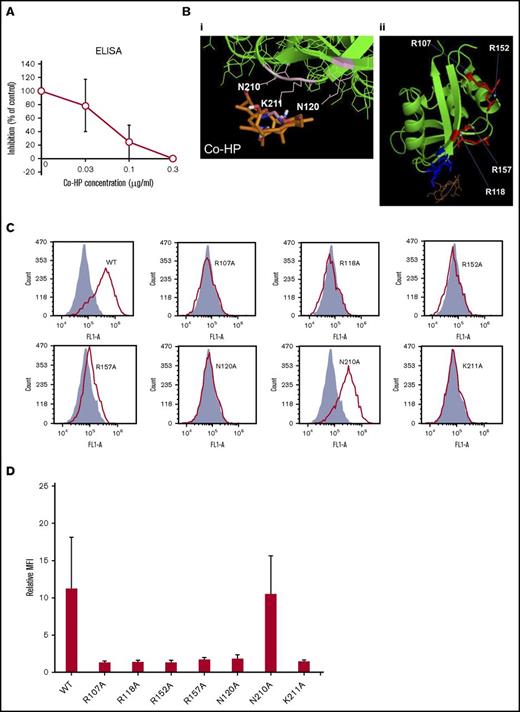
ELISA showed that Co-HP dose-dependently inhibited biotin-hPod-rFc2 binding to hCLEC-2–rFc2 with an 50% inhibitory concentration (IC50) of 0.058 μg/mL (88.5 nM) (Figure 2A).
Characterization of Co-HP. (A) ELISA-based IC50 curves for the association between hPod-hFc2 and immobilized hCLEC-2–rFc2 in the presence of Co-HP. (B) (i) Interaction between CLEC-2 (green) and Co-HP (brown). Amino acid residues involved in the Co-HP–CLEC-2 interaction are labeled and highlighted in pink. (ii) Interaction between CLEC-2 (green) and Co-HP (brown). Four arginine residues used for the podoplanin or rhodocytin–CLEC-2 interactions reported to date are labeled and highlighted in red. Amino acid residues involved in the Co-HP–CLEC-2 interactions are highlighted in blue. (C) Flow cytometric analysis using wild-type (WT) and mutant hCLEC-2–hFc2 in binding sites for podoplanin, rhodocytin, or Co-HP (R107A, R118A, R152A, R157A, N120A, N210A, and K211A) and podoplanin-expressing CHO cells. R107A, R118A, R152A, R157A, N120A, N210A, and K211A indicate that arginine, asparagine, and lysine residues in the indicated positions of the recombinant CLEC-2 are substituted with alanine residues. Fill, Purified hFc2; line, purified wild-type or mutant hCLEC-2–hFc2. (D) Binding of wild-type or mutant hCLEC-2–rFc2 to podoplanin-expressing CHO cells. Results are expressed as the relative MFI ± SD (n = 4, panel C) compared with MFI of control rFc2 binding.
Characterization of Co-HP. (A) ELISA-based IC50 curves for the association between hPod-hFc2 and immobilized hCLEC-2–rFc2 in the presence of Co-HP. (B) (i) Interaction between CLEC-2 (green) and Co-HP (brown). Amino acid residues involved in the Co-HP–CLEC-2 interaction are labeled and highlighted in pink. (ii) Interaction between CLEC-2 (green) and Co-HP (brown). Four arginine residues used for the podoplanin or rhodocytin–CLEC-2 interactions reported to date are labeled and highlighted in red. Amino acid residues involved in the Co-HP–CLEC-2 interactions are highlighted in blue. (C) Flow cytometric analysis using wild-type (WT) and mutant hCLEC-2–hFc2 in binding sites for podoplanin, rhodocytin, or Co-HP (R107A, R118A, R152A, R157A, N120A, N210A, and K211A) and podoplanin-expressing CHO cells. R107A, R118A, R152A, R157A, N120A, N210A, and K211A indicate that arginine, asparagine, and lysine residues in the indicated positions of the recombinant CLEC-2 are substituted with alanine residues. Fill, Purified hFc2; line, purified wild-type or mutant hCLEC-2–hFc2. (D) Binding of wild-type or mutant hCLEC-2–rFc2 to podoplanin-expressing CHO cells. Results are expressed as the relative MFI ± SD (n = 4, panel C) compared with MFI of control rFc2 binding.
Computational molecular docking studies showed that Co-HP binds to N120, N210, and K211 (Figure 2Bi). Four arginine residues, R107, R118, R152, and R157, have been reported to be binding sites for podoplanin or rhodocytin (Figure 2Bii),24 all of which differ from the Co-HP–binding residues. To investigate whether N120, N210, and K211 also participate in podoplanin binding, we generated hCLEC-2–rFc2 with N120A, N210A, K211A, and R107/118/152/157A. As reported,24 wild-type recombinant CLEC-2, but not that with the R107/118/152A mutation, bound to hPod-CHO, except for the R157A mutant, which showed only marginal binding (Figure 2C-D). For the Co-HP–binding site, hCLEC-2 with N210A bound to hPod-CHO, whereas those with N120A and K211A lost their ability to bind to hPod-CHO (Figure 2C-D). These findings suggest that N120 and K211 are required for binding to podoplanin, explaining the ability of Co-HP to block the CLEC-2–podoplanin interaction.
Co-HP potently and specifically inhibited platelet aggregation mediated by CLEC-2
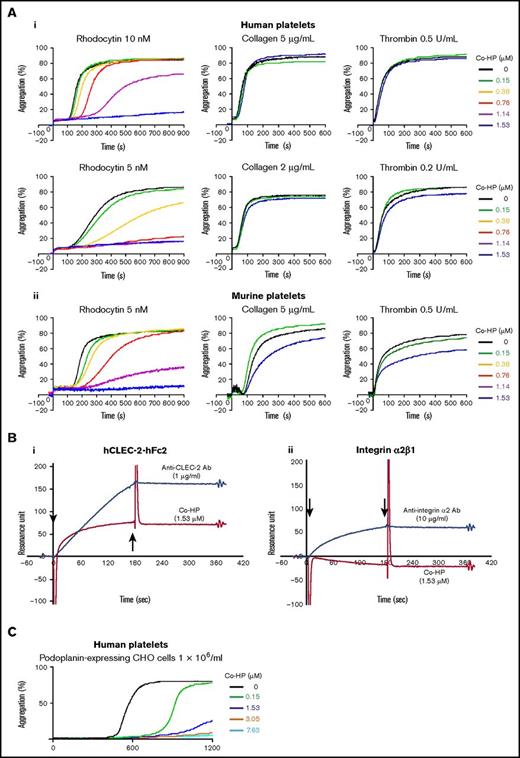
Co-HP (1.53 μM) completely inhibited rhodocytin-induced platelet aggregation in humans and mice (Figure 3A). At 10 nM rhodocytin in humans and 5 nM rhodocytin in mice, low-dose Co-HP delayed the onset of platelet aggregation, but Co-HP at a dose higher than 1.14 μM inhibited maximum aggregation. However, Co-HP did not inhibit collagen- or thrombin-induced platelet aggregation in humans (Figure 3Ai) and had marginal inhibitory effects in mice (Figure 3Aii).
Co-HP almost specifically inhibited rhodocytin-induced platelet aggregation by binding to CLEC-2. (A) Inhibitory effects of Co-HP on the aggregation of washed human (i) or murine (ii) platelets (2 × 108/mL) induced by rhodocytin, collagen, or thrombin. The results from 1 experiment, representative of 3 experiments, are shown. (B) Binding between Co-HP and hCLEC-2–hFc2/integrin α2β1 was analyzed by surface plasmon resonance assay. (i) Co-HP (red line) or anti–CLEC-2 antibody (blue line) were flowed over an immobilized hCLEC2-rFc2 or a control surface. (ii) Co-HP (red line) or anti-integrin α2β1 antibody (blue line) were flowed over an immobilized recombinant integrin α2β1 or a control surface. The arrows indicate the beginning and the end of analyte perfusion. The results from 1 experiment, representative of 3 experiments, are shown. (C) Inhibitory effects of Co-HP on the aggregation of washed human platelets (1 × 109/mL) induced by hPod-CHO (1 × 106/mL). The results from 1 experiment, representative of 3 experiments, are shown.
Co-HP almost specifically inhibited rhodocytin-induced platelet aggregation by binding to CLEC-2. (A) Inhibitory effects of Co-HP on the aggregation of washed human (i) or murine (ii) platelets (2 × 108/mL) induced by rhodocytin, collagen, or thrombin. The results from 1 experiment, representative of 3 experiments, are shown. (B) Binding between Co-HP and hCLEC-2–hFc2/integrin α2β1 was analyzed by surface plasmon resonance assay. (i) Co-HP (red line) or anti–CLEC-2 antibody (blue line) were flowed over an immobilized hCLEC2-rFc2 or a control surface. (ii) Co-HP (red line) or anti-integrin α2β1 antibody (blue line) were flowed over an immobilized recombinant integrin α2β1 or a control surface. The arrows indicate the beginning and the end of analyte perfusion. The results from 1 experiment, representative of 3 experiments, are shown. (C) Inhibitory effects of Co-HP on the aggregation of washed human platelets (1 × 109/mL) induced by hPod-CHO (1 × 106/mL). The results from 1 experiment, representative of 3 experiments, are shown.
Because rhodocytin interacts with integrin α2β1 in addition to CLEC-2,25,26 we performed a BiaCore assay. Co-HP was flowed onto hCLEC-2–human Fc2-coated surfaces. After perfusion, the resonance unit, which indicates binding of the analyte (flowing Co-HP) to the ligand (coated hCLEC-2–hFc2), gradually increased but did not decrease after the cessation of perfusion (Figure 3Bi). After perfusion of the anti–CLEC-2 antibody, the resonance unit sharply increased, but did not decrease after stopping perfusion, suggesting that the binding between Co-HP and CLEC-2 is as strong as that between the antibody and CLEC-2. Co-HP did not bind to recombinant integrin α2β1 (Figure 3Bii), suggesting that Co-HP inhibits rhodocytin-induced platelet aggregation by binding to CLEC-2.
As previously reported, hPod-CHO–induced aggregation of human platelets,27,28 which was dose-dependently inhibited by Co-HP (Figure 3C).
Taken together, these results indicate that Co-HP almost specifically inhibits platelet aggregation mediated through CLEC-2 in humans and mice.
Co-HP inhibited lung metastasis of the podoplanin-expressing B16F10 melanoma cell line, but not podoplanin-negative LLC in mice
It is well known that tumor cell-induced platelet aggregation facilitates the hematogenous tumor metastasis by increasing the survival of tumor cells by covering these cells with activated platelets and by attaching tumor cells to the vessel wall to form platelet aggregates.29 Because Co-HP inhibited hPod-CHO–induced platelet aggregation (Figure 3C), we investigated whether Co-HP inhibits hematogenous metastasis using a murine model of lung metastasis.
We first investigated whether IV injected Co-HP can inhibit CLEC-2–mediated platelet aggregation in mice. After administration of Co-HP, blood was drawn, and platelet aggregation was monitored. Five minutes after Co-HP injection, 2 nM rhodocytin- and 5 nM rhodocytin-induced platelet aggregation was completely and partially inhibited, respectively (Figure 4A). In contrast, platelet aggregation induced by collagen or thrombin receptor-activating PAR4-AP was not inhibited (Figure 4A). Forty-eight hours after Co-HP injection, marginal inhibitory effects were observed only in 2 nM rhodocytin-induced platelet aggregation (Figure 4B). These findings suggest that the inhibitory effects of Co-HP were still observed, but greatly decreased at 48 hours after IV administration. Therefore, we injected Co-HP into the mice every 48 hours.
IV-injected Co-HP inhibited rhodocytin-induced platelet aggregation. Five minutes (A) or 48 hours (B) after IV injection of 1% DMSO or Co-HP (estimated final concentration of 10 μg/mL [15 μM]), the mice were euthanized and the blood was drawn from the IVC with 5% final concentration of ACD. PRP was stimulated by the indicated concentrations of rhodocytin, collagen, or PAR4-AP (thrombin receptor activator peptide) and platelet aggregation was monitored for 10 minutes using a platelet aggregometer.
IV-injected Co-HP inhibited rhodocytin-induced platelet aggregation. Five minutes (A) or 48 hours (B) after IV injection of 1% DMSO or Co-HP (estimated final concentration of 10 μg/mL [15 μM]), the mice were euthanized and the blood was drawn from the IVC with 5% final concentration of ACD. PRP was stimulated by the indicated concentrations of rhodocytin, collagen, or PAR4-AP (thrombin receptor activator peptide) and platelet aggregation was monitored for 10 minutes using a platelet aggregometer.
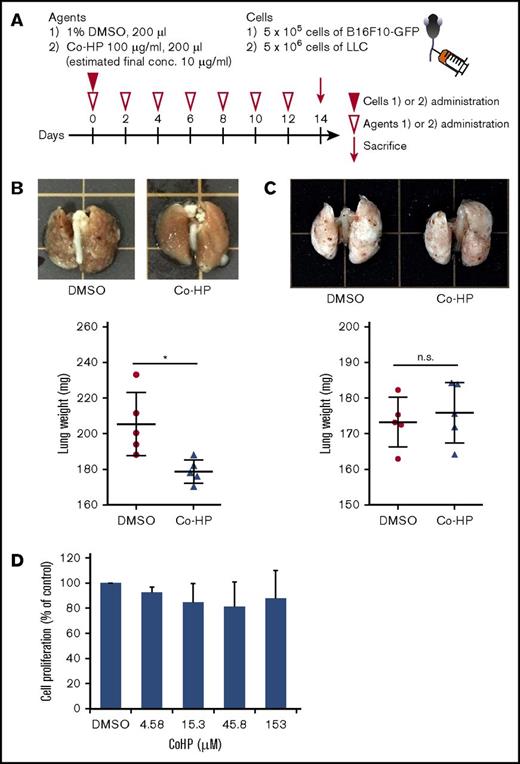
We injected podoplanin-expressing B16F10 cells or podoplanin-negative LLC cells into the mice through the tail vein and measured the lung weight 2 weeks later (Figure 5A). IV administration of Co-HP every 48 hours significantly inhibited lung metastasis of B16F10, but not that of LLC (Figure 5B-C). Importantly, Co-HP did not inhibit cell proliferation when cocultured with B16F10 in vitro (Figure 5D). Taken together, our data indicate that Co-HP inhibits hematogenous tumor metastasis by blocking the CLEC-2–podoplanin interaction.
Co-HP inhibited hematogenous metastasis of podoplanin-expressing B16F10, but not that of podoplanin-negative LLC. (A) Study design of experimental lung metastasis. Co-HP or 1% DMSO were injected every 48 hours. B16F10-GFP cells or LLC-GFP cells were IV injected into mice through the tail vein 30 minutes after the injection of the first inhibitor. (B-C, top) Representative images of the lungs excised from Co-HP– or DMSO-injected mice 14 days after tumor injection of B16F10 (B) or LLC (C). (Bottom) Weight of the lungs excised from Co-HP– or DMSO-injected mice (n = 5 in each group) 14 days after tumor injection of B16F10 (B) or LLC (C). Mean values ± SD are shown for each group (DMSO control, ●; Co-HP, ▲). Data were analyzed using the Student t test. *P < .05. (D) Proliferation of B16F10 cells in the presence of DMSO or Co-HP. The cells were counted 24 hours after 0.3% DMSO or Co-HP administration. The cell numbers in the presence of 0.3% DMSO were set to 100%. Mean values ± SD are shown for each group (n = 6 from 3 independent experiments). Data were analyzed using the Dunnett test. n.s., not significant.
Co-HP inhibited hematogenous metastasis of podoplanin-expressing B16F10, but not that of podoplanin-negative LLC. (A) Study design of experimental lung metastasis. Co-HP or 1% DMSO were injected every 48 hours. B16F10-GFP cells or LLC-GFP cells were IV injected into mice through the tail vein 30 minutes after the injection of the first inhibitor. (B-C, top) Representative images of the lungs excised from Co-HP– or DMSO-injected mice 14 days after tumor injection of B16F10 (B) or LLC (C). (Bottom) Weight of the lungs excised from Co-HP– or DMSO-injected mice (n = 5 in each group) 14 days after tumor injection of B16F10 (B) or LLC (C). Mean values ± SD are shown for each group (DMSO control, ●; Co-HP, ▲). Data were analyzed using the Student t test. *P < .05. (D) Proliferation of B16F10 cells in the presence of DMSO or Co-HP. The cells were counted 24 hours after 0.3% DMSO or Co-HP administration. The cell numbers in the presence of 0.3% DMSO were set to 100%. Mean values ± SD are shown for each group (n = 6 from 3 independent experiments). Data were analyzed using the Dunnett test. n.s., not significant.
Co-HP inhibited arterial or venous thrombosis and did not increase bleeding time in mice
Thrombus formation under flow in vivo and ex vivo was significantly inhibited in CLEC-2–deficient mice,10,11 although podoplanin is not expressed in the normal vessel wall. We then investigated the effect of Co-HP in arterial thrombosis. Thrombus formation in the femoral artery injured by topical application of FeCl3 causes vessel occlusion. As observed in CLEC-2–deficient mice, the time to occlusion was significantly prolonged in Co-HP–injected mice (Figure 6A).
Co-HP inhibited FeCl3-induced arterial thrombosis and DVT in vivo but did not show a significant increase in bleeding time. (A) Time required to cause vessel occlusion in the FeCl3 model; a 1 × 1 mm strip of filter paper saturated with 10% FeCl3 was applied to the adventitial surface of the exposed femoral artery of 1% DMSO- (●) or Co-HP–injected mice (▪; estimated final concentration of 10 μg/mL [15 μM]). The time to thrombotic occlusion of the artery was measured by monitoring femoral blood flow using a Doppler blood flow velocimeter. Each symbol represents 1 individual. **P < .01. Data were analyzed by the Student t test. (B) DVT model: 1% DMSO- (●) or Co-HP–injected mice (▲; estimated final concentration of 10 μg/mL [15 μM]) were subjected to IVC stenosis for 48 hours. Thrombus length (i), thrombus weight (ii), and thrombosis prevalence (iii) were measured. Each symbol represents 1 individual. Lines in dot plots represent medians. Data of the prevalence and of length/weight were analyzed by the Fisher exact test and the Mann-Whitney test, respectively. *P < .05; ***P < .005. (C) Time of bleeding from the cut tail of DMSO- (●) or Co-HP–injected mice (▪). A 1-mm segment of the tail tip was cut, and the tail tip was immersed in saline. Each symbol represents 1 individual. Data were analyzed by the Student t test.
Co-HP inhibited FeCl3-induced arterial thrombosis and DVT in vivo but did not show a significant increase in bleeding time. (A) Time required to cause vessel occlusion in the FeCl3 model; a 1 × 1 mm strip of filter paper saturated with 10% FeCl3 was applied to the adventitial surface of the exposed femoral artery of 1% DMSO- (●) or Co-HP–injected mice (▪; estimated final concentration of 10 μg/mL [15 μM]). The time to thrombotic occlusion of the artery was measured by monitoring femoral blood flow using a Doppler blood flow velocimeter. Each symbol represents 1 individual. **P < .01. Data were analyzed by the Student t test. (B) DVT model: 1% DMSO- (●) or Co-HP–injected mice (▲; estimated final concentration of 10 μg/mL [15 μM]) were subjected to IVC stenosis for 48 hours. Thrombus length (i), thrombus weight (ii), and thrombosis prevalence (iii) were measured. Each symbol represents 1 individual. Lines in dot plots represent medians. Data of the prevalence and of length/weight were analyzed by the Fisher exact test and the Mann-Whitney test, respectively. *P < .05; ***P < .005. (C) Time of bleeding from the cut tail of DMSO- (●) or Co-HP–injected mice (▪). A 1-mm segment of the tail tip was cut, and the tail tip was immersed in saline. Each symbol represents 1 individual. Data were analyzed by the Student t test.
Because a recent study reported that the association between CLEC-2 and podoplanin upregulated in the venous wall during thrombosis exacerbates DVT,14 we investigated the effects of Co-HP using a murine DVT model of IVC stenosis. Figure 6B shows that at 48 hours after stenosis, the thrombus length/weight (Figure 6Bi-ii) and prevalence of thrombi (Figure 6Biii) were significantly lower in Co-HP–injected mice.
Despite the significant decrease in arterial/venous thrombus formation, a significant increase in tail-bleeding time was not observed in Co-HP–injected mice (Figure 6C). These findings suggest that Co-HP–injected mice are a phenocopy of CLEC-2–deficient mice; Co-HP inhibits arterial and venous thrombosis without an apparent bleeding tendency in mice.
Discussion
We identified Co-HP as a candidate anti–CLEC-2 drug by screening a chemical library of small-molecular-weight compounds and optimization. Co-HP directly binds to CLEC-2, probably to N120, N210, and K211, among which N120 and K211 are identified as previously unknown binding sites for podoplanin. Co-HP inhibited podoplanin binding to CLEC-2 with an IC50 of 88.5 nM, as determined by ELISA. Co-HP at a concentration of 1.53 μM inhibited platelet aggregation induced by CLEC-2 agonists, but not that induced by other agonists. Moreover, Co-HP administered to wild-type mice mimicked the phenotype of CLEC-2–deficient mice, including the inhibition of rhodocytin/podoplanin-induced platelet aggregation, experimental lung metastasis, and in vivo arterial or venous thrombosis, without significantly increasing bleeding time. These findings indicate that Co-HP is a promising molecule for treating antitumor metastasis and antithrombosis, both of which are leading causes of death in developed countries.
Chang et al reported that 20 μM of the noncytotoxic 5-nitrobenzoate compound 2CP, which blocks the CLEC-2–podoplanin interaction, inhibited podoplanin-induced, but not rhodocytin (16.6 μM)-induced, platelet aggregation. Experimental lung metastasis was inhibited significantly, although only slightly inhibited by 2CP.27 Podoplanin, rhodocytin, and 2CP share 3 arginine residues, R107, R118, and R157, for their binding to CLEC-2, which could not explain the differences in their inhibitory effects. 2CP, podoplanin, and rhodocytin bind to CLEC-2 with Biacore-assessed dissociation constants (Kd) of 33.2 μM,27 24.5 μM,30 and 1 to 3 μM,24,31 respectively; based on this, Chang et al suggested that the difference in the inhibitory effect of 2CP is due to its low affinity compared with rhodocytin. In contrast, Co-HP inhibited both hPod-CHO– and rhodocytin (5 nM)-induced platelet aggregation in a similar range of concentrations (1.53-3.05 μM) (Figure 3). This discrepancy may be due to the higher affinity of Co-HP compared with 2CP, as described in the next paragraph.
Surface plasmon resonance showed that Co-HP binds directly to CLEC-2. We could not determine the Kd of Co-HP–CLEC-2 binding because Co-HP hardly dissociated from CLEC-2 after terminating perfusion, like the anti–CLEC-2 antibody (Figure 3Bi). These findings suggest that the avidity of Co-HP for CLEC-2 is as strong as that of the antibody, the estimated Kd of which is 10 to 100 pM. Although the precise Kd is unknown, Co-HP seems to have a higher affinity than 2CP (33.2 μM).
CLEC-2–binding sites for Co-HP are N120, N210, and K211, as determined by computer analysis (Figure 2C-D), differed from the previously reported podoplanin- and rhodocytin-binding sites R107, R118, R152, and R157.24 In the present study, we identified novel podoplanin-binding sites, N120 and K211 (Figure 2C-D). Nagae et al reported that CLEC-2 recognizes both the sialylated O-glycan and the adjoining Glu-Asp of podoplanin; the negatively charged patch of the Glu-Asp doublet accepts the positively charged patch formed by R107, R152, and R157 in CLEC-2.24 Moreover, the negatively charged sialylated O-glycan in podoplanin accepts the positively charged R118.24 N120 in CLEC-2 is highly conserved among species24 and was confirmed to be N-glycosylated in mice,32 suggesting that glycosylation of N120 is used for podoplanin binding. K211 is not conserved among species (K211 in humans and macaques, but R211 in mice and rats). However, both Lys and Arg are positively charged basic amino acids. Because podoplanin is a negatively charged protein, it is possible that the positively charged K211 or R211 in CLEC-2 are used for podoplanin binding. Our computer analysis showed that Co-HP binds to N120 and K211. Co-HP inhibited podoplanin binding to CLEC-2. Mutant CLEC-2, having N120A and K211A, failed to associate with podoplanin (Figure 2C-D). These findings suggest that Co-HP inhibits the podoplanin interaction by binding to N120 and K211, newly identified podoplanin-binding sites within CLEC-2. Distinct from 2CP, Co-HP inhibited both podoplanin- and rhodocytin-induced platelet aggregation and strongly inhibited experimental lung metastasis in mice. This difference may be because of the high affinity of Co-HP and/or because the blocking of N120 and K211 may more effectively inhibit podoplanin binding than blocking of R107, R118, R152, or R157.
Co-HP or CLEC-2 depletion inhibited FeCl3-induced arterial thrombus formation in vivo, although podoplanin is not expressed in the normal vessel wall. We previously proposed that CLEC-2 forms a homophilic association depending on platelet activation, thereby stabilizing thrombi under high arterial shear rates. However, Biacore analysis showed that Co-HP did not inhibit the homophilic association of CLEC-2 (data not shown). Recently, Haining et al reported that CLEC-2 adheres to unknown ligand(s) in an activation-independent manner during thrombus formation under flow and stabilizes thrombi.33 It is possible that Co-HP interferes with the binding of CLEC-2 to unidentified ligand(s). We showed that blocking CLEC-2 by Co-HP also inhibited venous thrombosis using a murine DVT model. Payne et al reported that mice with a deficiency in CLEC-2 are protected against DVT.14 Although podoplanin is not expressed in the normal vessel wall of veins, they showed that podoplanin expression was induced in the IVC wall during experimental IVC stenosis. Deficiency in CLEC-2 and inhibition of podoplanin were associated with reduced platelet accumulation at the IVC wall after stenosis, suggesting that CLEC-2 and upregulation of podoplanin in the venous wall exacerbate thrombus formation.14 Thus, CLEC-2–blocking therapy, including Co-HP, may be useful for preventing DVT.
It is known that distant metastasis, cachexia, and thrombosis are the main causes of death in cancer patients. Venous and arterial thrombosis frequently occur in tumor-bearing patients for various reasons, including procoagulant activity of tumor cells, chronic inflammation, and chemotherapy. We demonstrated that in the tumor-bearing state, CLEC-2–depleted mice showed significantly prolonged survival, less thrombus formation in the lungs, less plasma inflammatory cytokines, and less cachexia.21 Tumor-related thrombus formation may cause chronic inflammation and subsequent cancer-induced cachexia. The present study showed that IV administration of Co-HP inhibited both tumor metastasis and venous/arterial thrombus formation in mice (Figures 5 and 6). It is tempting to speculate that CLEC-2 inhibition by Co-HP may prolong the survival of cancer patients by inhibiting cachexia, metastasis, and thrombosis.
Protoporphyrins and hematoporphyrins are endogenous products present in vivo. Therefore, our finding that CLEC-2 has affinity for porphyrins may lead to new insights into the functions of CLEC-2.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors express gratitude to Uina Fukuda, Hisaichiro Nakazawa, Chiaki Komatsu, and Yukie Yoshioka for their excellent technical assistance.
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) Funding Program for Next Generation World-Leading Researchers (NEXT Program) (LS052). This work was also supported in part by the Strategic Project of Yamanashi University and a grant from the Takeda Science Foundation, as well as the Chemical Genomics Research Program (S.K.) from RIKEN.
Authorship
Contribution: N.T., M.O., T. Sasaki, and T. Shirai performed the experiments, analyzed data, and wrote the manuscript; K.S., O.I., N.U., and C.M. performed the experiments; T. Saito, S.K., and H.S. contributed vital new reagents and performed the first chemical screening; Y.O. analyzed the data and wrote the manuscript; K.S.-I. designed the study, analyzed data, wrote the manuscript, and supervised the study; and all authors discussed the results and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katsue Suzuki-Inoue, Department of Clinical and Laboratory Medicine, Faculty of Medicine, University of Yamanashi, 1110 Shimokato, Chuo, Yamanashi 409-3898, Japan; e-mail: katsuei@yamanashi.ac.jp.
References
Author notes
N.T., M.O., T. Sasaki, and T. Shirai contributed equally to this study.

![Figure 1. Inhibitory effects of porphyrins and rhodocytin on podoplanin binding to CLEC-2–expressing T-REx-293 cells. (A) Chemical formula of H2-PP. Results of flow cytometric assays for detecting the inhibitory effects of H2-PP (i) and rhodocytin (ii) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red line, DMSO + hpod-hFc2; blue line, H2-PP (i) or rhodocytin (ii) + hpod-hFc2. (B) The chemical formulae of Co-protoporphyrin IX (Co-PP) (i), Cu-protoporphyrin IX (Cu-PP) (ii), and Pd-protoporphyrin IX (Pd-PP) (iii). Results of flow cytometric assays for detecting the inhibitory effects of Co-PP (i), Cu-PP (ii), and Pd-PP (iii) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red, DMSO + hpod-hFc2; blue, 20 μg/mL [28.1-33.0 μM] compound + hpod-hFc2. (C) The chemical formulae of hematoporphyrin (H2-HP) (i), Co- hematoporphyrin (Co-HP) (ii), Cu-hematoporphyrin (Cu-HP) (iii), and Pd-hematoporphyrin (Pd-HP). Results of the flow cytometric assays for the detection of the inhibitory effects of H2-HP (i), Co-HP (ii), Cu-HP (iii), and Pd-HP (iv) on the binding of podoplanin to CLEC-2–expressing T-REx-293 cells. Fill, DMSO + hFc2; red, DMSO + hpod-hFc2; blue, 20 μg/mL [28.4-33.4 μM] compound + hpod-hFc2. (D) Binding of hpod-hFc2 to CLEC-2–expressing T-REx-293 cells in the presence of indicated chemicals or rhodocytin. Results are expressed as the relative mean fluorescence intensity (MFI) ± standard deviation (SD) (n = 4, panels A-C) compared with MFI of control hFc2 binding.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/17/10.1182_bloodadvances.2018016261/3/m_advances016261f1.jpeg?Expires=1769125678&Signature=DR75X0ldGeUfWArmfd67VQsxGcHJ-hXr~u2tIV6F7ICIWc6-ygjKgf98lj5k17kFN2KDh37Kmmm3cjXVXezmvAJoOVd3AStofSfCaICWm3JrtGdS4uzFw1GplUZmTnIpS7KV-DTWKIRXRLqdjCA~rI4aP4cZqYEtqkjSHisxmZ7SdKtP1Sui~T37-fGtA5C0XSFgoMMzaeJfdssLs48gdimZlIN8UA3b7dZ~ZfYFFuG0ZzjzDUU1l35bFhbF6sdQYtpQB7h5wn03NlH1HyK2ad~ZWypWYsyygX2KQziOui2GJ7XMcEqDtlYgIcvo3Qa4f8mn6yXD2Tygf~vaKny3Wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. IV-injected Co-HP inhibited rhodocytin-induced platelet aggregation. Five minutes (A) or 48 hours (B) after IV injection of 1% DMSO or Co-HP (estimated final concentration of 10 μg/mL [15 μM]), the mice were euthanized and the blood was drawn from the IVC with 5% final concentration of ACD. PRP was stimulated by the indicated concentrations of rhodocytin, collagen, or PAR4-AP (thrombin receptor activator peptide) and platelet aggregation was monitored for 10 minutes using a platelet aggregometer.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/17/10.1182_bloodadvances.2018016261/3/m_advances016261f4.jpeg?Expires=1769125678&Signature=sUgMqtVwAhbIMJjg08SLfzkrWz1Fbjgh7xx722u776DsQtL4Dx3bQ~1X2JK~c47pYze-1nI~TS9GEMuxgmqdZyp-QvMDr6ub2PPvCDegD9vDIA6dNOBXP3SUMdbVmm8M6EiQtCVqGjS5cGtproOKdX8m~zOBKLPR9t~RIq5qxtvQW~rQzVgrLbfnk2WRIosP55o1mDnuYfJwBaJsCY6AoK41KaGg9UJSpJCmNGPyZsD2Jr7rI3lHAXUsSdWLWt~iFFCMGG8QwR7ECUzed71gZUPJ4a2shXQYGc2xHHavNr0mLmt4aTXgjPpPGWk8Oe3BpkO2FGHbMQwB1v0prTFVWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Co-HP inhibited FeCl3-induced arterial thrombosis and DVT in vivo but did not show a significant increase in bleeding time. (A) Time required to cause vessel occlusion in the FeCl3 model; a 1 × 1 mm strip of filter paper saturated with 10% FeCl3 was applied to the adventitial surface of the exposed femoral artery of 1% DMSO- (●) or Co-HP–injected mice (▪; estimated final concentration of 10 μg/mL [15 μM]). The time to thrombotic occlusion of the artery was measured by monitoring femoral blood flow using a Doppler blood flow velocimeter. Each symbol represents 1 individual. **P < .01. Data were analyzed by the Student t test. (B) DVT model: 1% DMSO- (●) or Co-HP–injected mice (▲; estimated final concentration of 10 μg/mL [15 μM]) were subjected to IVC stenosis for 48 hours. Thrombus length (i), thrombus weight (ii), and thrombosis prevalence (iii) were measured. Each symbol represents 1 individual. Lines in dot plots represent medians. Data of the prevalence and of length/weight were analyzed by the Fisher exact test and the Mann-Whitney test, respectively. *P < .05; ***P < .005. (C) Time of bleeding from the cut tail of DMSO- (●) or Co-HP–injected mice (▪). A 1-mm segment of the tail tip was cut, and the tail tip was immersed in saline. Each symbol represents 1 individual. Data were analyzed by the Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/17/10.1182_bloodadvances.2018016261/3/m_advances016261f6.jpeg?Expires=1769125678&Signature=lq8ap7rdEGBz8ZQWEDS2QGyoarcNvayt2hNg9IecDZT6mhKnJ7EKpXiO055inRtWYxieE9zW5Om2yXZFF8vwBygRy62rw3xjn1Q9lMvguFuJOZx93655p4rCr5ikARKCXVrG4hxgXzdZZv-tqt7btAkbPKbciUGUKHRG-aXAXeio~JUiOxhPh9ZQefKVaFEO8ijCem5rKK56BfLkc1ejZUvoH5OCWpNSAraFeLUEFIkuWLUVRQ7HuLBHGqsX8moqWXY5umlJc7uuWTvW16g13g28dzoPCFgscdMaA40uSh3WQCKIlBe7dE~PwhILpHOeKQ625uLMSRLJc-3AiDBfQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)