Key Points
Targeting XBP-1 on B cells is sufficient to prevent cGVHD.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD while preserving GVL activity.
Abstract
Hematopoietic stem cell transplantation (HCT) is a curative procedure for hematological malignancies, but chronic graft-versus-host disease (cGVHD) remains a major complication after allogeneic HCT. Because donor B cells are essential for cGVHD development and B cells are sensitive to endoplasmic reticulum (ER) stress, we hypothesized that the IRE-1α/XBP-1 pathway is required for B-cell activation and function and for the development of cGVHD. To test this hypothesis, we used conditional knock-out mice deficient of XBP-1 specifically in B cells. Recipients transplanted with donor grafts containing XBP-1–deficient B cells displayed reduced cGVHD compared with controls. Reduction of cGVHD correlated with impaired B-cell functions, including reduced production of anti–double-stranded DNA immunoglobulin G antibodies, CD86, Fas, and GL7 surface expression, and impaired T-cell responses, including reduced interferon-γ production and follicular helper T cells. In a bronchiolitis obliterans cGVHD model, recipients of transplants containing XBP-1–deficient B cells demonstrated improved pulmonary function correlated with reduced donor splenic follicular helper T cells and increased B cells compared with those of wild-type control donor grafts. We then tested if XBP-1 blockade via an IRE-1α inhibitor, B-I09, would attenuate cGVHD and preserve the graft-versus-leukemia (GVL) effect. In a cutaneous cGVHD model, we found that prophylactic administration of B-I09 reduced clinical features of cGVHD, which correlated with reductions in donor T-cell and dendritic cell skin infiltrates. Inhibition of the IRE-1α/XBP-1 pathway also preserved the GVL effect against chronic myelogenous leukemia mediated by allogeneic splenocytes. Collectively, the ER stress response mediated by the IRE-1α/XBP-1 axis is required for cGVHD development but dispensable for GVL activity.
Introduction
Chronic graft-versus-host disease (cGVHD) remains a prominent cause of allogeneic hematopoietic stem cell transplantation (allo-HCT)-related morbidity and mortality even with available treatments. Despite this, the most effective treatment of hematological malignancies, including leukemia, lymphoma, and myeloma, is allo-HCT. Although there has been progress in understanding acute graft-versus-host disease (GVHD) development, mechanisms responsible for development of cGVHD are less understood and remain a major obstacle in providing optimal allo-HCT therapies.
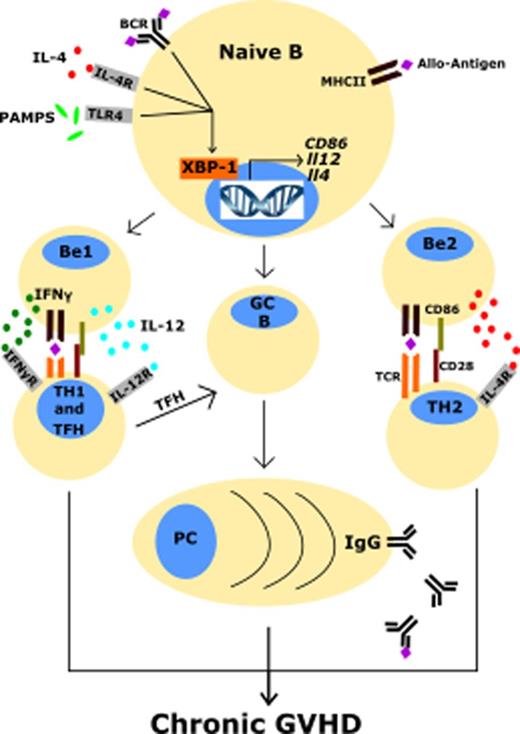
One potential unexplored strategy for combating cGVHD involves targeting the endoplasmic reticulum (ER) stress response. This approach is promising in the treatment of hematological malignancies.1-4 The ER stress response is employed by many types of immune cells to cope with cell stress to avoid apoptosis.5-11 The 3 primary regulators of the ER stress response are IRE-1α, PERK, and ATF6.12 IRE-1α is particularly critical for the function of plasma cells.13-15 When activated, IRE-1α converts unspliced XBP-1 (XBP-1u) messenger RNA (mRNA) into spliced XBP-1 (XBP1s) mRNA via its ribonuclease activity. XBP-1s is subsequently translated into XBP-1s protein, which acts as a transcription factor regulating genes for protein folding, protein degradation, and unfolded protein response function.13,14 Noncanonical functions, such as binding to promoters of genes encoding inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor (TNF) in macrophages, demonstrate the multifunctional nature of this protein.16
Here, we use both genetic and pharmacologic approaches to uncover the role of the IRE-1α/XBP-1 pathway in preclinical mouse models of cGVHD and present a potential therapeutic strategy to prevent cGVHD that is applicable in patients after allo-HCT.
Materials and methods
Mice
Female B10.D2 mice (H-2d, CD229.1−) were purchased from Jackson Laboratory (Bar Harbor, ME); BALB/c (H-2d, CD229.1+), B6.Ly5.1 (H-2b, CD45.1), and B6D2F1 (H-2b/d) were purchased from Charles River Laboratories (Wilmington, MA), and B10.BR (H-2k) were purchased from Jackson Laboratory and bred in a specific pathogen-free facility at the Medical University of South Carolina (MUSC, Charleston, SC). B-cell conditional XBP-1 knock-out (KO) strain (XBP-1fl/flCD19-Cre+) and littermate wild-type (WT) control strain (XBP-1fl/flCD19-Cre−) were generated by crossing XBP-1fl/fl mice with CD19-Cre mice on a B6 background described previously.17,18 Experimental animals were housed in the American Association for Laboratory Animal Care–accredited Animal Resource Center at MUSC. All animal experiments were approved by the MUSC Institutional Animal Care and Use Committee.
Allogeneic bone marrow transplantation (BMT)
T-cell depletion (TCD-BM) or T- and B-cell depletion (TBCD-BM) of bone marrow was performed for donor strains as described previously.19 In B6 to BALB/c and B10.D2 to BALB/c models, recipients were monitored with cGVHD clinical scoring system described previously.19 On day 30 or 60 posttransplant, recipient spleens and trunk skin were collected for flow cytometry analysis, and skin paraformaldehyde fixed and sectioned for hematoxylin and eosin staining. An independent pathologist scored skin sections for cGVHD as described previously.19 In the B6 to B10.BR model, recipients were given 120 mg/kg intraperitoneal (IP) cyclophosphamide on days −3, −2, and sublethal irradiation (700 cGy, X-ray source) on day −1 prior to BMT on day 0 as described previously.20 Unfractionated splenocytes from either XBP-1 WT or XBP-1 KO donors were pooled with respective TCD-BM from each strain and injected IV at a dose of 5 × 106 TCD-BM plus 0.15 × 106 splenocytes per mouse. B6 to B6D2F1 acute GVHD model was described previously.21
Treatment with B-I09
B-I09, a small-molecule inhibitor for the IRE-1α RNase, was developed and tested for inhibiting the expression of XBP-1s in vitro and in vivo, described previously.2 B-I09 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 50 to 100 μM, syringe filter sterilized, aliquoted, and stored at −80°C. Aliquots were brought to 3.33 mg/mL with 1× phosphate buffered saline, and animals were injected IP with 150 μL per mouse (25 mg/kg) 2 to 4 hours prior to allo-BMT or on day 21 after BMT for treatment, and followed with once daily injections until day 21 or day 42, respectively.
Graft-versus-leukemia (GVL) response
The blast-crisis chronic myelogenous leukemia (BC-CML) model was generated previously22,23 and was a gift from Shlomchik’s laboratory. B10.D2 donor grafts were given to lethally irradiated BALB/c recipients with or without BC-CML cells at a dose of 1 × 106 BC-CML cells per mouse together with TCD-BM (5 × 106 per mouse) with or without whole splenocytes (5 × 106 per mouse). Mice given TCD-BM plus BC-CML cells treated with either vehicle or B-I09 alone without splenocytes were used as controls. Peripheral blood from recipients was collected periodically starting 14 days after transplant, and splenocytes were collected on day 60 and analyzed via flow cytometry for expression of CD11b+ and GFP+ (green fluorescent protein) BC-CML cells.
FlexiVent pulmonary function tests
B10.BR recipients were anesthetized with isoflurane, weighed, and subjected to tracheostomy in order to evaluate lung function using a flexiVent device (SciReq, Montreal, QC, Canada) as described previously.20 Mice were ventilated at a tidal volume of 10 mL/kg body weight, a positive end-expiratory pressure of 3 cmH2O, and 150 breaths per minute (default). Spontaneous respiration was suppressed by IP injection of pancuronium bromide (0.08 mg/kg) and maintenance of the mice on 5% isoflurane. Compliance (ease of lung extension), airway resistance of the respiratory system as a whole (level of lung constriction) as well as quasistatic compliance (pressure of the lungs at a given volume), alveolar constriction (energy required to expand lung tissue), and elastance (rigidity or stiffness) of the lungs from each animal were determined using the Mouse Mechanics Scan script and flexiWare software version 7.6.
Flow cytometry and serum IgG detection
Splenocytes were analyzed for surface proteins and intracellular cytokines using standard flow cytometric protocols as previously described.19,24,25 Protocol for isolation of lymphocytes from lungs and trunk skin was adapted from a previous publication.26 The following antibodies were used for staining: Fixable Live/Dead yellow (BD Biosciences, San Jose, CA), anti-TCRβ–FITC or -PE–Cy7 (Clone: H57-597), anti-CD4–FITC, or –V450 (RM4-5), anti-CD8α–FITC, or -allophycocyanin-cy7 (53-6.7), anti-PD-1–PE or –PerCpCy5.5 (J43), anti-CXCR5–PE-Cy7 (SPRCL5), anti-B220–V450 (RA3-6B2), Annexin V-PE and 7AAD Apoptosis staining kit (BD Biosciences), anti-CD80–PE-Cy7 (16-10A1), anti-CD86–PE-Cy5 (GL1), anti-Fas (Jo2), anti-GL7–Alexa 647 (GL7), anti-immunoglobulin M (IgM)–PE-Cy7 (RMM-1), anti-CD229.1–Biotin or –PE (30C7), anti-CD11c–PE-Cy7 (N418), anti-CD11b–PerCpCy5.5 (M1/70) purchased from BD Biosciences, anti–interferon-γ (IFN-γ)–PE or –Per-cp5.5 (XMG1.2; BD Biosciences), anti–IL-12p35–eflour660 (4D10p35), and IL-12p40 Biotin (eBioscience), and the appropriate unstimulated controls to guarantee intracellular staining specificity. Cell isolates were analyzed using Diva software, LSR II (BD Biosciences), and FlowJo (TreeStar, Ashland, OR). Enzyme-linked immunosorbent assay (ELISA) using an IgG capture antibody (Biolegend) or double-stranded DNA (dsDNA) -coated plates to measure serum total IgG and anti-dsDNA IgG antibodies was carried out as described previously.19
Statistics
Data presentation and statistical analyses were carried out using Prism 6 software (GraphPad) and graphed as mean ± standard error of the mean. For cGVHD clinical scores, all animals were examined for cGVHD clinical signs on day 0 as a reference point, and nonreference point clinical score data were analyzed using a Mann-Whitney U test to determine any statistical significance between groups (P < .05), or an unpaired 2-tailed Student t test was used to analyze a single time point at the experiment endpoint. For all other data, differences among experimental groups were compared using an unpaired 2-tailed Student t test to determine any statistical significance (P < .05) between 2 groups, or when comparing >2 groups a 1-way analysis of variance using Bonferroni multiple comparisons test was performed to determine any statistical significance (P < .05) unless otherwise stated.
Results
B-cell–specific XBP-1 impairs pulmonary function in a bronchiolitis obliterans (BO) model of cGVHD
XBP-1 regulates processes in B cells such as BTK phosphorylation, B-cell receptor (BCR) activation, and plasma cell development,2,5,6,17,27 which are implicated in pathogenesis of cGVHD.19,28-31 We hypothesized conditional deletion of XBP-1 on donor allogeneic B lymphocytes would impair B-cell activation and development of cGVHD. To test this hypothesis, we used a BO model of pulmonary cGVHD (B6 to B10.BR) mediated by donor T cells and antigen-presenting cells (APCs).20 Recipients transplanted with allogeneic grafts from B-cell–specific XBP-1 conditional KO (XBP-1 KO) donors displayed less body weight loss compared with those that received WT (XBP-1 WT) grafts at several time points (Figure 1A). The recipients of XBP-1 KO donor grafts also developed reduced pulmonary cGVHD compared with those of XBP-1 WT donor grafts as indicated by measurements of pulmonary mechanics. The recipients of XBP-1 KO grafts demonstrated significantly decreased respiratory resistance and increased lung compliance (Figure 1B-C). These recipients also displayed increased static compliance, reduced alveolar constriction, and reduced lung elastance, indicating less injured lungs compared with those of XBP-1 WT grafts (Figure 1C). In agreement with previous reports, at baseline we characterized the B-cell–specific XBP-1 KO and XBP-1 WT donor cells and found no gross differences or abnormalities in the B-cell compartment (data not shown).2,6,17 Collectively, these data indicate that B-cell intrinsic XBP-1s plays a pathogenic role in the development of pulmonary cGVHD.
B-cell–specific XBP-1 impairs pulmonary function in a BO model of cGVHD. B10.BR recipients were treated twice with cyclophosphamide (120 mg/kg) 1 time per day on days −3, −2, and sublethally irradiated on day −1 before transplant. On day 0, mice were transplanted with TCD-BM (5 × 106 per mouse) from XBP-1flox/floxCD19Cre− (n = 10) or XBP-1flox/floxCD19Cre+ donors (n = 9) on a B6 background with (n = 19) or without (n = 4) whole splenocytes (Sp) at 0.15 × 106 per mouse. Mice were monitored for body weight loss until experiment endpoint (A), where recipients were anesthetized, given tracheostomy, and subjected to lung function analysis using a SciReq flexiVent device 28 days after transplant (B-C). Data from panels B and C are pooled from 2 separate experiments. Panel A shows representative body weight loss from 2 replicate experiments. Statistics were performed using 2-tailed Student t tests of each indicated time point, where P < .05 indicates statistical significance and an asterisk indicates statistical significance between XBP-1flox/floxCD19Cre− and XBP-1flox/floxCD19Cre+ groups: *P < .05. BM, bone marrow; Crs, compliance; Cst, quasistatic compliance; Rrs, airway resistance of the respiratory system as a whole.
B-cell–specific XBP-1 impairs pulmonary function in a BO model of cGVHD. B10.BR recipients were treated twice with cyclophosphamide (120 mg/kg) 1 time per day on days −3, −2, and sublethally irradiated on day −1 before transplant. On day 0, mice were transplanted with TCD-BM (5 × 106 per mouse) from XBP-1flox/floxCD19Cre− (n = 10) or XBP-1flox/floxCD19Cre+ donors (n = 9) on a B6 background with (n = 19) or without (n = 4) whole splenocytes (Sp) at 0.15 × 106 per mouse. Mice were monitored for body weight loss until experiment endpoint (A), where recipients were anesthetized, given tracheostomy, and subjected to lung function analysis using a SciReq flexiVent device 28 days after transplant (B-C). Data from panels B and C are pooled from 2 separate experiments. Panel A shows representative body weight loss from 2 replicate experiments. Statistics were performed using 2-tailed Student t tests of each indicated time point, where P < .05 indicates statistical significance and an asterisk indicates statistical significance between XBP-1flox/floxCD19Cre− and XBP-1flox/floxCD19Cre+ groups: *P < .05. BM, bone marrow; Crs, compliance; Cst, quasistatic compliance; Rrs, airway resistance of the respiratory system as a whole.
XBP-1 decreases B-cell recovery and promotes the TFH cell subset after allo-BMT
We further investigated potential mechanisms of the impact of XBP-1 on cGVHD using the B6 to B10.BR model. On day 28 after allo-BMT, recipients transplanted with XBP-1 KO donor grafts had higher frequencies of donor-derived B cells in the spleens (Figure 2A-B), which correlated with reduced frequencies of the splenic follicular helper T-cell (TFH) subset (Figure 2A,C). Isolated lung lymphocytes showed that recipients of XBP-1 KO grafts had significantly increased frequencies of donor-derived B cells in the lungs compared with XBP-1 WT recipients (Figure 2D-E). The XBP-1 KO donor B cells within the recipient lungs were also characterized by significantly lower frequencies of apoptotic markers Annexin V and 7AAD compared with donor XBP-1 WT B cells (Figure 2D,F). Together, these results indicate that XBP-1 contributes to impaired reconstitution of B cells in lymphoid and nonlymphoid organs after allo-BMT, at least in part because of apoptosis of B cells in nonlymphoid organs such as the lungs. Furthermore, deficiency of XBP-1 in B cells also reduced differentiation of TFH cells, a subset attributable to cGVHD pathogenesis.32-34
XBP-1 decreases B-cell recovery and promotes the TFH cell subset after allo-HCT. B10.BR recipients were treated twice with cyclophosphamide (120 mg/kg) 1 time per day on days −3, −2, and sublethally irradiated on day −1 before transplant. On day 0, mice were transplanted with TCD-BM (5 × 106 per mouse) from XBP-1flox/floxCD19Cre− (n = 10) or XBP-1flox/floxCD19Cre+ donors (n = 9) on a B6 background with (n = 19) or without (n = 4) whole splenocytes at 0.15 × 106 per mouse. On day 28 after allo-HCT, recipient mice were killed and spleens excised and processed into single-cell suspensions for flow cytometry analysis of B220+ B cells (A-B) and donor CD4+ TFH cells (PD-1hiCXCR5+) (A,C). Lungs were excised, and lymphocytes were isolated and stained for B220+ B cells (D-E) and apoptotic B cells (Annexin V+7AAD+) (D-F). Data shown in panels A and D are individual flow cytometry plots representative of each group. Data shown in panels B, C, E, and F are pooled from 2 replicate experiments. P < .05 indicates statistical significance. SSC-A, side scatter area.
XBP-1 decreases B-cell recovery and promotes the TFH cell subset after allo-HCT. B10.BR recipients were treated twice with cyclophosphamide (120 mg/kg) 1 time per day on days −3, −2, and sublethally irradiated on day −1 before transplant. On day 0, mice were transplanted with TCD-BM (5 × 106 per mouse) from XBP-1flox/floxCD19Cre− (n = 10) or XBP-1flox/floxCD19Cre+ donors (n = 9) on a B6 background with (n = 19) or without (n = 4) whole splenocytes at 0.15 × 106 per mouse. On day 28 after allo-HCT, recipient mice were killed and spleens excised and processed into single-cell suspensions for flow cytometry analysis of B220+ B cells (A-B) and donor CD4+ TFH cells (PD-1hiCXCR5+) (A,C). Lungs were excised, and lymphocytes were isolated and stained for B220+ B cells (D-E) and apoptotic B cells (Annexin V+7AAD+) (D-F). Data shown in panels A and D are individual flow cytometry plots representative of each group. Data shown in panels B, C, E, and F are pooled from 2 replicate experiments. P < .05 indicates statistical significance. SSC-A, side scatter area.
XBP-1 mediates B-cell activation and cGVHD pathogenicity
We used a previously described second model of cGVHD (B6 to BALB/c) to further investigate the role of XBP-1 in cGVHD.19,35 Recipients given XBP-1 KO donor grafts developed significantly reduced cGVHD compared with those that received XBP-1 WT donor grafts indicated by cGVHD clinical scores (Figure 3A). Within the clinical scores, recipients of XBP-1 KO donor grafts showed reduced diarrhea, eye inflammation, and conjunctivitis, and improved posture and fur integrity. Similar to the B6 to B10.BR model, XBP-1 KO recipients displayed a significant increase in the frequency of splenic B cells compared with XBP-1 WT donor-graft recipients 30 days after BMT (Figure 3B). In XBP-1 KO donor-graft recipients, B cells displayed significantly lower expression of CD86 (Figure 3C) and lower percentage of Fas+GL7+ cells (Figure 3D-E), suggesting a reduction in costimulatory activity and germinal center (GC) B cells compared with those of XBP-1 WT B cells, respectively. B cells in recipients of XBP-1 KO grafts expressed significantly higher levels of surface IgM compared with those in XBP-1 WT recipients (Figure 3D,F). We then measured serum autoantibodies against dsDNA via ELISA, and recipients of XBP-1 KO grafts had significantly decreased levels of serum anti-dsDNA IgG compared with those of WT grafts (Figure 3G). B cells in the recipients of XBP-1 KO grafts produced significantly lower levels of IL-12p35 (Figure 3H-I) and IFN-γ (Figure 3H,J) compared with B cells from those of XBP-1 WT grafts. Taken together, these data indicate that cGVHD was reduced in the absence of XBP-1 in donor B cells, and that B-cell intrinsic XBP-1 contributes to a diverse repertoire of B-cell functions, such as activation, differentiation, and cytokine production in the context of allo-BMT.
XBP-1 mediates B-cell activation and cGVHD pathogenicity. Lethally irradiated BALB/c mice were transplanted with TCD-BM (5 × 106 per mouse) from XBP-1flox/floxCD19Cre− (n = 21), XBP-1flox/floxCD19Cre+ (n = 15), or TBCD-BM B6Ly5.1+ (n = 10) donors on a B6 background with (n = 40) or without (n = 6) between 0.5 and 1 × 106 whole splenocytes. Mice were monitored for cGVHD clinical scores until day 60 after allo-HCT, and statistics were performed using a Mann-Whitney U test of the entire time course (A). Subsets of recipient mice were killed on day 30, and spleens were excised and processed into single-cell suspensions for flow cytometric analysis of B220+ B cells (B), CD86-expressing B cells (C), GC B cells (Fas+GL7+) (D-E), and B-cell surface IgM (D,F). Serum from peripheral blood was collected on day 30 and assayed for anti-dsDNA autoantibodies using ELISA (G). On day 30, 2 × 106 recipient splenocytes from each mouse were stimulated with lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), and Ionomycin, and B cells were intracellularly stained and analyzed for IL-12p35 and IFN-γ cytokine production (H-J). Data shown in panel A are representative clinical scoring of 3 replicate experiments. Data in panels B-D and F-J were collected from 1 subset of mice (n = 10) out of n = 40 recipients. Data in panel E are pooled from 2 independent experiments. P < .05 indicates statistical significance. MFI, mean fluorescence intensity; O.D., optical density.
XBP-1 mediates B-cell activation and cGVHD pathogenicity. Lethally irradiated BALB/c mice were transplanted with TCD-BM (5 × 106 per mouse) from XBP-1flox/floxCD19Cre− (n = 21), XBP-1flox/floxCD19Cre+ (n = 15), or TBCD-BM B6Ly5.1+ (n = 10) donors on a B6 background with (n = 40) or without (n = 6) between 0.5 and 1 × 106 whole splenocytes. Mice were monitored for cGVHD clinical scores until day 60 after allo-HCT, and statistics were performed using a Mann-Whitney U test of the entire time course (A). Subsets of recipient mice were killed on day 30, and spleens were excised and processed into single-cell suspensions for flow cytometric analysis of B220+ B cells (B), CD86-expressing B cells (C), GC B cells (Fas+GL7+) (D-E), and B-cell surface IgM (D,F). Serum from peripheral blood was collected on day 30 and assayed for anti-dsDNA autoantibodies using ELISA (G). On day 30, 2 × 106 recipient splenocytes from each mouse were stimulated with lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), and Ionomycin, and B cells were intracellularly stained and analyzed for IL-12p35 and IFN-γ cytokine production (H-J). Data shown in panel A are representative clinical scoring of 3 replicate experiments. Data in panels B-D and F-J were collected from 1 subset of mice (n = 10) out of n = 40 recipients. Data in panel E are pooled from 2 independent experiments. P < .05 indicates statistical significance. MFI, mean fluorescence intensity; O.D., optical density.
B-cell XBP-1 modulates T-cell activation during cGVHD
We then asked whether or how XBP-1 deficiency in B cells impacts T cells during cGVHD development using the B6 to BALB/c cGVHD model. Corresponding with our long-term data, mice that received XBP-1 KO donor grafts had significantly lower cGVHD clinical scores compared with those that received B-cell XBP-1 WT grafts (Figure 4A). This result was associated with reduced surface expression of PD-1 on donor CD8 T cells compared with those from the recipients of XBP-1 WT grafts (Figure 4B-C). Recipients of XBP-1 KO grafts also had significantly lower frequencies of donor-derived IFN-γ–producing CD4 (Figure 4B,D) and CD8 (Figure 4B,E) T cells in their spleens compared with those of XBP-1 WT grafts. In agreement with our data from the B6 to B10.BR model, frequencies of the TFH cell subset were also reduced in the recipients of XBP-1 KO grafts compared with those of XBP-1 WT grafts (Figure 4F).
B-cell XBP-1 modulates T-cell activation during cGVHD. Lethally irradiated BALB/c mice were transplanted with TBCD-BM (5 × 106 per mouse) from B6Ly5.1+ mice (n = 10) with 1 × 106 whole splenocytes from XBP-1flox/floxCD19Cre-− (n = 5) or XBP-1flox/floxCD19Cre+ (n = 5), donors on a B6 background. Mice were monitored for cGVHD clinical scores after allo-HCT (A) (indicated statistical significance is a 2-tailed Student t test performed for the day 30 time point). The recipient mice were killed on day 30, and their spleens were excised and processed into single-cell suspensions for direct flow cytometric analysis of donor H2Kb PD-1 expressing CD8+ T cells (B-C) or were stimulated with LPS, PMA, and Ionomycin to detect IFN-γ–producing CD4+ (B,D) and CD8+ T cells (B,E). Data in panels A-F were collected from 1 cohort of mice (n = 10). P < .05 indicates statistical significance.
B-cell XBP-1 modulates T-cell activation during cGVHD. Lethally irradiated BALB/c mice were transplanted with TBCD-BM (5 × 106 per mouse) from B6Ly5.1+ mice (n = 10) with 1 × 106 whole splenocytes from XBP-1flox/floxCD19Cre-− (n = 5) or XBP-1flox/floxCD19Cre+ (n = 5), donors on a B6 background. Mice were monitored for cGVHD clinical scores after allo-HCT (A) (indicated statistical significance is a 2-tailed Student t test performed for the day 30 time point). The recipient mice were killed on day 30, and their spleens were excised and processed into single-cell suspensions for direct flow cytometric analysis of donor H2Kb PD-1 expressing CD8+ T cells (B-C) or were stimulated with LPS, PMA, and Ionomycin to detect IFN-γ–producing CD4+ (B,D) and CD8+ T cells (B,E). Data in panels A-F were collected from 1 cohort of mice (n = 10). P < .05 indicates statistical significance.
In the acute GVHD setting, transplant of XBP-1 KO B cells, relative to XBP-1 WT B cells, was associated with significant reduction of IFN-γ–producing CD4+ and CD8+ cells and IL-17A–producing CD4+ T cells in the spleens of mice given acute GVHD in B6D2F1 recipients. However, T cells in mesenteric lymph nodes or gut were unaffected, and more importantly, acute GVHD pathology was not impacted (supplemental Figure 1A), consistent with literature showing that B cells do not significantly contribute to acute GVHD.36 By day 14 posttransplant, no death was observed in any groups tested, and body weight loss was comparable between the recipients with WT vs KO grafts (supplemental Figure 1B). Collectively, these data suggest the ER stress response mediated by XBP-1 specifically in B cells can significantly alter the activation status of both CD4+ and CD8+ T cells as well as differentiation of TFH cells during chronic GVHD, but not necessarily in acute GVHD.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD
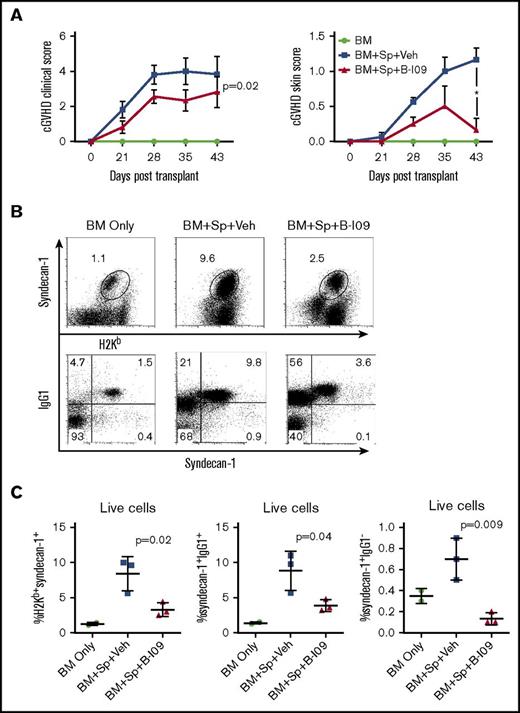
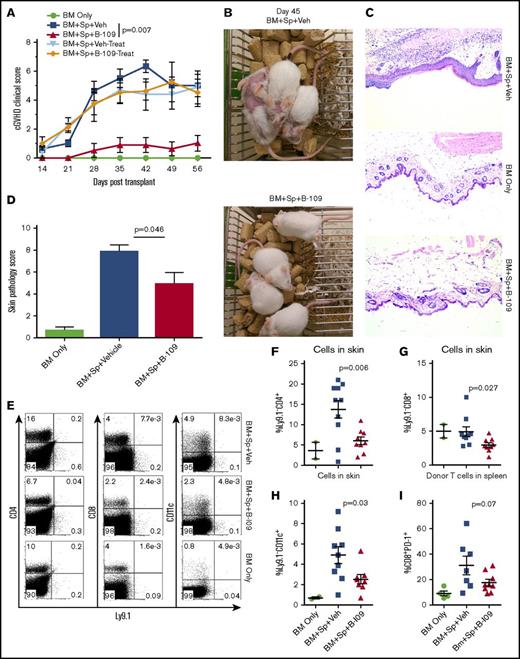
We then tested the ability of a potent IRE-1α inhibitor, B-I09, to prevent cGVHD. B-I09 blocks RNAse activity of IRE-1α and prevents its ability to splice XBP-1 mRNA into the active XBP-1 transcription factor.2 We tested this inhibitor as a prophylactic in MHC-mismatched BMT model (B6 to BALB/c), and as both a prophylactic and a treatment in MHC-matched BMT model of cGVHD (B10.D2 to BALB/c), where skin damage is the most prominent feature. We found that recipients prophylactically injected with B-I09 exhibited significantly reduced cGVHD clinical scores compared with those injected with vehicle in both MHC-mismatched (Figure 5A) and MHC-matched BMT models (Figure 6A). Within the clinical scores, the recipients treated with B-I09 showed reduced skin damage, diarrhea, eye inflammation, and conjunctivitis and improved fur integrity and mobility. Although T helper (Th1) and Th17 cells in the spleens and peripheral lymph nodes were not impacted (data not shown), antibody-secreting plasma cells were significantly reduced in recipient spleens by B-I09 prophylaxis compared with vehicle controls in B6 to BALB/c model as measured by surface syndecan-1 (CD138) and intracellular IgG1 on day 50 after BMT (Figure 5B-C). Images showing disease status in mice prophylactically treated with vehicle or B-I09 using the B10.D2 to BALB/c model were taken on day 45 posttransplant (Figure 6B). On day 60 after allo-BMT, skin biopsies were excised for pathologic analysis (Figure 6C), indicating that recipients treated with B-I09 displayed significantly reduced skin pathology scores consisting of reduced epidermal thickening, fat loss, inflammation, follicular loss, and dermal fibrosis parameters compared with vehicle recipients (Figure 6C-D). Although the use of B-I09 was highly effective at preventing cGVHD when used as a prophylactic, a delayed treatment strategy was not able to ameliorate already established cGVHD when treatment was initiated at day 21 posttransplant (Figure 6A). We then found that the recipients given B-I09 prophylaxis had significantly lower frequencies of donor-derived CD4+ cells (Figure 6E-F), CD8+ cells (Figure 6E,G), and CD11c+ cells in their skin compared with those treated with vehicle (Figure 6E,H). In agreement with our data from using B-cell conditional KO mice (Figure 4), splenic donor CD8+ T cells from recipients treated with B-I09 displayed reduced PD-1 expression on day 60 compared with those treated with vehicle control, although not statistically different (Figure 6I). Collectively, these results indicate that targeting the IRE-1α/XBP-1 pathway pharmacologically is able to prevent development of sclerodermatous cGVHD, primarily through the reduction of infiltration of donor T cells and DCs into the skin.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD in MHC-mismatched BMT model. B6 to BALB/c BMT was carried out as in Figure 3. The recipients were left without treatment (n = 4) or IP injected daily with DMSO vehicle or with B-I09 at a dose of 25 mg/kg starting on day 0 and continued for 3 weeks (n = 8 per group). BMT recipients (n = 19) were monitored for cGVHD clinical scores and skin scores (A) and killed on day 30 (n = 4 per group) or 50 (n = 4 per group) after transplant, and spleens were subjected to flow cytometric analysis for surface expression of syndecan-1 and intracellular IgG1. Representative flow plots from day 50 for each group are depicted in panel B. Data from each group are quantified in panel C. Overall cGVHD clinical scores for the entire experimental time course were analyzed using a paired Student t test. For skin cGVHD clinical scores, a 2-tailed Student t test was performed for the final time point. P < .05 indicates statistical significance. Data were collected from n = 19 mice from 1 representative experiment. Veh, vehicle.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD in MHC-mismatched BMT model. B6 to BALB/c BMT was carried out as in Figure 3. The recipients were left without treatment (n = 4) or IP injected daily with DMSO vehicle or with B-I09 at a dose of 25 mg/kg starting on day 0 and continued for 3 weeks (n = 8 per group). BMT recipients (n = 19) were monitored for cGVHD clinical scores and skin scores (A) and killed on day 30 (n = 4 per group) or 50 (n = 4 per group) after transplant, and spleens were subjected to flow cytometric analysis for surface expression of syndecan-1 and intracellular IgG1. Representative flow plots from day 50 for each group are depicted in panel B. Data from each group are quantified in panel C. Overall cGVHD clinical scores for the entire experimental time course were analyzed using a paired Student t test. For skin cGVHD clinical scores, a 2-tailed Student t test was performed for the final time point. P < .05 indicates statistical significance. Data were collected from n = 19 mice from 1 representative experiment. Veh, vehicle.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD in MHC-matched BMT model. Lethally irradiated BALB/c recipients were transplanted with TCD-BM (5 × 106/mouse) from B10.D2 donors with (n = 30) or without (n = 4) whole splenocytes at 5 × 106/mouse. Groups were either given no treatment (n = 4), given daily IP injection of DMSO vehicle alone starting on day 0 and continued for 3 weeks (days 0-21; n = 10) or beginning on day 21 (days 21-42; n = 5), or IP injected with B-I09 at a dose of 25 mg/kg beginning at day 0 and continued for 3 weeks (days 0-21; n = 10), or beginning on day 21 (days 21-42; n = 5). Recipient mice were monitored for cGVHD clinical scores (A) until experiment endpoint on day 60. On day 45, images were taken of prophylactically treated vehicle and B-I09 groups (B). Skin biopsies were sectioned and stained with hematoxylin and eosin (magnification ×200) (C) and analyzed by an independent pathologist for signs of cGVHD skin damage (D). On day 60, mice were killed, and spleens and trunk skin were excised for processing into single-cell suspension for flow cytometric analysis of donor CD229.1− (Ly9.1−) CD4, CD8, and CD11c lymphocyte skin infiltrates (E). Quantification of CD4 (F), CD8 (G), and CD11c (H) cells in skin is shown. Splenic donor CD8+ T cells were analyzed via flow cytometry for expression of PD-1 (I). Data shown in panels A-C are representative of 2 separate experiments. Data shown in panel D are pooled from 2 replicate experiments. P < .05 indicates statistical significance. Data in panel E are representative flow plots from 2 separate experiments, which are quantified as pooled data in panels F-I. Statistical analysis of cGVHD clinical scores was performed using a Mann-Whitney U test of the entire experimental time course. P < .05 indicates statistical significance.
Pharmacologic inhibition of IRE-1α/XBP-1 prevents cGVHD in MHC-matched BMT model. Lethally irradiated BALB/c recipients were transplanted with TCD-BM (5 × 106/mouse) from B10.D2 donors with (n = 30) or without (n = 4) whole splenocytes at 5 × 106/mouse. Groups were either given no treatment (n = 4), given daily IP injection of DMSO vehicle alone starting on day 0 and continued for 3 weeks (days 0-21; n = 10) or beginning on day 21 (days 21-42; n = 5), or IP injected with B-I09 at a dose of 25 mg/kg beginning at day 0 and continued for 3 weeks (days 0-21; n = 10), or beginning on day 21 (days 21-42; n = 5). Recipient mice were monitored for cGVHD clinical scores (A) until experiment endpoint on day 60. On day 45, images were taken of prophylactically treated vehicle and B-I09 groups (B). Skin biopsies were sectioned and stained with hematoxylin and eosin (magnification ×200) (C) and analyzed by an independent pathologist for signs of cGVHD skin damage (D). On day 60, mice were killed, and spleens and trunk skin were excised for processing into single-cell suspension for flow cytometric analysis of donor CD229.1− (Ly9.1−) CD4, CD8, and CD11c lymphocyte skin infiltrates (E). Quantification of CD4 (F), CD8 (G), and CD11c (H) cells in skin is shown. Splenic donor CD8+ T cells were analyzed via flow cytometry for expression of PD-1 (I). Data shown in panels A-C are representative of 2 separate experiments. Data shown in panel D are pooled from 2 replicate experiments. P < .05 indicates statistical significance. Data in panel E are representative flow plots from 2 separate experiments, which are quantified as pooled data in panels F-I. Statistical analysis of cGVHD clinical scores was performed using a Mann-Whitney U test of the entire experimental time course. P < .05 indicates statistical significance.
XBP-1 is required for B-cell activation and differentiation
To further ask how XBP-1 affects B-cell activation and differentiation in general, B cells were activated with LPS + IL-4 in vitro. B cells deficient for XBP-1 differentiated into Fas+GL7+ GC B cells at a significantly lower frequency (supplemental Figure 2A-B) and expressed lower CD86, ICOS-L, and MHCII (supplemental Figure 2A-C) as compared with WT B cells. Inhibition of XBP-1 with B-I09 also reduced GC B-cell differentiation and expression of costimulatory molecules, although less profound than XBP-1 deficiency (supplemental Figure 2A-B). Consistent with our observation in vivo (Figure 3), XBP-1 KO B cells secreted significantly lower total IgG antibodies into cell culture media, which was phenocopied by XBP-1 inhibition with B-I09 (supplemental Figure 2D). Furthermore, both XBP-1 deficiency and treatment with B-I09 impaired the ability of B cells to produce IL-4/5 and IL-12p40 cytokines after stimulation when compared with XBP-1 WT B cells or vehicle controls (supplemental 2E-F). Collectively, these in vitro studies suggest that XBP-1 plays a critical role in B-cell activation and differentiation into cytokine-secreting or GC B cells, and that B-I09 can phenocopy multiple aspects of XBP-1 deficiency in B cells.
Prophylactic B-I09 administration does not impair the GVL effect
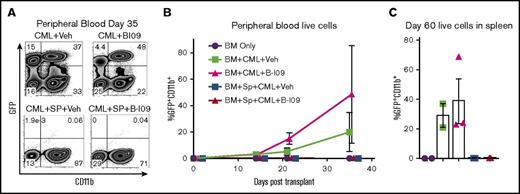
Here, we used a transplantable BC-CML model to test the impact of B-I09 administration and XBP-1 inhibition on the GVL effect. In this model, allogeneic T cells contained in donor B10.D2 splenocytes mediate an antitumor response against tumor cells expressing disparate minor histocompatibility antigens as the host (BALB/c).22 As expected, recipients that received allogeneic BM alone without splenocytes developed GFP-expressing CML cells in both vehicle- and B-I09-treated conditions (Figure 7A-B). The recipients that received allogeneic BM plus splenocytes and treated with vehicle control did not display any signs of tumor growth. Similarly, the recipients that received allogeneic BM plus splenocytes and treated with B-I09 displayed potent antitumor responses with no evident impairment of GVL activity in peripheral blood measured until day 35 (Figure 7A-B) or in spleen measured on day 60 (Figure 7C). Although B-I09 treatment alone without splenocytes did not suppress CML growth in peripheral blood or the spleens, these recipients did not succumb to tumor mortality compared with recipients of vehicle alone without splenocytes, which displayed 33% tumor mortality (data not shown). To test how XBP-1 affects B-cell–mediated antigen presentation, we compared ability of XBP-1 WT vs KO B cells to stimulate allogeneic T cells in vitro. Under this condition, XBP-1 in B cells had no effect on T-cell proliferation, activation, and cytolytic potential (Granzyme B) (supplemental Figure 3). These data support that inhibition of the IRE-1α/XBP-1 pathway may not impair the GVL effect.
Prophylactic B-I09 administration does not impair the GVL effect. Lethally irradiated BALB/c recipients were transplanted with TCD-BM from B10.D2 donors with (n = 20) or without (n = 13) whole splenocytes. Mice were also cotransplanted with either no blast crisis chronic myeloid leukemia cells (BC-CML) (n = 4) or 1 × 106 BC-CML splenocytes (n = 29). Groups subsequently either received no treatment (n = 4), received daily IP injection of DMSO vehicle (n = 16), or received 25 mg/kg B-I09 (n = 13) on day 0 and continued for 3 weeks. Peripheral blood was collected and analyzed for GFP+CD11b+ expressing BC-CML cells via flow cytometry (A) periodically after transplant until day 35 and is shown as a time course of GFP+CD11b+ cells in each group (B). On day 60, mice were killed, and splenocytes were analyzed for the presence of BC-CML cells indicated by GFP+CD11b+ expression (C). Flow cytometry plot from panel A is representative of 2 replicate experiments. Data from panels B and C are pooled from 2 replicate experiments.
Prophylactic B-I09 administration does not impair the GVL effect. Lethally irradiated BALB/c recipients were transplanted with TCD-BM from B10.D2 donors with (n = 20) or without (n = 13) whole splenocytes. Mice were also cotransplanted with either no blast crisis chronic myeloid leukemia cells (BC-CML) (n = 4) or 1 × 106 BC-CML splenocytes (n = 29). Groups subsequently either received no treatment (n = 4), received daily IP injection of DMSO vehicle (n = 16), or received 25 mg/kg B-I09 (n = 13) on day 0 and continued for 3 weeks. Peripheral blood was collected and analyzed for GFP+CD11b+ expressing BC-CML cells via flow cytometry (A) periodically after transplant until day 35 and is shown as a time course of GFP+CD11b+ cells in each group (B). On day 60, mice were killed, and splenocytes were analyzed for the presence of BC-CML cells indicated by GFP+CD11b+ expression (C). Flow cytometry plot from panel A is representative of 2 replicate experiments. Data from panels B and C are pooled from 2 replicate experiments.
Discussion
Here, targeting XBP-1 specifically in B cells using a conditional KO strategy was sufficient to prevent cGVHD in 2 preclinical mouse models. Prophylactic administration of an IRE-1α/XBP-1 pathway inhibitor (B-I09) during allo-BMT prevented cGVHD development in both MHC-matched and MHC-mismatched cGVHD models with minimal toxicity. In vitro assays revealed that XBP-1 mediates optimal TLR4 signaling in B cells and is required for generation of IL-12p40–producing B-effector 1 cells and IL-4/5-producing B-effector 2 cells in response to LPS and IL-4 stimulation.
Reduction of cGVHD in recipients of XBP-1 KO donor grafts was characterized by improved pulmonary function tests, suggesting XBP-1 contributes to pulmonary fibrosis. Lung trichrome staining showed no significant differences in type I collagen deposition between groups (data not shown), suggesting lung function might be mediated by other types of collagen rather than type I, as previously described.37,38 XBP-1–deficient B cells also exhibited reduced apoptosis in lungs but not in the spleens, in agreement with the literature demonstrating that GVHD patients have impaired B-cell reconstitution attributable to active GVHD or treatments for GVHD, in addition to Fas- and IFN-γ–mediated apoptosis of B cells by donor T cells.5,39-44
Costimulation of T cells via CD86 expressed by APCs delivers a strong costimulatory signal to cognate T cells and has been shown to be critical for development of cGVHD.45-47 In light of our in vivo and in vitro data on costimulatory molecules and given previous reports that IL-4 rapidly induces XBP-1 gene expression through STAT6 signaling,5 we propose that XBP-1 promotes the expression of costimulatory molecules CD86, MHCII, and ICOS-L via IL-4/STAT6-dependent signaling through IL-4.48 Although it is known that IL-4/STAT6 signaling regulates transcription of CD86 and MHCII genes in response to IL-4,49-51 the role of XBP-1 in this B-cell process has not been clearly established. Activated B cells that produce cytokines have previously been shown to enhance Th1 differentiation and promote autoimmunity.45,52,53 IL-12p40 mediates cGVHD as shown previously,54,55 and IL-4/5 drives fibrosis.56 In our study, XBP-1–deficient B cells produced significantly less IL-12p40 and IL-4/5 cytokines after LPS and IL-4 stimulation, suggesting that XBP-1 regulates both type 1 and 2 effector B-cell differentiation. Although some of our B-cell data can be connected to an intrinsic defect of BCR signaling and S1P1 from XBP-1 deficiency,17 we have demonstrated that XBP-1 also plays a critical role in several “innate” B-cell functions that do not rely on classical activation of the BCR, such as IL-12 and IL-4/5 cytokine production after TLR4 and IL-4 stimulation.57
Differentiation of B cells into GC B cells has been shown to be highly correlated with cGVHD development.32,34 XBP-1–deficient B cells displayed reduced differentiation into GC B cells both in vivo and in vitro, and B-I09 treatment of B cells in vitro also demonstrated this effect. Antibodies from donor B cells have recently been directly correlated with cGVHD development.29 Here, XBP-1–deficient B cells secreted less antibodies in response to both in vitro and in vivo stimuli, and both in vitro and in vivo treatment with B-I09 phenocopied this effect. XBP-1 KO B cells also engaged in less class-switch recombination after allo-BMT compared with XBP-1 WT B cells, where higher retention of surface IgM was consistent with reduced levels of serum anti-dsDNA IgG, consistent with a previous report that XBP-1 KO B cells express less activation-induced cytidine deaminase.17 Because reduction in antibody secretion and plasma cell numbers can potentially cause vulnerability to pathogens, targeting XBP-1 should likely be considered as a short-term strategy to prevent cGVHD. Nevertheless, a comparable strategy using ibrutinib to target B cells did not cause infections for the majority of patients.58
TFH are a T-cell subset that has recently been shown to be correlated with cGVHD pathogenesis.32,34 Although the role of TFH and GC B cells in murine models of cGVHD is still under debate,29,59 Forcade et al recently indicated a key role of TFH, which strongly correlated with cGVHD in human patients.33 Accordingly, in our study murine recipients of XBP-1 KO B cells displayed an indirect impact on TFH cell differentiation, where donor TFH cells were reduced in the spleens. CD8+ T cells are also critically involved in cGVHD, where secretion of inflammatory cytokines and cytolytic molecules can lead to tissue fibrosis and organ failure.28,60 In our study, recipients of XBP-1 KO donor grafts or recipients treated with an IRE-1α/XBP-1 pathway inhibitor B-I09 displayed reduced chronic CD8+ T-cell activation, indicated via lower PD-1 surface expression, a coinhibitory receptor that can serve as a marker of T-cell receptor ligation and chronic T-cell activation as well as T-cell exhaustion.61,62 This finding also occurred in conjunction with reduced intracellular production of IFN-γ in both CD4+ and CD8+ T cells in the recipients of XBP-1 KO donor grafts. These findings suggest that reduction in PD-1 on CD8+ T cells does not necessarily exacerbate GVHD as reported previously by others.63
One of the most notable features of human cGVHD is skin damage and scleroderma, which occur in up to 75% of cGVHD patients.64 Thus, we sought to test if targeting XBP-1 would also be efficacious in a minor-antigen mismatched cutaneous model of cGVHD. Using this model, we found that blocking the IRE-1α/XBP-1 pathway with an inhibitor, B-I09, dramatically reduced sclerodermatous cGVHD. In contrast, delayed treatment with B-I09 did not suppress cGVHD, and XBP-1 KO B cells did not impact acute GVHD development, suggesting that the IRE1-α/XBP-1 pathway mediates cGVHD pathogenicity during the transition from the acute to chronic phase. Supporting our findings, a recent report found experimental-autoimmune-encephalitis development was significantly delayed in mice prophylactically treated with an ER stress inhibitor (tauroursodeoxycholic acid); however, experimental autoimmune encephalitis was not ameliorated with delayed administration because of no effect on early Th17 differentiation.65 In our study, prophylactic B-I09 reduced infiltration of donor CD4+, CD8+, and CD11c+ lymphocytes into recipient skin. This result could be attributed to XBP-1–dependent defects in dendritic cell–derived type I IFN signaling, which regulates T-cell survival,10,66 from chemotactic signals such as CXCL16 produced by nonhematopoietic cells, also regulated by XBP-1,67 or from antibody-mediated cutaneous infiltration of pathogenic Th17 cells.29,65 In spleens, frequency of total granulocytes was significantly reduced after B-I09 administration in the B10.D2 to BALB/c model (data not shown), in agreement with previous reports indicating that XBP-1 is critically involved in the differentiation and recruitment of granulocyte subsets into tissues leading to inflammatory fibrosis,11,68,69 and that these subsets are involved in cGVHD.70,71 Current leading therapies for cGVHD directly target B cells or T cells,31,60 whereas the current study and previous literature suggest that inhibition of the IRE-1α/XBP-1 pathway via B-I09 could directly impact other cell subsets involved in cGVHD development in addition to B cells and T cells,65 such as dendritic cells,10 granulocytes,11 and even nonhematopoietic cells.67
The ultimate goal of the HCT field is to cure cancers such as leukemia without producing deleterious GVHD. Along these lines, prophylactically targeting the IRE-1α/XBP-1 pathway using B-I09 or using XBP-1 KO B cells as APCs in vitro did not impair the GVL effect or antitumor functions of T cells in agreement with previous reports that XBP-1 deficiency had little to no direct impact on T-cell responses against infection and did not impair proliferation, activation, or differentiation of Th1 and Th2 cells.16,65,72,73 Furthermore, administration of B-I09 in a previous study was able to directly inhibit tumor growth of B-cell leukemia, lymphoma, and multiple myeloma.2 Thus, inhibition of IRE-1α/XBP-1 in vivo likely does not have a direct impact on antitumor functions in T cells.
Taken together, our results demonstrate that in the context of allo-BMT, XBP-1 regulates a diverse repertoire of B-cell functions via antibody-dependent and antibody-independent mechanisms that correlate with the alleviation of cGVHD. Furthermore, systemic administration of an IRE-1α/XBP-1 pathway inhibitor, B-I09, is able to prevent disease progression and severity in 2 preclinical models of cGVHD. Inhibition of the IRE-1α/XBP-1 pathway with B-I09 was also able to preserve the GVL activity against BC-CML and produced a durable and long-lasting antitumor response in recipients. These results provide strong rationale for additional investigation of the potential for targeting the ER stress response mediated by the IRE-1α/XBP-1 pathway to prevent GVHD while also preserving the GVL effect after allo-HCT in the clinic.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful for the technical support of the Division of Laboratory Animal Resources.
The research presented in this article was supported in part by the Flow Cytometry & Cell Sorting Unit as part of the Hollings Cancer Center at the MUSC, which is funded by Cancer Center Support Grant P30 CA138313 from the National Institutes of Health, National Cancer Institute (NCI). This work was supported in part by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 Al118305) and NCI (R01 CA169116 and R01 CA118116) (X.-Z.Y.). This work was also supported in part by NCI grants R01 CA163910 (C.-C.A.H.), R01 CA190860 (C.-C.A.H. and J.R.D.V.), and R21 CA199553 (J.R.D.V.).
Authorship
Contribution: S.D.S. participated in experimental design, performed research, collected, analyzed, and interpreted data, performed statistical analysis, and drafted and revised the manuscript; Y.W. participated in experimental design, performed research, collected, analyzed, and interpreted data, and edited the manuscript; C.-H.A.T. performed genotyping of mouse strains, prepared mouse spleens and bone marrow for transplantation, interpreted data, and edited the manuscript; D.B., H.N., M.H.S., and M.Z. performed research, interpreted data, and edited the manuscript; C.L. and K.H. performed histological scoring of mouse tissues; C.W. participated in experimental design, performed pulmonary function tests, and edited the manuscript; L.M.S. participated in experimental design, interpreted data, and revised the manuscript; J.R.D.V. synthesized the IRE-1α RNase inhibitor, B-I09, participated in experimental design, interpreted data, and revised the manuscript; and C.-C.A.H. and X.-Z.Y. designed research, interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xue-Zhong Yu, Department of Microbiology & Immunology, Medical University of South Carolina, HCC350, MSC 955, 86 Jonathan Lucas St, Charleston, SC 29425; e-mail: yux@musc.edu.