Key Points
α genes and CAR haplotypes independently impact hemolytic anemia severity; low G6PD-activity impacts anemia severity in CAR/CAR patients.
BEN/BEN patients have a higher prevalence of the favorable BCL11A/rs1427407 T allele and a better response to HU than CAR/CAR patients.
Abstract
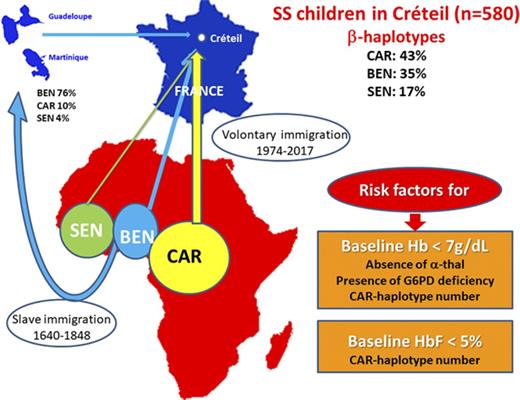
Sickle cell anemia (SCA), albeit monogenic, has heterogeneous phenotypic expression, mainly related to the level of hemoglobin F (HbF). No large cohort studies have ever compared biological parameters in patients with major β-globin haplotypes; ie, Senegal (SEN), Benin (BEN), and Bantu/Central African Republic (CAR). The aim of this study was to evaluate the biological impact of α genes, β haplotypes, and glucose-6-phosphate dehydrogenase (G6PD) activity at baseline and with hydroxyurea (HU). Homozygous HbS patients from the Créteil pediatric cohort with available α-gene and β-haplotype data were included (n = 580; 301 females and 279 males) in this retrospective study. Homozygous β-haplotype patients represented 74% of cases (37.4% CAR/CAR, 24.3% BEN/BEN, and 12.1% SEN/SEN). HU was given to 168 cohort SCA children. Hematological parameters were recorded when HbF was maximal, and changes (ΔHU-T0) were calculated. At baseline, CAR-haplotype and α-gene numbers were independently and negatively correlated with Hb and positively correlated with lactate dehydrogenase. HbF was negatively correlated with CAR-haplotype numbers and positively with BEN- and SEN-haplotype numbers. The BCL11A/rs1427407 “T” allele, which is favorable for HbF expression, was positively correlated with BEN- and negatively correlated with CAR-haplotype numbers. With HU treatment, Δ and HbF values were positively correlated with the BEN-haplotype number. BEN/BEN patients had higher HbF and Hb levels than CAR/CAR and SEN/SEN patients. In conclusion, we show that BEN/BEN patients have the best response on HU and suggest that this could be related to the higher prevalence of the favorable BCL11A/rs1427407/T/allele for HbF expression in these patients.
Introduction
Despite being a monogenic disease, sickle cell anemia (SCA; homozygous hemoglobin S [HbS]) is remarkably heterogeneous in its phenotypic expression. Additional genetic markers such as α-thalassemia coinheritance, β-globin haplotypes, and glucose-6-phosphate dehydrogenase (G6PD) deficiency presence may have an influence on the course of the disease by modulating biological parameters such as the levels of HbF or the degree of hemolytic anemia severity. High HbF concentrations dilute the amount of HbS but also disrupt HbS polymerization. A high level of HbF is associated with improved survival1 and lower rates of vaso-occlusive crisis (VOC) or acute chest syndrome (ACS),2 whereas severe hemolytic anemia is associated with a higher risk of cerebral vasculopathy,3 leg ulcers, priapism, and pulmonary hypertension.2 The impact of G6PD deficiency on the clinical and biological expression of SCA has been controversial, as several studies reported no significant impact,4,5 whereas others showed more severe anemia3,6-8 and a higher risk of abnormal velocities on transcranial Doppler (TCD).3 The presence of α thalassemia in SCA patients is known to reduce HbS polymerization and hemolysis by lowering intracellular Hb concentration9-11 and improve anemia, and it has been reported to be associated with improved survival.12
The HbS gene emerged and expanded through the selective pressure of Plasmodium falciparum malaria in separate geographic locations of Africa.13 These distinct mutational events were identified by linked DNA polymorphic sites, located on the β-globin cluster, and defined as the Senegal (SEN), Benin (BEN), Bantu/Central African Republic (CAR), and Cameroon14 haplotypes. Only a few older studies investigated the role of β haplotypes,15-17 but the positive association between HbF level and the number of SEN haplotypes was clearly demonstrated.15,16 In the United States, the BEN haplotype is largely predominant, the CAR haplotype is rarely observed, and patients are often compound heterozygotes.18 In Africa, environmental, nutritional, and infectious factors make it difficult to distinguish the role of β haplotypes in modulating SCA hematological characteristics. In France, SCA patients often come from West and Central Africa, and all 3 major β haplotypes are well represented and frequently homozygous in this population, making it possible to determine the impact of the β haplotypes on hematological parameters recorded at baseline and during hydroxyurea (HU) treatment. The HbF (α2γ2) level and its distribution among erythrocytes are genetically modulated. A single-nucleotide polymorphism (SNP) located 5′ to HBG2 (rs7482144, originally known as the −158C>T Xmn1 restriction enzyme site) in carriers of SEN and Arab-Indian haplotypes is strongly associated with HbF levels and the concentration of Gγ-globin chains.19,20 Genome-wide association studies have highlighted other genetic markers known to influence HbF levels, such as BCL11A,21,22 a transacting factor involved in HbF regulation, and the intergenic sequence between HBS1L and c-MYB (HMIP), where several SNPs have been linked to the modulation of fetal globin-gene expression.23
Our goal was to determine in a large cohort where all 3 major β haplotypes were well represented the predictive risk factors for low HbF and Hb levels and the responses on HU.
Patients and methods
This retrospective study includes all homozygous HbS patients from the Centre Hospitalier Intercommunal Créteil cohort with available assessment of α genes and β haplotypes and parental written consent (n = 580). The globin α gene3.7, the most frequent α-thalassemic trait found in people of African origin, was detected using a multiplexed GAP polymerase chain reaction procedure15 that was modified to detect the αanti-3.7 triplication.24 β-Globin haplotypes were determined by Sanger sequencing of the “Pre Gγ framework” region, which is located 1 kb upstream of the Gγ globin gene (HBG2) and contains polymorphisms (namely, rs10126653 T/G at −1450, rs2855121G/A at −1280, and rs2855122 G/A at −1225 with respect to HBG2 cap sites). Combination of these 3 SNPs have been found to make 4 different frames, each being associated with 1 of the 4 main SCD haplotypes in Africa (BEN, SEN, CAR, and Cameroon).16 SNPs related to HbF expression, previously selected to define a genetic score predicting severity in thalassemic patients,25 were assessed using different methods depending on the SNP; ie, a specific probes-based assay for β globin cluster rs7482144 (C/T), BCL11A rs10189857 (G/A), and rs1427407 (G/T) (Kasp genotyping assay; LGC group, Middlesex, United Kingdom) and Sanger sequencing for HMIP rs9399137 (T/C).
Baseline biological parameters and G6PD activity were prospectively recorded at least 1 month away from VOC, 3 months from transfusion, and before HU treatment or other intensive therapy such as chronic transfusion or stem cell transplantation. When present, iron and folic acid deficiencies were first corrected before recording all parameters. All parameters at baseline and with HU were measured in the same laboratory. HbF was assessed by high-performance liquid chromatography. G6PD activity was measured with a spectrophotometric assay 3 months away from transfusion, and hexokinase activity and/or reticulocyte counts were assessed simultaneously. Normal ranges were 11 to 17 IU/g Hb for G6PD activity and 0.74 to 1.15 IU/g Hb for hexokinase. SCA patients were considered to have G6PD deficiency when the G6PD activity was low and concomitant hexokinase activity and/or reticulocyte counts were high.
HU was prescribed to children older than 3 years of age who had experienced at least 3 VOCs per year or 2 ACSs, had severe anemia (baseline Hb <7 g/dL), or had a history of abnormal velocities by TCD that had normalized with chronic transfusion but had no stenosis. The initial dose of 11.5 mg/kg per day was given for 1 month. The dose was increased to 23 mg/kg per day the following month and thereafter until the maximum tolerated dose was reached, defined by neutrophil counts of 2 to 4 × 109/L in the absence of hematologic toxicity: Hb <7 g/dL with reticulocytes <100 × 109/L or decrease by >20% from value at T0 with reticulocytes <100 × 109/L or reticulocytes <80 × 109/L or platelets <80 × 109/L). Values of biological parameters were recorded after at least 3 months of HU treatment and when HbF was maximal. Changes with HU treatment (Δ) were calculated as value on HU − value at HU initiation (T0). Use of the database was approved for this project by the Créteil institutional review board.
Participant baseline characteristics were summarized through the use of percentages, and mean (standard deviation [SD]) or median (interquartile range). Fisher exact tests were used to compare proportions, and the Student t test or Wilcoxon rank sum tests were used to compare continuous distributions. Pearson correlations between the different biological parameters and the number of α genes and β haplotypes were evaluated. Significant variables (P < .1) in the univariate analysis were submitted to multivariate linear regression analysis. Logistic regression analyses were performed to evaluate the predictive risk factors for low Hb and HbF. Ninety-five percent confidence intervals (CIs) around point estimates were computed. All statistical tests were 2 sided, with P ≤ .05 denoting statistical significance. To control for the multiplicity of inferences that could possibly result in an increased false positive rate, we modified the P value threshold for statistical significance when appropriate. Statistical analysis was performed with SPSS version 22.
Results
Patient characteristics at baseline
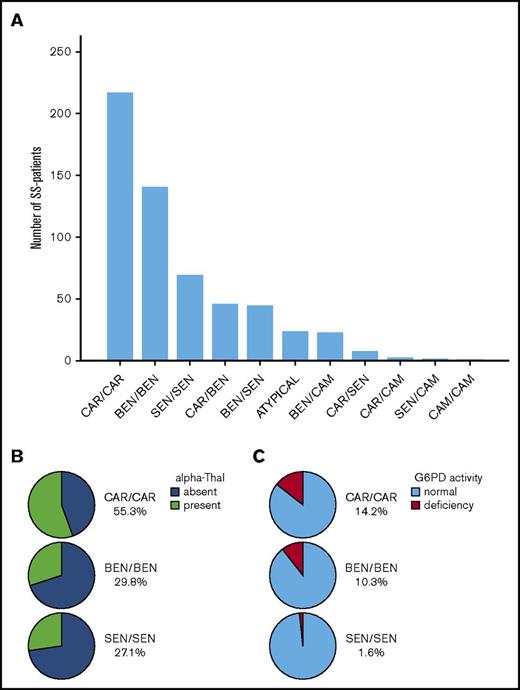
The α- and β-haplotype evaluation was available in 580 children (301 females and 279 males) from the overall Centre Hospitalier Intercommunal Créteil SCA cohort. The distribution of the different β haplotypes and the prevalence of α-thalassemia and G6PD deficiency in the cohort and in the 3 major homozygous β haplotypes are presented in Figure 1A-C.
Distribution of the different β haplotypes in the SCA cohort as a function of α thalassemia and G6PD deficiency in the 3 major homozygous β haplotypes. (A) Number of patients with the different combinations of β haplotypes. The CAR haplotype was the most prevalent allele (43.2%), followed by BEN (34.6%), SEN (17.0%), CAM (2.7%), and atypical (2.5%). Homozygosity for β haplotype was present in 74% of SS patients; ie, CAR/CAR (37.4%), BEN/BEN (24.3%), SEN/SEN (12.1%), and CAM/CAM (0.2%). (B) Prevalence of α thalassemia in the 3 major homozygous β haplotypes. α thalassemia was present in 44.1% of the patients, with 32.8% being heterozygous for the α−3.7 deletion (3 α genes) and 11.4% being homozygous (2 α genes), whereas 0.5% had 5 α genes (patient heterozygous for the anti-3.7 triplication). The prevalence of α thalassemia was significantly higher (P < .001) in CAR/CAR patients (55.3%) than in any other homozygous β haplotypes (BEN/BE, 29.8%; SEN/SEN, 27.1%). (C) Prevalence of G6PD deficiency in the 3 major homozygous β haplotypes. The overall prevalence of G6PD deficiency was 10.6% and was similar in patients with (10.9%) or without α thalassemia (10.4%). However its prevalence was significantly lower (P = .01) in SEN/SEN patients (1.6%) than in CAR/CAR (14.2%) and BEN/BEN (10.3%) patients. The prevalence of G6PD deficiency was significantly lower (P = .01) in SEN/SEN patients (1.6%) than in CAR/CAR (14.2%) and BEN/BEN (10.3%) patients.
Distribution of the different β haplotypes in the SCA cohort as a function of α thalassemia and G6PD deficiency in the 3 major homozygous β haplotypes. (A) Number of patients with the different combinations of β haplotypes. The CAR haplotype was the most prevalent allele (43.2%), followed by BEN (34.6%), SEN (17.0%), CAM (2.7%), and atypical (2.5%). Homozygosity for β haplotype was present in 74% of SS patients; ie, CAR/CAR (37.4%), BEN/BEN (24.3%), SEN/SEN (12.1%), and CAM/CAM (0.2%). (B) Prevalence of α thalassemia in the 3 major homozygous β haplotypes. α thalassemia was present in 44.1% of the patients, with 32.8% being heterozygous for the α−3.7 deletion (3 α genes) and 11.4% being homozygous (2 α genes), whereas 0.5% had 5 α genes (patient heterozygous for the anti-3.7 triplication). The prevalence of α thalassemia was significantly higher (P < .001) in CAR/CAR patients (55.3%) than in any other homozygous β haplotypes (BEN/BE, 29.8%; SEN/SEN, 27.1%). (C) Prevalence of G6PD deficiency in the 3 major homozygous β haplotypes. The overall prevalence of G6PD deficiency was 10.6% and was similar in patients with (10.9%) or without α thalassemia (10.4%). However its prevalence was significantly lower (P = .01) in SEN/SEN patients (1.6%) than in CAR/CAR (14.2%) and BEN/BEN (10.3%) patients. The prevalence of G6PD deficiency was significantly lower (P = .01) in SEN/SEN patients (1.6%) than in CAR/CAR (14.2%) and BEN/BEN (10.3%) patients.
Baseline biological parameters
The baseline biological parameters, recorded at the mean (SD) age of 5.8 (4.8) years, are shown for the entire cohort and as a function of the presence or absence of G6PD deficiency or of α thalassemia and for the 3 major homozygous β haplotypes (Table 1). After adjustment for age at baseline, their association with the genetic markers was assessed by univariate and multivariate analyses
Baseline biological parameters in the overall cohort and as a function of presence or absence of G6PD deficiency, α thalassemia, and 3 major homozygous β haplotypes
| . | . | G6PD . | α Thalassemia . | β Haplotypes . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall . | Normal . | Deficient . | Normal vs deficient, P . | Absent . | Present . | Absent vs present, P . | CAR/CAR . | BEN/BEN . | SEN/SEN . | BEN/BEN vs CAR/CAR, P . | BEN/BEN vs SEN/SEN, P . | |
| No. of patients | 580 | 429 | 51 | — | 324 | 256 | — | 217 | 141 | 70 | — | — |
| Leukocytes, ×109/L | 13.7 ± 4.7 | 13.6 ± 4.7 | 14.2 ± 4.9 | NS | 14.3 ± 4.8 | 12.9 ± 4.5 | .002 | 13.3 ± 4.8 | 13.8 ± 4.5 | 13.5 ± 4.6 | NS | NS |
| Neutrophils, ×109/L | 5.9 ± 3.0 | 5.9 ± 3.0 | 5.4 ± 2.2 | NS | 5.9 ± 3.0 | 5.8 ± 2.9 | NS | 5.9 ± 3.1 | 5.9 ± 3.0 | 5.1 ± 2.5 | NS | NS |
| Platelets, ×109/L | 365 ± 120 | 366 ± 118 | 351 ± 132 | NS | 374 ± 119 | 352 ± 120 | .042 | 358 ± 115 | 376 ± 124 | 363 ± 109 | NS | NS |
| Hemoglobin, g/dL | 8.1 ± 1.3 | 8.1 ± 1.2 | 7.7 ± 1.1 | .011 | 8.0 ± 1.3 | 8.3 ± 1.2 | .021 | 7.9 ± 1.1 | 8.2 ± 1.3 | 8.4 ± 1.5 | .006 | NS |
| Reticulocytes, ×109/L | 285 ± 115 | 284 ± 110 | 286 ± 142 | NS | 298 ± 119 | 267 ± 108 | .004 | 286 ± 99 | 290 ± 128 | 270 ± 115 | NS | NS |
| MCV, fL | 79.2 ± 9.0 | 79.2 ± 9.1 | 79.0 ± 7.3 | NS | 82.6 ± 8.2 | 74.8 ± 8.0 | <.001 | 77.5 ± 8.8 | 81.5 ± 9.0 | 80.8 ± 7.9 | <.001 | NS |
| HbF, % | 12.8 ± 8.1 | 13.2 ± 7.9 | 10.2 ± 7.0 | .012 | 13.7 ± 8.5 | 11.7 ± 7.5 | .010 | 10.1 ± 6.7 | 14.6 ± 8.6 | 17.2 ± 8.7 | <.001 | .058 |
| Bilirubin, mmol/L | 37.2 ± 20.9 | 37.8 ± 20.7 | 40.0 ± 23.2 | NS | 39.0 ± 21.4 | 34.6 ± 19.9 | NS | 39.4 ± 18.9 | 39.4 ± 18.9 | 35.0 ± 24.9 | NS | NS |
| LDH, IU/L | 852 ± 384 | 855 ± 369 | 981 ± 554 | NS | 885 ± 402 | 809 ± 355 | NS | 902 ± 423 | 821 ± 336 | 763 ± 324 | NS | NS |
| . | . | G6PD . | α Thalassemia . | β Haplotypes . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall . | Normal . | Deficient . | Normal vs deficient, P . | Absent . | Present . | Absent vs present, P . | CAR/CAR . | BEN/BEN . | SEN/SEN . | BEN/BEN vs CAR/CAR, P . | BEN/BEN vs SEN/SEN, P . | |
| No. of patients | 580 | 429 | 51 | — | 324 | 256 | — | 217 | 141 | 70 | — | — |
| Leukocytes, ×109/L | 13.7 ± 4.7 | 13.6 ± 4.7 | 14.2 ± 4.9 | NS | 14.3 ± 4.8 | 12.9 ± 4.5 | .002 | 13.3 ± 4.8 | 13.8 ± 4.5 | 13.5 ± 4.6 | NS | NS |
| Neutrophils, ×109/L | 5.9 ± 3.0 | 5.9 ± 3.0 | 5.4 ± 2.2 | NS | 5.9 ± 3.0 | 5.8 ± 2.9 | NS | 5.9 ± 3.1 | 5.9 ± 3.0 | 5.1 ± 2.5 | NS | NS |
| Platelets, ×109/L | 365 ± 120 | 366 ± 118 | 351 ± 132 | NS | 374 ± 119 | 352 ± 120 | .042 | 358 ± 115 | 376 ± 124 | 363 ± 109 | NS | NS |
| Hemoglobin, g/dL | 8.1 ± 1.3 | 8.1 ± 1.2 | 7.7 ± 1.1 | .011 | 8.0 ± 1.3 | 8.3 ± 1.2 | .021 | 7.9 ± 1.1 | 8.2 ± 1.3 | 8.4 ± 1.5 | .006 | NS |
| Reticulocytes, ×109/L | 285 ± 115 | 284 ± 110 | 286 ± 142 | NS | 298 ± 119 | 267 ± 108 | .004 | 286 ± 99 | 290 ± 128 | 270 ± 115 | NS | NS |
| MCV, fL | 79.2 ± 9.0 | 79.2 ± 9.1 | 79.0 ± 7.3 | NS | 82.6 ± 8.2 | 74.8 ± 8.0 | <.001 | 77.5 ± 8.8 | 81.5 ± 9.0 | 80.8 ± 7.9 | <.001 | NS |
| HbF, % | 12.8 ± 8.1 | 13.2 ± 7.9 | 10.2 ± 7.0 | .012 | 13.7 ± 8.5 | 11.7 ± 7.5 | .010 | 10.1 ± 6.7 | 14.6 ± 8.6 | 17.2 ± 8.7 | <.001 | .058 |
| Bilirubin, mmol/L | 37.2 ± 20.9 | 37.8 ± 20.7 | 40.0 ± 23.2 | NS | 39.0 ± 21.4 | 34.6 ± 19.9 | NS | 39.4 ± 18.9 | 39.4 ± 18.9 | 35.0 ± 24.9 | NS | NS |
| LDH, IU/L | 852 ± 384 | 855 ± 369 | 981 ± 554 | NS | 885 ± 402 | 809 ± 355 | NS | 902 ± 423 | 821 ± 336 | 763 ± 324 | NS | NS |
Data are presented as mean ± SD unless indicated otherwise. G6PD activity was only available in 480 of 580 patients because splenic sequestration or abnormal cerebral velocities requiring immediate transfusion prevented accurate measures of activity.
NS, not significant.
Impact of G6PD deficiency.
G6PD activity was found abnormally low in 51 of the 480 assessed patients (10.6%). The prevalence of G6PD deficiency was 16.5% (38/231) in males and 5.2% (13/249) in females. Patients with G6PD deficiency had lower Hb levels than those with normal activity (Table 1) in the overall cohort (7.7 ± 1.1 vs 8.1 ± 1.2 g/dL, P = .011). However, the impact of G6PD deficiency was different in the 3 major homozygous β haplotypes, as Hb (mean ± SD) was significantly lower in CAR/CAR patients with G6PD deficiency (7.3 ± 0.9 vs 7.9 ± 1.0 g/dL, P = .005), whereas no difference was observed in BEN/BEN and SEN/SEN patients (Figure 2A).
Biological parameters at baseline. Impact of G6PD deficiency and the number of α genes and β haplotypes (boxplots showing the median and interquartile range for each biological parameter). (A) Hb at baseline as a function of the presence or absence of G6PD deficiency in the 3 major homozygous β haplotypes. Median (quartile 1 [Q1] to Q3) hemoglobin was significantly lower in CAR/CAR patients with G6PD deficiency (7.3 [6.6-7.8] g/dL) than in CAR/CAR patients with normal G6PD activity (7.9 [7.2-8.5] g/dL; P = .005), whereas no such difference was observed in BEN/BEN and SEN/SEN patients. (B) MCV at baseline as a function of the number of present α genes. In the absence of α thalassemia (4 α genes), median (Q1-Q3) MCV was 82.8 (77.8-88.0) fL, whereas MCV was significantly lower in patients with α thalassemia (76.8 [73.0-81.3] fL in patients with 1 deleted gene and 67.5 [63.1-70.2] fL in those with 2 deleted genes). (C) HbF at baseline as a function of the major homozygous β haplotypes. Median (Q1-Q3) HbF was significantly lower in CAR/CAR patients (8.8% [4.8-14.0%]) than in BEN/BEN patients (12.8% [7.9-20.0%]; P < .001) and SEN/SEN patients (15.1% [10.0-23.5%]; P < .001), whereas the difference between BEN/BEN and SEN/SEN was not significant (P = .058). (D) Hb at baseline as a function of α-thalassemia presence or absence in the 3 major homozygous β haplotypes. The impact of α thalassemia on Hb was higher in CAR/CAR patients. Median (Q1-Q3) hemoglobin was 7.9 (7.4-8.5) g/dL in patients with α thalassemia vs 7.4 (6.8-8.1) g/dL in those without α thalassemia (P < .001). No significant difference was observed among BEN/BEN and SEN/SEN patients. Hb was significantly lower in CAR/CAR patients than in BEN/BEN patients in those with (P = .022) or without α thalassemia (P = .003). (E) LDH at baseline as a function of the number of α genes in the 3 major homozygous β haplotypes. The impact of α thalassemia on LDH was higher in CAR/CAR patients. Median (Q1-Q3) LDH was 773 (564-1018) IU/L in patients with α thalassemia vs 946 (686-1274) IU/L in those without α thalassemia (P = .005). No significant difference was observed among BEN/BEN and SEN/SEN patients. LDH was significantly higher in CAR/CAR patients than in BEN/BEN patients with or without α thalassemia (P = .014).
Biological parameters at baseline. Impact of G6PD deficiency and the number of α genes and β haplotypes (boxplots showing the median and interquartile range for each biological parameter). (A) Hb at baseline as a function of the presence or absence of G6PD deficiency in the 3 major homozygous β haplotypes. Median (quartile 1 [Q1] to Q3) hemoglobin was significantly lower in CAR/CAR patients with G6PD deficiency (7.3 [6.6-7.8] g/dL) than in CAR/CAR patients with normal G6PD activity (7.9 [7.2-8.5] g/dL; P = .005), whereas no such difference was observed in BEN/BEN and SEN/SEN patients. (B) MCV at baseline as a function of the number of present α genes. In the absence of α thalassemia (4 α genes), median (Q1-Q3) MCV was 82.8 (77.8-88.0) fL, whereas MCV was significantly lower in patients with α thalassemia (76.8 [73.0-81.3] fL in patients with 1 deleted gene and 67.5 [63.1-70.2] fL in those with 2 deleted genes). (C) HbF at baseline as a function of the major homozygous β haplotypes. Median (Q1-Q3) HbF was significantly lower in CAR/CAR patients (8.8% [4.8-14.0%]) than in BEN/BEN patients (12.8% [7.9-20.0%]; P < .001) and SEN/SEN patients (15.1% [10.0-23.5%]; P < .001), whereas the difference between BEN/BEN and SEN/SEN was not significant (P = .058). (D) Hb at baseline as a function of α-thalassemia presence or absence in the 3 major homozygous β haplotypes. The impact of α thalassemia on Hb was higher in CAR/CAR patients. Median (Q1-Q3) hemoglobin was 7.9 (7.4-8.5) g/dL in patients with α thalassemia vs 7.4 (6.8-8.1) g/dL in those without α thalassemia (P < .001). No significant difference was observed among BEN/BEN and SEN/SEN patients. Hb was significantly lower in CAR/CAR patients than in BEN/BEN patients in those with (P = .022) or without α thalassemia (P = .003). (E) LDH at baseline as a function of the number of α genes in the 3 major homozygous β haplotypes. The impact of α thalassemia on LDH was higher in CAR/CAR patients. Median (Q1-Q3) LDH was 773 (564-1018) IU/L in patients with α thalassemia vs 946 (686-1274) IU/L in those without α thalassemia (P = .005). No significant difference was observed among BEN/BEN and SEN/SEN patients. LDH was significantly higher in CAR/CAR patients than in BEN/BEN patients with or without α thalassemia (P = .014).
Impact of α thalassemia.
The mean corpuscular volume (MCV) was particularly influenced by the number of α genes (Figure 2B). Multivariate linear regression analysis showed that only the number of α genes had a significant and positive impact on MCV (β = 0.503, P < 10−31), leukocytes (β = 0.167, P = .0002), platelets (β = 0.163, P = .0004), and reticulocytes (β = 0.162, P = .001), whereas it was negatively correlated with Hb (β = −0.215, P < 10−5) and positively correlated with lactate dehydrogenase (LDH) (β = 0.206, P = .0002) independently of CAR-haplotype numbers.
Impact of β haplotypes.
HbF was positively correlated with BEN and SEN haplotypes and negatively correlated with CAR-haplotype numbers in univariate analysis, and multivariate analysis showed that HbF was independently correlated with CAR- (β = −0.196, P = 10−5) and SEN-haplotype numbers (β = 0.134, P = .003). Thus, CAR/CAR patients had the lowest HbF levels (Figure 2C). The number of CAR haplotypes was negatively correlated with Hb (β = −0.235, P < 10−6) and positively correlated with LDH (β = 0.178, P = .001) independent of the number of α genes.
Impact of genetic markers on known severity criteria for SCA (severe anemia or low HbF)
The baseline levels of Hb were <7 g/dL in 16.8% of the cohort. Absence of α thalassemia (odds ratio [OR] = 3.9; 95% CI, 2.1-7.2; P < .001), presence of G6PD deficiency (OR = 2.105; 95% CI, 1.004-4.428; P = .049), and the number of CAR haplotypes (OR = 1.4; 95% CI, 1.01-1.8; P = .043) were retained as significant and independent predictive risk factors for baseline Hb <7 g/dL by multivariate logistic regression analysis. Thus, as shown in Figure 2D-E, CAR/CAR patients without α thalassemia had the most severe hemolytic anemia; ie, lowest Hb levels (P < .001) and highest LDH levels (P = .001).
Baseline HbF levels were <5% in 17.1% of the cohort. Multivariate logistic regression analysis retained only the number of CAR haplotypes as a significant risk factor for baseline HbF <5% (OR = 1.58; 95% CI, 1.12-2.21; P = .009).
Prevalence of different SNPs in the 3 major homozygous β haplotypes
SNPs were assessed in 222 newborn SS patients recently referred to the Department of Genetics (Henri Mondor Hospital, University Paris Est), including 167 with homozygous β haplotypes (ie, CAR/CAR [n = 88], BEN/BEN [n = 65], and SEN/SEN [n = 14]) (Table 2).
Prevalence in the homozygous β haplotypes of the different alleles in the 3 loci known to impact HbF expression
| Patients (n = 222) . | CAR/CAR (n = 88), % . | BEN/BEN (n = 65), % . | SEN/SEN (n = 14), % . | |||
|---|---|---|---|---|---|---|
| Loci . | Allele . | |||||
| HBG2 | rs7482144 | chr11:5276169 | C/C | 100 | 100 | 0 |
| C/T | 0 | 0 | 0 | |||
| T/T | 0 | 0 | 100 | |||
| BCL11A | rs10189857 | chr2:60713235 | A/A | 44.3 | 53.8 | 57.1 |
| A/G | 45.5 | 40.0 | 35.8 | |||
| G/G | 10.2 | 6.2 | 7.1 | |||
| rs1427407 | chr2:60718043 | G/G | 65.9 | 40 | 42.9 | |
| G/T | 23.9 | 38.5 | 35.7 | |||
| T/T | 10.2 | 21.5 | 21.4 | |||
| HBS1L-MYB | rs9399137 | chr6:135419018 | T/T | 92 | 87.7 | 78.6 |
| C/T | 6.9 | 12.3 | 21.4 | |||
| C/C | 1.1 | 0 | 0 | |||
| Patients (n = 222) . | CAR/CAR (n = 88), % . | BEN/BEN (n = 65), % . | SEN/SEN (n = 14), % . | |||
|---|---|---|---|---|---|---|
| Loci . | Allele . | |||||
| HBG2 | rs7482144 | chr11:5276169 | C/C | 100 | 100 | 0 |
| C/T | 0 | 0 | 0 | |||
| T/T | 0 | 0 | 100 | |||
| BCL11A | rs10189857 | chr2:60713235 | A/A | 44.3 | 53.8 | 57.1 |
| A/G | 45.5 | 40.0 | 35.8 | |||
| G/G | 10.2 | 6.2 | 7.1 | |||
| rs1427407 | chr2:60718043 | G/G | 65.9 | 40 | 42.9 | |
| G/T | 23.9 | 38.5 | 35.7 | |||
| T/T | 10.2 | 21.5 | 21.4 | |||
| HBS1L-MYB | rs9399137 | chr6:135419018 | T/T | 92 | 87.7 | 78.6 |
| C/T | 6.9 | 12.3 | 21.4 | |||
| C/C | 1.1 | 0 | 0 | |||
For each locus, the favorable allele for HbF expression is in bold.
β-Globin cluster: HBG2 promoter (HBG2:g −158 C>T).
For the rs7482144 C/T SNP, the favorable allele for HbF expression (T) was only and exclusively present as homozygous T/T in SEN/SEN patients.
BCL11A.
For the rs10189857 A/G SNP, no significant association was found between the favorable allele A and β haplotypes. In contrast, for the rs1427407 G/T SNP, the favorable T allele for HbF expression was significantly positively correlated with the number of BEN haplotypes (r = 0.180, P = .009) and negatively correlated with the number of CAR haplotypes (r = −0.176, P = .011) and was not correlated with SEN-haplotype and CAM-haplotype numbers. The prevalence of the favorable T allele was significantly higher in BEN/BEN patients than in CAR/CAR patients (P = .006).
HMIP region.
For the rs9399137 T/C SNP, no significant association was found between the favorable C allele and β haplotypes.
Indications for HU treatment
In this cohort, 168 patients (85 females and 83 males) were treated with HU at the median dose of 26 mg/kg per day for at least 1 year between February 1993 and December 2014 and at the mean ± SD age of 7.5 ± 3.6 years. Patients with a history of frequent VOC associated or not with ACS (n = 109), isolated severe anemia (n = 13), a history of abnormal but normalized- TCD on chronic transfusion (n = 44), and priapism (n = 2) were treated with HU. The prevalence of CAR/CAR was significantly higher (42.9% vs 32.6%, P = .02) and that of SEN/SEN lower (9.4% vs 15.2%, P = .053 [NS]) in HU-treated patients than in nontreated patients, reflecting the degree of severity of these homozygous β haplotypes.
Biological parameters with HU treatment
Changes from HU initiation T0 (Δ HU-T0) and parameter values for HU treatment (Table 3) were highly correlated with their value at T0. Thus, in order to evaluate the impact of genetic markers, changes and values with HU treatment were adjusted with their values at T0 in the multivariate linear regression analysis.
Biological parameters during HU treatment: changes from T0 (ΔHU-T0) and values in patients with or without α thalassemia and in the 3 major homozygous β haplotypes
| . | α Thalassemia . | β Haplotypes . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | |||||
| Absent . | Present . | Abs vs present, P . | Absent . | Present . | Absent vs present, P . | CAR/CAR . | BEN/BEN . | SEN/SEN . | CAR/CAR vs BEN/BEN, P . | |||||
| No. of patients | 84 | 69 | — | 84 | 69 | — | 77 | 41 | 12 | — | — | |||
| Leukocytes, ×109/L | −5.3 ± 4.8 | −5.7 ± 5.8 | NS | 8.9 ± 3.6 | 7.8 ± 4.2 | NS | −5.8 ± 5.1 | 7.5 ± 3.4 | −6.3 ± 5.6 | 8.7 ± 4.1 | −1.9 ± 3.1 | 11.2 ± 4.2 | NS | NS |
| Neutrophils, ×109/L | −2.8 ± 6.3 | −3.1 ± 3.9 | NS | 4.7 ± 4.7 | 3.7 ± 2.3 | NS | −3.5 ± 3.7 | 3.4 ± 1.6 | −3.0 ± 3.4 | 3.9 ± 2.8 | −1.2 ± 3.3 | 6.2 ± 3.4 | NS | NS |
| Platelets, ×109/L | −43 ± 127 | −88 ± 112 | .023 | 344 ± 121 | 285 ± 119 | .002 | −51 ± 112 | 305 ± 118 | −105 ± 137 | 319 ± 132 | −5 ± 163 | 389 ± 161 | .037 | NS |
| Hb, g/dL | 0.7 ± 1.2 | 0.7 ± 1.3 | NS | 8.7 ± 1.2 | 8.8 ± 1.2 | NS | 0.7 ± 1.2 | 8.5 ± 1.1 | 1.0 ± 1.3 | 9.2 ± 1.3 | 0.8 ± 0.8 | 8.9 ± 1.4 | NS | .007 |
| Reticulocytes, ×109/L | −142 ± 122 | −117 ± 101 | NS | 184 ± 90 | 158 ± 73 | .048 | −129 ± 96 | 162 ± 71 | −144 ± 120 | 168 ± 82 | −89 ± 109 | 210 ± 98 | NS | NS |
| MCV, fL | 16.1 ± 9.6 | 11.7 ± 8.0 | .003 | 100.4 ± 12.1 | 86.1 ± 9.2 | <.001 | 13.6 ± 9.9 | 91.5 ± 13.3 | 17.2 ± 9.1 | 99.3 ± 13.3 | 12.0 ± 4.3 | 91 ± 11.8 | NS | .003 |
| HbF, % | 10.1 ± 6.8 | 8.1 ± 7.7 | NS | 18.7 ± 8.2 | 17.1 ± 8.1 | NS | 8.3 ± 7.4 | 16.6 ± 7.4 | 12.1 ± 8.4 | 21.2 ± 9.6 | 8.3 ± 7.7 | 19.1 ± 9.2 | .026 | .005 |
| Bilirubin, mmol/L | −11.0 ± 29.1 | −7.5 ± 22.1 | NS | 38.8 ± 28.6 | 32.9 ± 21.0 | NS | −9.3 ± 25.8 | 37 ± 24 | −14.7 ± 20.8 | 30 ± 31 | −5.1 ± 27.3 | 39 ± 33 | NS | NS |
| LDH, IU/L | −207 ± 340 | −177 ± 256 | NS | 696 ± 669 | 575 ± 264 | NS | −181 ± 280 | 604 ± 281 | −298 ± 347 | 734 ± 929 | −63 ± 101 | 520 ± 234 | NS | NS |
| . | α Thalassemia . | β Haplotypes . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | ΔHU-T0 . | With HU . | |||||
| Absent . | Present . | Abs vs present, P . | Absent . | Present . | Absent vs present, P . | CAR/CAR . | BEN/BEN . | SEN/SEN . | CAR/CAR vs BEN/BEN, P . | |||||
| No. of patients | 84 | 69 | — | 84 | 69 | — | 77 | 41 | 12 | — | — | |||
| Leukocytes, ×109/L | −5.3 ± 4.8 | −5.7 ± 5.8 | NS | 8.9 ± 3.6 | 7.8 ± 4.2 | NS | −5.8 ± 5.1 | 7.5 ± 3.4 | −6.3 ± 5.6 | 8.7 ± 4.1 | −1.9 ± 3.1 | 11.2 ± 4.2 | NS | NS |
| Neutrophils, ×109/L | −2.8 ± 6.3 | −3.1 ± 3.9 | NS | 4.7 ± 4.7 | 3.7 ± 2.3 | NS | −3.5 ± 3.7 | 3.4 ± 1.6 | −3.0 ± 3.4 | 3.9 ± 2.8 | −1.2 ± 3.3 | 6.2 ± 3.4 | NS | NS |
| Platelets, ×109/L | −43 ± 127 | −88 ± 112 | .023 | 344 ± 121 | 285 ± 119 | .002 | −51 ± 112 | 305 ± 118 | −105 ± 137 | 319 ± 132 | −5 ± 163 | 389 ± 161 | .037 | NS |
| Hb, g/dL | 0.7 ± 1.2 | 0.7 ± 1.3 | NS | 8.7 ± 1.2 | 8.8 ± 1.2 | NS | 0.7 ± 1.2 | 8.5 ± 1.1 | 1.0 ± 1.3 | 9.2 ± 1.3 | 0.8 ± 0.8 | 8.9 ± 1.4 | NS | .007 |
| Reticulocytes, ×109/L | −142 ± 122 | −117 ± 101 | NS | 184 ± 90 | 158 ± 73 | .048 | −129 ± 96 | 162 ± 71 | −144 ± 120 | 168 ± 82 | −89 ± 109 | 210 ± 98 | NS | NS |
| MCV, fL | 16.1 ± 9.6 | 11.7 ± 8.0 | .003 | 100.4 ± 12.1 | 86.1 ± 9.2 | <.001 | 13.6 ± 9.9 | 91.5 ± 13.3 | 17.2 ± 9.1 | 99.3 ± 13.3 | 12.0 ± 4.3 | 91 ± 11.8 | NS | .003 |
| HbF, % | 10.1 ± 6.8 | 8.1 ± 7.7 | NS | 18.7 ± 8.2 | 17.1 ± 8.1 | NS | 8.3 ± 7.4 | 16.6 ± 7.4 | 12.1 ± 8.4 | 21.2 ± 9.6 | 8.3 ± 7.7 | 19.1 ± 9.2 | .026 | .005 |
| Bilirubin, mmol/L | −11.0 ± 29.1 | −7.5 ± 22.1 | NS | 38.8 ± 28.6 | 32.9 ± 21.0 | NS | −9.3 ± 25.8 | 37 ± 24 | −14.7 ± 20.8 | 30 ± 31 | −5.1 ± 27.3 | 39 ± 33 | NS | NS |
| LDH, IU/L | −207 ± 340 | −177 ± 256 | NS | 696 ± 669 | 575 ± 264 | NS | −181 ± 280 | 604 ± 281 | −298 ± 347 | 734 ± 929 | −63 ± 101 | 520 ± 234 | NS | NS |
Data are presented as mean ± SD unless indicated otherwise.
No significant difference was found in the dose of HU independent of α- or β-haplotype status.
None of the changes in and values of biological parameters during HU treatment were significantly impacted by the presence or absence of G6PD deficiency.
The number of α genes was correlated with the change in (β = 0.505, P < 10−5) and value of MCV (β = 0.370, P < 10−5) and the value of platelets (β = 0.202, P = .005) with HU treatment. Thus, the increase in MCV was significantly lower in patients with α thalassemia than in those without α thalassemia (11.7 ± 8.0 vs 16.1± 9.6 fL, P = .003), thereby increasing the MCV difference between patients with or without α thalassemia who were receiving HU (86.1 ± 9.2fL vs 100.4 ± 12.1, P < .001). As shown in Figure 3A, MCV was <82 fL in 75% of the patients with 2 deleted α genes, while lower than 95fL in 75% of those with 1 deleted gene. As shown in Figure 3B, HU-treated patients with α thalassemia had a greater decrease in platelets.
Biological parameters with HU therapy. (A) MCV during HU therapy as a function of the number of α genes. In the absence of α thalassemia, (4 α genes), median (Q1-Q3) MCV during HU treatment was 100 (93-106.9) fL, and it was significantly lower (P < .001) in α-thalassemia patients with 1 deleted gene (88.1 [84.9-96.0] fL) or 2 deleted genes (74.7 [70.7-82.3] fL). (B) Platelets during HU therapy as a function of the number of α genes. Platelet counts were significantly lower in HU-treated patients with α thalassemia. Median (Q1-Q3) platelet count was 217 × 109/L (172-372) in patients with 2 deleted genes, 284 × 109/L (217-378) in those with 1 deleted gene, and 350 × 109/L (274-408) in those without α thalassemia. (C) Delta HbF on HU was significantly higher (P = .026) in BEN/BEN than in CAR/CAR patients. (D) HbF during HU treatment in the 3 major homozygous β haplotypes. Median (Q1-Q3) HbF with HU therapy was significantly higher in BEN/BEN patients (21.1% [13.7-27.6%] than in SEN/SEN patients (20.9% [11.6-24.8%]) (P = .016) and CAR/CAR patients (16.7% [11.4-20.1%] (P = .005). (E) Hb levels with HU treatment in the 3 major homozygous β haplotypes. Median (Q1-Q3) Hb levels during HU therapy were significantly higher in BEN/BEN patients (9.4 [8.1-10.1] g/dL) than in SEN/SEN patients (8.7 [7.8-9.8] g/dL) (P = .017) and CAR/CAR patients (8.4 [7.8-9.2] g/dL) (P = .007).
Biological parameters with HU therapy. (A) MCV during HU therapy as a function of the number of α genes. In the absence of α thalassemia, (4 α genes), median (Q1-Q3) MCV during HU treatment was 100 (93-106.9) fL, and it was significantly lower (P < .001) in α-thalassemia patients with 1 deleted gene (88.1 [84.9-96.0] fL) or 2 deleted genes (74.7 [70.7-82.3] fL). (B) Platelets during HU therapy as a function of the number of α genes. Platelet counts were significantly lower in HU-treated patients with α thalassemia. Median (Q1-Q3) platelet count was 217 × 109/L (172-372) in patients with 2 deleted genes, 284 × 109/L (217-378) in those with 1 deleted gene, and 350 × 109/L (274-408) in those without α thalassemia. (C) Delta HbF on HU was significantly higher (P = .026) in BEN/BEN than in CAR/CAR patients. (D) HbF during HU treatment in the 3 major homozygous β haplotypes. Median (Q1-Q3) HbF with HU therapy was significantly higher in BEN/BEN patients (21.1% [13.7-27.6%] than in SEN/SEN patients (20.9% [11.6-24.8%]) (P = .016) and CAR/CAR patients (16.7% [11.4-20.1%] (P = .005). (E) Hb levels with HU treatment in the 3 major homozygous β haplotypes. Median (Q1-Q3) Hb levels during HU therapy were significantly higher in BEN/BEN patients (9.4 [8.1-10.1] g/dL) than in SEN/SEN patients (8.7 [7.8-9.8] g/dL) (P = .017) and CAR/CAR patients (8.4 [7.8-9.2] g/dL) (P = .007).
The univariate regression analysis showed that BEN- and CAR-haplotype numbers were inversely correlated with the changes and values of HbF, but multivariate regression analysis retained only the number of BEN haplotypes as a significant factor positively associated with HbF increase (β = 0.200, P = .014) and value (β = 0.174, P = .014) with HU. Thus, the mean ± SD increase of HbF with HU (Δ HU-T0) was significantly more increased in BEN/BEN patients than in CAR/CAR patients (+12.1 ± 8.4% vs +8.3 ± 7.4%, respectively; P = .026) (Figure 3C). BEN/BEN patients had a significantly higher HbF levels than CAR/CAR patients (21.2 ± 9.6 vs 16.6 ± 7.4, P = .005) and even SEN/SEN patients (19.1 ± 9.2%, P = .014) (Figure 3D). With HU treatment, HbF levels were still <20% in 70% of CAR/CAR patients vs 39% of BEN/BEN and 42% SEN/SEN patients (P = .003). BEN/BEN patients were significantly less anemic than CAR/CAR (P = .007) and SEN/SEN patients (P = .017) (Figure 3E).
Discussion
Most published studies that reported on β haplotypes were until now from cohorts in the United States, Jamaica, and West Indies, where the BEN haplotype is largely predominant. In a US study by Powars et al,26 among 221 SS patients from a Los Angeles cohort, 38% were BEN/BEN, 5% were CAR/CAR, and only 1% was SEN/SEN; all others were heterozygous. Thereafter, similar percentages of β haplotypes were reported in cohorts from Jamaica27 and West Indies.28 In contrast, Brazilian studies reported a number of CAR homozygotes comparable to our cohort (34%) but no SEN haplotypes.29 Only studies from Venezuela30 reported the presence of all 3 β haplotypes (ie, BEN [51%], CAR [29.5%], SEN [12.5%], and CAM [2.5%]); however, only 8.6% were BEN/BEN, reflecting the very high frequency of admixture in their population of African origin. There are only few studies on β haplotypes in Africa. In Cameroon,31 the BEN haplotype is the most prevalent (66.3%), whereas in Congo-Brazzaville32 and in Uganda,33 91% and 94% of SS patients, respectively, are CAR/CAR. In the present French cohort of 580 SS children, all 3 major β haplotypes are well represented, with a high proportion of homozygosity (74%) and a predominance of CAR/CAR (37.4%), followed by BEN/BEN (24.3%), and SEN/SEN (12.1%). This allowed us to determine the relative impact of G6PD, α genes, and the 3 major β haplotypes on biological parameters at baseline and with HU treatment.
At baseline, we found here that CAR/CAR patients with G6PD deficiency had significantly lower Hb levels than those with normal G6PD activity, whereas no such difference was observed in BEN/BEN patients. These results can explain some of the discrepancies between several previous studies.3-8 Steinberg et al reported no significant impact of G6PD deficiency on the biological parameters in SCA males from the Cooperative Study of Sickle Cell Disease; however, homozygous CAR was rare (3%), and the BEN haplotype was highly predominant.4 In agreement with the report from Nouraie et al,8 we did not find that hemolysis was greater in patients with G6PD deficiency than in those with normal activity (i.e., the reticulocyte count and bilirubin and LDH levels were similar in both groups), suggesting that anemia in these patients was most likely due to a decreased production of erythrocytes, as reported by Paglialunga et al.34
The number of α genes had a significant and positive impact by multivariate analyses on baseline MCV, leukocyte and platelet counts, and bilirubin and LDH levels and a negative impact on baseline Hb levels independent of age at baseline. Thus, patients with α thalassemia had less severe hemolytic anemia and lower leukocyte and platelet counts. To our knowledge, this impact on leukocytes and platelets has not been reported before and could possibly contribute to the beneficial effects of α thalassemia on patient survival12 and frequency of crises35 by lowering viscosity. At baseline, in the Baby-Hug trial,36 patients with α thalassemia (75/189) had significantly lower MCV, mean corpuscular Hb, reticulocytes, and bilirubin levels, but leukocyte and platelet counts were not reported, and the presence of α thalassemia had no effect on Hb levels, probably in part because of the lower number of patients in their cohort, but the favorable impact of α thalassemia on Hb was only observed in CAR/CAR patients and not in BEN/BEN patients in our study. The favorable impact of α thalassemia in SCA (ie, decreased hemolysis9 and improved survival12 ) was reported some 30 years ago. Studies have shown that the presence of α thalassemia inhibits the formation of high density and high mean corpuscular Hb concentration sickle red blood cells.11 However, the higher Hb level due to α thalassemia may also increase the rate of VOC events37 and osteonecrosis.38 We confirm here previous reports showing that the effect of α thalassemia on HbF found by univariate analysis is no longer significant after adjusting for β haplotypes39 and show that this apparent influence of α thalassemia is related to its variable prevalence in the different β haplotypes.
The impact of β haplotypes was highly significant in multivariate analyses. In the present cohort, we confirm that HbF is highest in SEN/SEN, lowest in CAR/CAR, and intermediate in BEN/BEN patients, in agreement with older studies.40 Furthermore, we show that HbF level is independently positively correlated with SEN-haplotype numbers and strongly negatively correlated with CAR-haplotype numbers. The association between HbF level and XmnI polymorphism (rs7482144) in the proximal promoter of the Gγ-globin (HBG2) gene, present in the SEN and Arab-Indian haplotypes, is well documented.41 However, we demonstrate for the first time that in the BCL11A locus (rs1427407), the favorable T allele for HbF expression, is significantly positively correlated with BEN-haplotype numbers and negatively correlated with CAR-haplotype numbers, providing a first possible explanation for higher HbF levels in patients with BEN compared with those with CAR haplotypes.
We clearly show in the present cohort that the number of CAR haplotypes is negatively correlated with Hb and positively with LDH, independent of α-gene status. Thus, CAR/CAR patients had the most severe hemolytic anemia (particularly in the absence of α thalassemia) and the lowest HbF level. These results are in agreement with the observation by Powars et al that patients with CAR haplotypes have early-onset irreversible organ failure. The degrees of anemia and hemolysis are the major risk factors for cerebral vasculopathy7,42-46 ; thus, the high prevalence of CAR haplotypes in our cohort may explain the high incidence of cerebral vasculopathy.7,44,46
This study shows the important impact of the number of α-genes on HU treatment, which positively correlated with changes in and values of MCV and platelets. Thus, patients with α thalassemia had a lower increase in MCV while taking HU. This highlights the importance of determining the α-gene status in patients treated with HU before raising the issue of noncompliance on MCV and to preferentially use the neutrophil count as a marker of compliance. This result is in agreement with one report47 and contrasts with another one,36 which showed no difference, but the number of patients with α thalassemia was lower than in our cohort. We also found a significant impact of β haplotypes. The number of BEN haplotypes positively influenced the increase in and value of HbF during HU treatment. This effect of BEN haplotypes on the HbF levels during HU treatment has not been reported in US studies,36,48 probably because of the high predominance of BEN haplotypes and the rarity of CAR haplotypes in US cohorts. Our observation that the BCL11a rs1427407 T allele, which is favorable for HbF expression, was positively associated with the BEN haplotypes and negatively correlated with CAR haplotypes could be a novel explanation for the differences observed in HbF level at baseline and with HU treatment in BEN/BEN and CAR/CAR patients. It was recently reported in a Brazilian cohort49 that 2 BCL11A SNPs (rs1427407 and rs4671393) were associated with increased baseline and maximum tolerated dose HbF, but they did not report data on β haplotypes. Nevertheless, it is known that the prevalence of SEN haplotypes is very low in Brazil, whereas that of CAR/CAR patients is similar to that of our cohort. In our cohort, no link was found with the HMIP/rs9399137/C, but that may be related to its low prevalence in the African population (rs9399137/C frequency: 0.0416 from the “1000Genome” database and 0.0398 from the HAPMAP-YRI database). Despite a significant increase in Hb levels with HU in our study, CAR/CAR patients still had the most severe anemia. Considering the significant impact of the degree of anemia3,7 on the risk of abnormal TCD, this finding may explain the risk of abnormal TCD recurrence (13/45) observed in our cohort following a switch to HU,46 contrary to the TWiTCH trial50 performed in a US cohort with a high BEN-haplotype predominance in which noninferiority of HU compared with chronic transfusion was demonstrated.
This study has several limitations. The Sanger sequencing of the “Pre Gγ framework” region used for β-globin haplotype assessment does not allow differentiating the Arab India (AI) haplotype from the SEN one. This limitation should not affect our study, because (1) the AI haplotype has only been found in tribes in India and in population of the eastern part of Saudi Arabia, and most of our patients originate from Western and Central Africa. In addition, none of the published studies in SCD patients from Eastern Africa have reported the presence of the AI haplotype51-54 ; and (2) the SEN and the AI haplotypes share the rs 7482144 C/T, which is the strongest predictor of HbF expression, and is located on chromosome 11, even in the AI group55-57 In our study, G6PD deficiency was not defined by gene analysis but by abnormally low G6PD activity. As G6PD deficiency is caused by mutations in the X-linked G6PD gene, heterozygous females may have normal G6PD activity or be deficient.57 Thus, gene analysis does not allow detection of G6PD deficiency in heterozygous girls. Moreover, even if the G6PD A− allele, which contains 2 mutations, G376A and G202A, is the most common G6PD deficiency variant in Africa, 160 mutations of the G6PD gene have been identified,58-61 justifying that G6PD deficiency be detected by using methods measuring activity such as a spectrophotometric assay, provided that reticulocytosis is assessed and the comparison with hexokinase activity is done to control for the effects of cell age. HU was only prescribed to patients with severe disease, and results might have been different in a nonselected population. Moreover, we can speculate that earlier initiation or higher doses or longer treatment times of HU could have improved the response. SNPs were only available in patients recently referred to the Department of Genetics (Henri Mondor Hospital, University Paris Est) (newborns for whom baseline HbF and HbF response to HU are not yet available). Thus, we can only suggest that the higher HbF increase observed in BEN/BEN patients could be partly explained by a higher prevalence of the favorable BCL11a/rs1427407/T allele. To further assess the impact of BCL11A on baseline HbF and response to HU in the recent newborn cohort, a longer follow-up will be required.
Moreover, the associations between biological parameters and genetic markers described here are only descriptive and do not demonstrate a causal relationship. We also acknowledge that our results will need to be further validated in independent cohorts. Finally, only the association of the 4 SNPs known to influence HbF level with the different β haplotypes was investigated. Until now, apart from the well-known rs7482144/C, no SNP or SNPs sets have been validated in SCA as outcome predictors as in β thalassemia, for which a severity score was used to predict age at first transfusion.25 The choice of HMIP-SNP was derived from that of thalassemic patients25 and appears to be inaccurate for the present African SCA cohort. Considering the striking differences in α-thalassemia and G6PD-deficiency prevalence observed in the different β haplotypes, one could speculate that similar differences exist in BCL11A and HMIP variants. However, genome-wide association studies require complete clinical and biological data for valid statistical analysis, which may be difficult to obtain in SCA patients. For this reason, we believe that the baseline biological parameters recorded before initiating HU or other intensive therapy may have an important predictive value, as they are representative of the influence of all genetic biomarkers on the different components of the blood.
In conclusion, this large cohort study with all major β haplotypes well represented shows that the number of CAR haplotypes is the strongest risk factor associated with low baseline HbF, whereas the severity of hemolytic anemia is positively and independently correlated with α-gene and CAR-haplotype numbers. We confirm the efficiency of HU at increasing HbF, Hb, and MCV and decreasing leukocytes, platelets, reticulocytes, bilirubin, and LDH in all patients independently of their α status or β haplotype and that HU treatment should not be limited to certain genetic subsets. However, we show that the increase and value of HbF with HU treatment are positively correlated with the BEN-haplotype number and that BEN/BEN patients have the highest HbF while taking HU. This could be partly explained by our findings that the BEN and CAR haplotypes are inversely correlated with the favorable BCL11a/rs1427407/T allele for HbF expression, which is more prevalent in BEN/BEN patients than in CAR/CAR patients. Nevertheless, CAR/CAR patients taking HU have the most severe anemia and the lowest HbF level. This could explain the smaller favorable effect of HU observed in our French cohort in patients with cerebral vasculopathy,46 and it suggests that HU treatment might have a less beneficial impact in patients from countries in Central Africa, where the CAR haplotype is almost exclusively present.
Acknowledgments
The authors thank Martine Torres for her critical reading of the manuscript and editorial assistance.
This work was supported in part by the French Ministry of Health (institutional grant IDF05001).
Authorship
Contribution: F.B., C.A., A.K., C.P., and S.P. conceived and designed the study; S.P. performed the molecular biology studies and analyzed the data; F.L. assayed the hematological parameters and analyzed the data; C.A., A.K., C.P., F.B., and S.P. collected and interpreted the data; F.B. performed the statistical analyses; F.B. and S.P. wrote the manuscript in collaboration with C.A., A.K., and C.P.; C.A., A.K., I.H., C.P., R.E., and F.B. participated in the management of patient care; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Bernaudin, Pédiatrie, Centre de Référence des Syndromes Drépanocytaires Majeurs, Centre Hospitalier Intercommunal de Créteil, 40 Ave de Verdun, 94010 Créteil, France; e-mail: francoise.bernaudin@chicreteil.fr.

![Figure 2. Biological parameters at baseline. Impact of G6PD deficiency and the number of α genes and β haplotypes (boxplots showing the median and interquartile range for each biological parameter). (A) Hb at baseline as a function of the presence or absence of G6PD deficiency in the 3 major homozygous β haplotypes. Median (quartile 1 [Q1] to Q3) hemoglobin was significantly lower in CAR/CAR patients with G6PD deficiency (7.3 [6.6-7.8] g/dL) than in CAR/CAR patients with normal G6PD activity (7.9 [7.2-8.5] g/dL; P = .005), whereas no such difference was observed in BEN/BEN and SEN/SEN patients. (B) MCV at baseline as a function of the number of present α genes. In the absence of α thalassemia (4 α genes), median (Q1-Q3) MCV was 82.8 (77.8-88.0) fL, whereas MCV was significantly lower in patients with α thalassemia (76.8 [73.0-81.3] fL in patients with 1 deleted gene and 67.5 [63.1-70.2] fL in those with 2 deleted genes). (C) HbF at baseline as a function of the major homozygous β haplotypes. Median (Q1-Q3) HbF was significantly lower in CAR/CAR patients (8.8% [4.8-14.0%]) than in BEN/BEN patients (12.8% [7.9-20.0%]; P < .001) and SEN/SEN patients (15.1% [10.0-23.5%]; P < .001), whereas the difference between BEN/BEN and SEN/SEN was not significant (P = .058). (D) Hb at baseline as a function of α-thalassemia presence or absence in the 3 major homozygous β haplotypes. The impact of α thalassemia on Hb was higher in CAR/CAR patients. Median (Q1-Q3) hemoglobin was 7.9 (7.4-8.5) g/dL in patients with α thalassemia vs 7.4 (6.8-8.1) g/dL in those without α thalassemia (P < .001). No significant difference was observed among BEN/BEN and SEN/SEN patients. Hb was significantly lower in CAR/CAR patients than in BEN/BEN patients in those with (P = .022) or without α thalassemia (P = .003). (E) LDH at baseline as a function of the number of α genes in the 3 major homozygous β haplotypes. The impact of α thalassemia on LDH was higher in CAR/CAR patients. Median (Q1-Q3) LDH was 773 (564-1018) IU/L in patients with α thalassemia vs 946 (686-1274) IU/L in those without α thalassemia (P = .005). No significant difference was observed among BEN/BEN and SEN/SEN patients. LDH was significantly higher in CAR/CAR patients than in BEN/BEN patients with or without α thalassemia (P = .014).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/6/10.1182_bloodadvances.2017014555/4/m_advances014555f2.jpeg?Expires=1771336641&Signature=SYhJmwkB22whko2fQG0OaD93jFmN6kRzImakEKRFOJ8Alr5ICPfZRGTlztHGZ5Kp1WSd8yuoeVCWUbuHCd63~zNGIHRWUWl3fiogCea9EK7O3DIsFV6iU1oi15fjKtzDeiCBQywevTRgmQ3ktKIkO~E7JGsiC~dnW9O6QCd8eYXyZ8YPyM7y-kPqnLLq3KvFWXy4ObuGpMZb00gC7wwYotNNJ1wSpjW2VctLgmAO-gw~5BsK1RccfXp1RZUFxLqQwjnKWGf6gk0mVgNRJyttv3Fabxo12tgdwuvd8GSMpkGtVbiyUeVz425w-So98PtpdRZ-8Fm8Op~mKyx~~3rIyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Biological parameters with HU therapy. (A) MCV during HU therapy as a function of the number of α genes. In the absence of α thalassemia, (4 α genes), median (Q1-Q3) MCV during HU treatment was 100 (93-106.9) fL, and it was significantly lower (P < .001) in α-thalassemia patients with 1 deleted gene (88.1 [84.9-96.0] fL) or 2 deleted genes (74.7 [70.7-82.3] fL). (B) Platelets during HU therapy as a function of the number of α genes. Platelet counts were significantly lower in HU-treated patients with α thalassemia. Median (Q1-Q3) platelet count was 217 × 109/L (172-372) in patients with 2 deleted genes, 284 × 109/L (217-378) in those with 1 deleted gene, and 350 × 109/L (274-408) in those without α thalassemia. (C) Delta HbF on HU was significantly higher (P = .026) in BEN/BEN than in CAR/CAR patients. (D) HbF during HU treatment in the 3 major homozygous β haplotypes. Median (Q1-Q3) HbF with HU therapy was significantly higher in BEN/BEN patients (21.1% [13.7-27.6%] than in SEN/SEN patients (20.9% [11.6-24.8%]) (P = .016) and CAR/CAR patients (16.7% [11.4-20.1%] (P = .005). (E) Hb levels with HU treatment in the 3 major homozygous β haplotypes. Median (Q1-Q3) Hb levels during HU therapy were significantly higher in BEN/BEN patients (9.4 [8.1-10.1] g/dL) than in SEN/SEN patients (8.7 [7.8-9.8] g/dL) (P = .017) and CAR/CAR patients (8.4 [7.8-9.2] g/dL) (P = .007).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/6/10.1182_bloodadvances.2017014555/4/m_advances014555f3.jpeg?Expires=1771336641&Signature=0xsdvk~ZF5KXR6vHW7UAg9V~g4sVqdsUTLi0JieYmmLOXuTdOBUsq~H2G6k0gZeznDq9xUy62GGhY8H-oGXBsnh8Z3yLYCNsLQ-fLjrPY92zW6PQHx~IbZyVehaK3drogsfwJK6abZZoVlrx1sTT9L7wRfFyy6-EC2ZGIHXv9yN3yf0han1P6C7zQDd2mOyFum4UxccQw0d-x8sMDGtnHLDQhuaWxPd892csD9IJAwqrxnSl2ZJcbt4MmPZdzWKRAZ2cEh1QRm9hz~Iuims2dc8vMR37l1dCAu1nZZnugMyD0Dm8YQHB1XErq-qXxPCMM5Jeq2BYUU1oiMoDTJ2OTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)