Key Points
SUV on PET imaging is a predictive factor of OS for patients with MALT lymphoma.
Large cell transformation and worse OS are more common in patients with SUV ≥10 on their initial PET scan.
Abstract
The role of [18F]fluorodeoxyglucose (FDG) positron-emission tomography (PET) in mucosa-associated lymphoid tissue (MALT) of marginal zone lymphoma remains poorly defined. We correlated initial PET with pathology, clinical factors, and outcome. From January 2001 to July 2012, 173 MALT lymphoma patients with a biopsied lesion identified on PET within 90 days of tissue biopsy were analyzed. PET positivity and intensity of FDG uptake were correlated with clinical factors and patient outcome. Among 173 accrued cases, biopsied site was PET avid in 123 patients (71%); median standardized uptake value (SUV) was 6.0 (range: 0.7-28.0), and SUV >10.0 in 20 patients (16%). PET avidity varied by organ sites. PET positivity correlated with higher International Prognostic Index, but not with 5-year overall survival (OS; 96% vs 88%, PET negative vs positive, P = .229) or 5-year progression-free survival (67% vs 56%, P = .493). SUV was an independent prognostic factor of OS, and an increased SUV was associated with a decreasing 5-year OS. Patients who presented with SUV ≥10 had a higher rate of subsequent large cell transformation (20% vs 5%, P = .035) and inferior OS (78% vs 92%, P = .008). The exact role of FDG PET in the management of MALT lymphoma, beyond initial staging, remains to be defined.
Introduction
Marginal zone lymphoma is the third most common subtype of non-Hodgkin lymphoma, accounting for 10% of all non-Hodgkin lymphoma cases.1 As the most common entity of marginal zone lymphoma, the mucosa-associated lymphoid tissue subtype (MALT) has defined etiology with antigenic stimulation in many cases, is extranodal, and is associated with an excellent treatment outcome when localized.2 MALT lymphoma is considered an indolent lymphoma, yet one-third of patients will present with disseminated disease, including multiple mucosal sites.3-6
A unique and challenging issue for MALT lymphoma is staging.7 Current computed tomography (CT)–based staging workup is limited in evaluating extranodal disease involvement. [18F]fluorodeoxyglucose (FDG) positron-emission tomography (PET) has many advantages in lymphoma evaluation.8,9 In untreated patients, FDG PET leads to upstaging in as many as 30%; for extranodal involvement, the sensitivity of PET/CT was almost twofold better than that of CT (88% vs 50%), although the specificity was similar (100% vs 90%).10 Although PET may potentially be useful for MALT lymphoma evaluation, PET avidity is histopathologic subtype dependent.9,11,12 In general, indolent lymphomas tend to have lower FDG uptake than aggressive lymphomas11,13,14 ; high uptake can be observed in patients with indolent lymphomas who undergo transformation.15,16
Further complicating the role of PET imaging in MALT, the pathogenesis of MALT lymphoma is often associated with infection and inflammatory conditions, which are invariably FDG avid. Many extranodal MALT organs have background physiological metabolic uptake, such as the digestive tract and salivary glands. Thus, it may be challenging to differentiate between abundant FDG uptake in extranodal sites with rich lymphoid tissue and inflammation vs uptake in the tumor itself. In fact, tumor uptake may sometimes be lower than uptake related to these other causes. Previous studies investigating the role of FDG PET in MALT had various designs with ambiguous definition of positive lesions, making interpretation and comparison of the results difficult. Some studies included many histological subtypes other than MALT lymphoma.14,17,18 Most studies accrued relatively small and heterogeneous populations referred for initial staging, evaluation of treatment outcome, or restaging after recurrence.19,20 The value of PET in routine disease evaluation for MALT remains controversial.8,12,21 Moreover, prognostic factors for overall survival (OS) in MALT are still needed despite the results of retrospective case studies with large numbers. Therefore, the objective of the current study was to correlate initial diagnostic PET/CT in histologically proven MALT lymphoma with clinical characteristics, including disease prognosis.
Patients and methods
This retrospective data analysis was approved by the Institutional Review Board. We retrospectively reviewed all MALT lymphoma cases initially treated at Memorial Sloan Kettering Cancer Center (MSKCC) between January 2001 and July 2012. The diagnosis was confirmed by a dedicated MSKCC hematopathologist. We applied the following inclusion criteria: (1) an FDG PET scan was carried out at MSKCC or outside facility with the original PET/CT images available, (2) the PET/CT was done within 90 days of tissue biopsy, (3) lesion described in the pathologic report could be accurately identified in PET imaging, (4) no antilymphoma treatment was administered before PET/CT scanning, and (5) no other malignant disease was evident, aside from MALT lymphoma. Cases that underwent an excisional biopsy before PET/CT scanning were excluded. Cases with only bone marrow disease were also excluded.
Image review, interpretation, and data collection
All PET/CT images were reviewed specifically for this study using the PET VCAR display and analysis application (GE Healthcare). Each PET/CT was reviewed independently by 2 nuclear medicine physicians (M.Y.H. and H.S.). The specific biopsied site of the lesion was provided to the reviewing physician. Regions of interest were placed in these predefined sites as well as in liver and mediastinal blood pool for background measurements. In patients who had >1 lesion biopsied, we only recorded data of the predominant lesion. Suspicious MALT lymphoma lesions on imaging without pathology confirmation were not included in the analysis, even when they had intense FDG uptake. A positive or negative PET was defined based on a visual qualitative assessment. Any focal FDG uptake greater than local background activity in the regions of interest defined by the nuclear physician was considered positive. For lesions in organs frequently showing physiological FDG uptake, or at sites with known inflammation, particular attention was paid to ensure presence of focal uptake. Standardized uptake values (SUV), normalized to body weight, were recorded. Data on clinical presentation, treatment, and follow-up were retrieved from the electronic medical record system.
Statistical analysis
We analyzed varied PET-positive rate using the Fisher’s exact test and χ2 Student t test. SUV values of the various subgroups were compared by 1-way analysis of variance with a post hoc Tukey test used for multiple comparisons. We calculated the duration of OS from the date of diagnosis until the time of death or last follow-up, and the duration of progression-free survival (PFS) from the date of diagnosis until the date of progression, death, or time of last follow-up. Survival curves were evaluated using the Kaplan-Meier method and compared between groups using log-rank tests for P values. Univariate hazard estimates were generated with unadjusted Cox proportional hazards models. A stepwise Cox proportional hazards model was used for multivariable survival analysis, with factors significant on univariable analysis entered in a hierarchical fashion using forward selection of the covariates’ likelihood ratios (P < .10 for inclusion). To assess the shape of the association between SUV and survival, univariable and multivariable models were constructed, where SUVmax was modeled as a continuous variable using restricted cubic splines to account for nonlinear relationships. Restricted cubic splines were performed using the RMS package in R version 3.3.3 (http://www.r-project.org/); other analyses were performed using IBM SPSS Statistics, version 20.0. P < .05 was considered statistically significant.
Results
Patient characteristics and treatment
From January 2001 to July 2012, 582 patients were diagnosed with MALT lymphoma at MSKCC. Among these 582 patients, 314 (54%) were excluded from analysis because they had no initial PET/CT imaging evaluation (n = 129) or had initial PET/CT done outside without available imaging for analysis (n = 185). An additional 69 patients (12%) were excluded because the initial PET was done after a complete resection, and 26 patients (4%) were excluded because their initial PET/CT predated the date of biopsy by >90 days. Therefore, a total of 173 patients (30% of the original patient pool) met eligibility criteria and were included in this study.
Table 1 lists the basic demographic and clinical characteristics of these 173 patients. Most cases presented with normal LDH (87%), rare bone marrow involvement (4%), and low International Prognostic Index (IPI; score 0 or 1 in 64%). High-risk MALT-IPI22 (≥2) was only found in 28 cases (16%).
Basic characteristics of studied population
| . | Total (n = 173) . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 61 (21-88) | |
| Mean (± SD) | 59.8 (±14.7) | |
| <70 | 124 | 71.7 |
| ≥70 | 49 | 28.3 |
| Sex | ||
| Male | 82 | 47.4 |
| Female | 91 | 52.6 |
| Primary site | ||
| Stomach | 42 | 24.3 |
| Lung | 34 | 19.7 |
| Skin | 22 | 12.7 |
| Orbit | 21 | 12.1 |
| Rare site | ||
| Head and neck | 11 | 6.4 |
| Soft tissue | 10 | 5.8 |
| Parotid gland | 7 | 4.0 |
| CNS | 5 | 2.9 |
| Thyroid | 4 | 2.3 |
| Bowel | 3 | 1.7 |
| Breast | 3 | 1.7 |
| Others | 11 | 6.4 |
| ECOG PS | ||
| 0 | 113 | 65.3 |
| 1 | 56 | 32.4 |
| 2 | 4 | 2.3 |
| Stage | ||
| I | 99 | 57.2 |
| II | 19 | 11.0 |
| III | 5 | 2.9 |
| IV | 50 | 28.9 |
| LDH | ||
| Normal | 151 | 87.3 |
| Elevated | 22 | 12.7 |
| BM involvement | ||
| No | 96 | 55.5 |
| Yes | 7 | 4.0 |
| Unknown | 70 | 40.5 |
| IPI | ||
| 0 | 55 | 31.8 |
| 1 | 55 | 31.8 |
| 2 | 33 | 19.1 |
| 3 | 27 | 15.6 |
| 4 | 3 | 1.7 |
| MALT-IPI | ||
| 0 | 76 | 43.9 |
| 1 | 69 | 39.9 |
| ≥2 | 28 | 16.2 |
| . | Total (n = 173) . | % . |
|---|---|---|
| Age, y | ||
| Median (range) | 61 (21-88) | |
| Mean (± SD) | 59.8 (±14.7) | |
| <70 | 124 | 71.7 |
| ≥70 | 49 | 28.3 |
| Sex | ||
| Male | 82 | 47.4 |
| Female | 91 | 52.6 |
| Primary site | ||
| Stomach | 42 | 24.3 |
| Lung | 34 | 19.7 |
| Skin | 22 | 12.7 |
| Orbit | 21 | 12.1 |
| Rare site | ||
| Head and neck | 11 | 6.4 |
| Soft tissue | 10 | 5.8 |
| Parotid gland | 7 | 4.0 |
| CNS | 5 | 2.9 |
| Thyroid | 4 | 2.3 |
| Bowel | 3 | 1.7 |
| Breast | 3 | 1.7 |
| Others | 11 | 6.4 |
| ECOG PS | ||
| 0 | 113 | 65.3 |
| 1 | 56 | 32.4 |
| 2 | 4 | 2.3 |
| Stage | ||
| I | 99 | 57.2 |
| II | 19 | 11.0 |
| III | 5 | 2.9 |
| IV | 50 | 28.9 |
| LDH | ||
| Normal | 151 | 87.3 |
| Elevated | 22 | 12.7 |
| BM involvement | ||
| No | 96 | 55.5 |
| Yes | 7 | 4.0 |
| Unknown | 70 | 40.5 |
| IPI | ||
| 0 | 55 | 31.8 |
| 1 | 55 | 31.8 |
| 2 | 33 | 19.1 |
| 3 | 27 | 15.6 |
| 4 | 3 | 1.7 |
| MALT-IPI | ||
| 0 | 76 | 43.9 |
| 1 | 69 | 39.9 |
| ≥2 | 28 | 16.2 |
BM, bone marrow; CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; PS, performance status; SD, standard deviation.
Of the 118 patients with stage I/II disease, 91 (77%) received primary localized treatment with radiotherapy (n = 67; 57%) or surgery (n = 24; 20%). Other patients were either observed (n = 14; 12%) or underwent chemotherapy (n = 4; 3%) or immunotherapy (n = 9; 8%). Among the 55 patients with advanced stage, 26 (47%) cases received systemic treatment, 20 (36%) had active surveillance, and 9 (16%) received localized palliative treatment (8 on radiotherapy, 1 on surgery).
PET positivity and relevant clinical factors
In 123 of the 173 pathologically confirmed lesions (71%), the FDG PET scan was positive. PET scan failed to detect a true lesion in 50 cases (29%). PET-positive reading varied according to different disease-related factors, as listed in Table 2. PET avidity differed with respect to the organ site of biopsy (eg, lung MALT lesions were 100% PET positive, but skin lesions were only 23% positive). For some rare MALT sites, PET sensitivity also differed considerably. PET positive rate was very high in soft tissue, parotid gland, thyroid, and lymph node lesions, and particularly low in central nervous system and breast, although numbers were small for these. Patients with higher IPI and MALT-IPI showed higher FDG PET–positive rate than those with lower IPI and MALT-IPI.
Incidence and intensity of PET positivity according to disease characteristics in studied population
| . | PET . | . | . | . | |
|---|---|---|---|---|---|
| . | Positive (n = 123), n (%) . | Negative (n = 50), n (%) . | P . | SUV (mean ± SD) . | P . |
| Origin site | .000 | .032 | |||
| Stomach | 21 (50.0) | 21 (50.0) | 5.8 ± 1.9 | ||
| Lung | 34 (100.0) | 0 | 5.9 ± 4.4 | ||
| Skin | 5 (22.7) | 17 (77.3) | 3.7 ± 3.9 | ||
| Orbit | 15 (71.4) | 6 (28.6) | 6.3 ± 4.1 | ||
| Rare site | |||||
| Head and neck | 11 (100.0) | 0 | 7.9 ± 3.9 | ||
| Soft tissue | 10 (100) | 0 | 7.3 ± 3.9 | ||
| Parotid gland | 5 (71.4) | 2 (28.6) | 7.7 ± 3.7 | ||
| CNS | 3 (60.0) | 2 (40.0) | 5.2 ± 2.2 | ||
| Thyroid | 4 (100.0) | 0 | 14.8 ± 6.2 | ||
| Bowel | 3 (100.0) | 0 | 6.9 ± 3.2 | ||
| Breast | 1 (33.3) | 2 (66.7) | 2.6 | ||
| Others | 11 (100.0) | 0 | 8.9 ± 7.5 | ||
| Age, y | .055 | .295 | |||
| <70 | 83 (33.1) | 41 (66.9) | 4.6 ± 4.9 | ||
| ≥70 | 40 (18.4) | 9 (81.6) | 5.4 ± 5.0 | ||
| ECOG PS | .323 | .140 | |||
| 0-1 | 118 (70.2) | 50 (29.8) | 6.6 ± 4.5 | ||
| ≥2 | 5 (100) | 0 | 9.7 ± 4.7 | ||
| Stage | .161 | .058 | |||
| I-II | 80 (67.8) | 38 (32.2) | 4.3 ± 4.6 | ||
| III-IV | 43 (78.2) | 12 (21.8) | 5.8 ± 5.3 | ||
| LDH | .317 | .390 | |||
| Normal | 105 (69.5) | 46 (30.5) | 6.7 ± 4.4 | ||
| Elevated | 18 (81.8) | 4 (18.2) | 7.6 ± 5.1 | ||
| IPI | .000 | .578 | |||
| 0 | 28 (50.9) | 27 (49.1) | 6.1 ± 4.6 | ||
| 1 | 41 (74.5) | 14 (25.5) | 6.8 ± 4.5 | ||
| 2 | 27 (81.8) | 6 (18.2) | 6.5 ± 5.2 | ||
| ≥3 | 27 (90.0) | 3 (10.0) | 7.7 ± 3.8 | ||
| MALT-IPI | .035 | .034 | |||
| 0 | 47 (61.8) | 29 (38.2) | 3.8 ± 4.6 | ||
| 1 | 52 (75.4) | 17 (24.6) | 5.3 ± 4.6 | ||
| ≥2 | 24 (85.7) | 4 (14.3) | 6.4 ± 5.9 | ||
| . | PET . | . | . | . | |
|---|---|---|---|---|---|
| . | Positive (n = 123), n (%) . | Negative (n = 50), n (%) . | P . | SUV (mean ± SD) . | P . |
| Origin site | .000 | .032 | |||
| Stomach | 21 (50.0) | 21 (50.0) | 5.8 ± 1.9 | ||
| Lung | 34 (100.0) | 0 | 5.9 ± 4.4 | ||
| Skin | 5 (22.7) | 17 (77.3) | 3.7 ± 3.9 | ||
| Orbit | 15 (71.4) | 6 (28.6) | 6.3 ± 4.1 | ||
| Rare site | |||||
| Head and neck | 11 (100.0) | 0 | 7.9 ± 3.9 | ||
| Soft tissue | 10 (100) | 0 | 7.3 ± 3.9 | ||
| Parotid gland | 5 (71.4) | 2 (28.6) | 7.7 ± 3.7 | ||
| CNS | 3 (60.0) | 2 (40.0) | 5.2 ± 2.2 | ||
| Thyroid | 4 (100.0) | 0 | 14.8 ± 6.2 | ||
| Bowel | 3 (100.0) | 0 | 6.9 ± 3.2 | ||
| Breast | 1 (33.3) | 2 (66.7) | 2.6 | ||
| Others | 11 (100.0) | 0 | 8.9 ± 7.5 | ||
| Age, y | .055 | .295 | |||
| <70 | 83 (33.1) | 41 (66.9) | 4.6 ± 4.9 | ||
| ≥70 | 40 (18.4) | 9 (81.6) | 5.4 ± 5.0 | ||
| ECOG PS | .323 | .140 | |||
| 0-1 | 118 (70.2) | 50 (29.8) | 6.6 ± 4.5 | ||
| ≥2 | 5 (100) | 0 | 9.7 ± 4.7 | ||
| Stage | .161 | .058 | |||
| I-II | 80 (67.8) | 38 (32.2) | 4.3 ± 4.6 | ||
| III-IV | 43 (78.2) | 12 (21.8) | 5.8 ± 5.3 | ||
| LDH | .317 | .390 | |||
| Normal | 105 (69.5) | 46 (30.5) | 6.7 ± 4.4 | ||
| Elevated | 18 (81.8) | 4 (18.2) | 7.6 ± 5.1 | ||
| IPI | .000 | .578 | |||
| 0 | 28 (50.9) | 27 (49.1) | 6.1 ± 4.6 | ||
| 1 | 41 (74.5) | 14 (25.5) | 6.8 ± 4.5 | ||
| 2 | 27 (81.8) | 6 (18.2) | 6.5 ± 5.2 | ||
| ≥3 | 27 (90.0) | 3 (10.0) | 7.7 ± 3.8 | ||
| MALT-IPI | .035 | .034 | |||
| 0 | 47 (61.8) | 29 (38.2) | 3.8 ± 4.6 | ||
| 1 | 52 (75.4) | 17 (24.6) | 5.3 ± 4.6 | ||
| ≥2 | 24 (85.7) | 4 (14.3) | 6.4 ± 5.9 | ||
SUV and relevant clinical factors
The median SUV for the biopsied lesion measured in the 123 PET-positive cases was 6.0 (range: 0.7 to 28.0). Among these 123 patients, only 20 (16%) presented with an SUV >10.0, whereas 36 (29%) had an SUV <4.0 (frequency distribution is shown in Figure 1). Different origin site was significantly correlated with SUV (Table 2), with skin lesions presenting with low FDG uptake (mean SUV, 3.7), whereas thyroid (mean SUV, 14.8) presented with high FDG uptake. High MALT-IPI score correlated with high FDG uptake. IPI scores, stage, elevated LDH, and ECOG PS did not show significant correlation with SUV (Table 2).
SUV distribution histogram in FDG-avid MALT lymphoma. Frequency of SUV distribution by SUV level.
SUV distribution histogram in FDG-avid MALT lymphoma. Frequency of SUV distribution by SUV level.
Prognostic role of PET presentation
With a median follow-up of 45 months among survivors, 51 (30%) developed progression after initial treatment, 12 (7%) developed large cell transformation, and 18 (10%) died. The median time to transformation from diagnosis was 25 months, ranging from 0.3 to 94 months. The 5-year OS was 90% (95% confidence interval [CI], 83% to 94%), and 5-year PFS was 60% (95% CI, 50% to 68%). PET positivity did not predict OS (5-year OS, PET negative vs PET positive, 96% vs 88%, P = .229) or PFS (5-year PFS, PET negative vs PET positive, 67% vs 56%, P = .493).
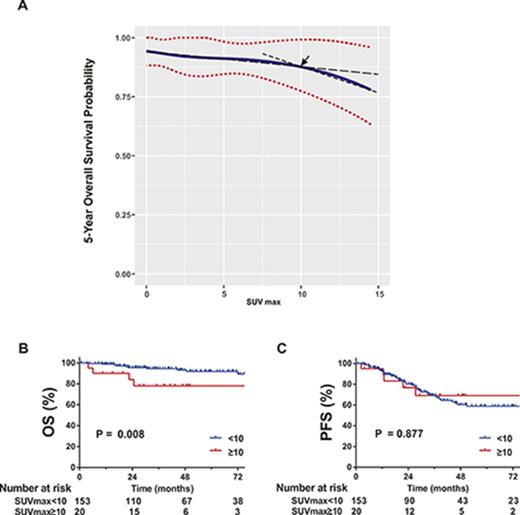
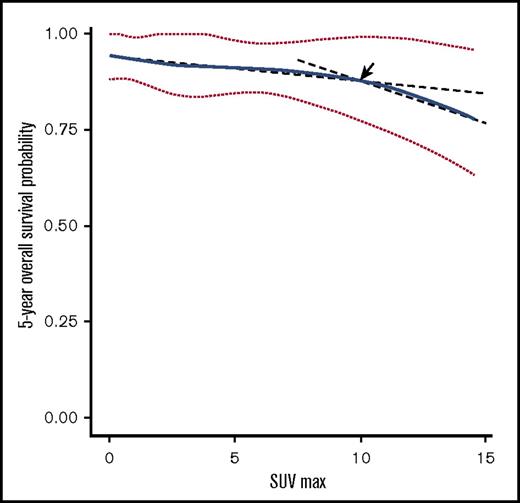
However, SUV was a significant prognostic factor for 5-year OS by univariate (hazard ratio [HR], 1.12; 95% CI, 1.04 to 1.21; P = .003) and multivariate analysis (HR, 1.11; 95% CI, 1.03 to 1.20; P = .010), in addition to age and large cell transformation. SUV was not predictive of 5-year PFS (supplemental Table 1; Figure 3). In the RMS analysis assessing the relationship between SUV and 5-year OS, the survival curve decreased linearly, indicating increased SUV is negatively associated with OS (Figure 2). Using a series of cutoff values for SUV from 5 to 10, the higher SUV group consistently showed significantly or borderline significantly worse OS than the lower SUV group and negative PET group. Cutoff point of 10 resulted in the maximal significance level (ie, smallest P value). Patients with SUV ≥10 had a significantly worse 5-year OS than those with SUV <10.
Five-year OS probability curve by continuous SUV increase using cubic splines in MALT lymphoma. The association of SUV and estimated 5-year OS (solid line) with 95% confidence intervals (dotted lines) based on the model of a cubic spline. The arrow indicates a visual turning point of the survival probability curve, cutting line into 2 segments with different slopes.
Five-year OS probability curve by continuous SUV increase using cubic splines in MALT lymphoma. The association of SUV and estimated 5-year OS (solid line) with 95% confidence intervals (dotted lines) based on the model of a cubic spline. The arrow indicates a visual turning point of the survival probability curve, cutting line into 2 segments with different slopes.
The 5-year OS was 78% (95% CI, 51% to 91%) for patients with SUV ≥10 and 92% (95% CI, 84% to 96%) for patients with SUV <10 (P = .008; Figure 3). The incidence rate of subsequent large cell transformation was significantly higher in SUV ≥10 cases than those with SUV <10 (20% vs 5%, P = .035).
OS and PFS according to SUV groups (SUV ≥10 vs SUV <10). Kaplan-Meier estimates for OS (A) and PFS (B).
OS and PFS according to SUV groups (SUV ≥10 vs SUV <10). Kaplan-Meier estimates for OS (A) and PFS (B).
We also compared survival of stage I patients with PET/CT workup vs without PET workup among the 582 MALT lymphoma patients. No significant difference in either OS or PFS was detected between the 2 groups (data not shown).
Correlation of SUV with proliferative index
We correlated the SUV of the biopsied lesion with the proliferative index as measured by Ki-67 in the marginal zone. Ki-67 was available in 103 patients with a median level of 5% (range: 1% to 80%). The Ki-67 was <30%, the typical cutoff for indolent lymphoma in 88 cases (85%). Patients with higher Ki-67 level (grouping with different cutoff values of 15%, 20%, and 30%, respectively) showed similar PET-positive rate and similar high FDG-avid rate (SUV ≥10) to those with lower Ki-67 level. This included the 15 patients with Ki-67 >30% (supplemental Table 2).
Discussion
In this large series of patients, we correlated biopsy-proven lesions with PET/CT imaging at presentation to determine the prognostic value of PET/CT in MALT. Predefined criteria for PET positivity were used, taking into account the physiologic or inflammatory uptake in the background. Our results showed a 71% PET-positive rate for MALT lymphoma, with extensive diversity among different involved organs. IPI had close correlation with PET positivity. SUV in MALT lymphoma was relatively low, with a median value of 6.0; although the range was wide (0.7 to 28.0), only 15% presented SUV >10. SUV was an independent prognostic factor of OS in univariate and multivariate analysis, with increased SUV associated with decreasing OS. Patients with SUV ≥10 had a higher incident rate of large cell transformation and inferior OS. In patients with available Ki-67 information, the proliferation index showed no association with PET avidity.
Sensitivity of PET in MALT lymphoma is highly divergent, ranging from 50% to 80%,11,12,14,16,23-25 71% in current study and 81% in a previous report from MSKCC by Beal et al.12 The difference across studies may be due to the diverse definitions of positivity and different composition of lesion sites. In general, the PET-positive rate was much lower compared with Hodgkin lymphoma, diffuse large B-cell lymphoma, and follicular lymphoma.9,11,26,27 According to our study and others, bone marrow, skin, and gastrointestinal tract commonly presented with low PET positivity, whereas lung and soft tissue were more often noticed with high FDG avidity.19,20,25,28,29 The relatively low diagnostic sensitivity and low SUV uptake in an inflammatory and physiological uptake background may limit the role of PET in staging and baseline evaluation, given 30% of cases would have a false negativity with PET in MALT lymphoma. Because pulmonary MALT often presents as multiple lesions within lung and biopsy remains challenging in more central locations, the high sensitivity of PET (100% detection rate in lung in our series) may provide a role in diagnosis, staging, and treatment monitoring.
MALT lymphoma has an excellent prognosis.22,30-32 However, ∼1.5% to 10% of patients have large cell transformation,4,6,22,30,32 and 10% of patients die of disease according to SEER database analysis.31Previously, age was the only consistently reported prognostic factor of OS for localized and advanced stage MALT lymphoma.22,30,32 SUV was not evaluated in these studies. Our study found that SUV is an independent prognostic factor for OS but not for PFS. Patients with an SUV ≥10 had a higher rate of large cell transformation and worse survival. As intensified therapy regimens for these patients may result in complete response and prolonged survival, the prognostic effect of SUV deserves validation, because the number of cases with SUV ≥10 in our series is small (n = 20). Several studies on follicular lymphoma in combination with other indolent lymphoma showed a significantly higher SUV of transformed lesions compared with indolent lesions, suggesting PET can be used to direct the site of biopsy and confirm aggressive disease when clinically appropriate.13,33
Finally, we correlated pathologic findings of growth fraction by Ki-67 with SUV of the biopsied lesion. Unlike other studies,34,35 our analysis showed a nonsignificant correlation of Ki-67 with FDG avidity. This may partially be explained by the limited numbers and retrospectively record-reviewing nature.
In conclusion, a 71% rate of PET avidity in biopsy-confirmed MALT lymphoma was recorded in our series. FDG avidity was correlated with tumor location and tumor burden. SUV was a significant prognostic factor for 5-year OS because patients with an SUV ≥10 had a higher incident rate of large cell transformation and inferior OS.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by research funding from the National Cancer Institute, National Institutes of Health Cancer Center Support Grant P 30 CA008748 (J.Y.), Connecticut Cancer Foundation (J.Y.), and the Lymphoma Foundation (J.Y.). S.Q. was a Mortimer J. Lacher Lymphoma Fellow at Memorial Sloan Kettering Cancer Center.
Authorship
Contribution: S.Q. and J.Y. designed the research project, interpreted the data, and wrote the manuscript; M.Y.H., Y.Y., H.S., S.T., and K.C. collected, edited, and analyzed data; S.Q. and Y.Y. performed statistical analyses and created the tables and figures; and A.N. and H.S. contributed to data interpretation and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joachim Yahalom, Department of Radiation Oncology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: yahalomj@mskcc.org.