Key Points
λ Light chain AL amyloidosis is associated with a shorter PFS and OS compared with κ.
Light chain type predicts likelihood of organ involvement in AL amyloidosis.
Abstract
We evaluated the impact of light chain type, lambda (λ) or kappa (κ), on disease features and outcomes in patients with immunoglobulin light chain (AL) amyloidosis receiving stem cell transplant at the Mayo Clinic between October 2002 and August 2016. Patients with λ AL amyloidosis had higher rates of renal and neurological involvement (λ 69% vs κ 57%, P = .02 and λ 16% vs κ 9%, P = .03, respectively). Patients with κ AL amyloidosis had more hepatic involvement (λ 7% vs κ 18%, P = .0003). Complete response rate was 43% for both groups and overall response rates were similar (λ 85% vs κ 91%, P = .12). Patients with κ light chain amyloidosis had better progression-free and overall survival (PFS: λ 74 months vs κ 101 months, P = .0064 and OS: λ 121 months vs κ not reached, P = .003). Mayo stage 2004 was more predictive of survival in the λ cohort (median OS of 143 months stage I vs 77 months stage II vs 33 months stage III, P < .0001) than in the κ cohort (median OS not reached for stage I and II and 102 months for stage III, P = .044). Conditioning dose predicted survival in the λ cohort only (median OS 149 months for melphalan 200 mg/m2 vs 50 months for melphalan <200 mg/m2, P < .0001; median OS κ not reached for melphalan 200 mg/m2 or <200 mg/m2, P = .38). On multivariate analysis, light chain type remained an independent predictor of survival. Light chain type predicts organ involvement and survival in patients with AL amyloidosis receiving stem cell transplant.
Introduction
The systemic amyloidoses refer to a group of disorders that share in their pathophysiology the extracellular deposition of pathologic insoluble fibrillar proteins.1 The classification of amyloidosis is based on the nature of the protein precursor undergoing aggregation and deposition in organs and tissues.2 Each subtype has distinct clinical features, and more importantly, therapeutic approaches. Thus, correct subtyping of the protein is critical to management decisions. Identifying the specific type of amyloid protein has evolved from clinicopathologic criteria to highly sensitive and specific proteomic analysis using laser microdissection and mass spectrometry.3 The immunoglobulin light chain protein is central to the pathophysiology of systemic immunoglobulin light chain (AL) amyloidosis. An abnormal plasma cell or B-cell clone produces the amyloidogenic immunoglobulin light chain protein, lambda (λ) or kappa (κ), which then deposits in organs leading to organ dysfunction. Risk stratification for patients with AL amyloidosis has focused on organ involvement and biology of the plasma cell clone with features such as the Mayo Staging system, proportion of marrow plasma cells and concurrent multiple myeloma being prognostic.4-7 Eradication of the plasma or B-cell clone responsible for light chain production has been the primary focus of treatment of AL amyloidosis. To this end, autologous stem cell transplantation (ASCT) has been used for >2 decades to treat AL amyloidosis, and although early experience was marred by high rates of treatment-related mortality, a better understanding of high-risk features has led to improved safety and efficacy.8-11 In addition to ASCT, a number of novel agents, including immunomodulatory drugs and proteasome inhibitors, are now available with proven efficacy in AL amyloidosis.12-15 Despite this, ASCT remains an integral part of treatment of eligible patients with AL amyloidosis.
Although protein subtype plays a central role in amyloidosis, there are very little data on clinical features or outcomes of AL amyloidosis according to light chain subtype. The importance of the serum free light chain assay and the difference between involved and uninvolved light chains (dFLC) as a prognostic marker has been previously described with this being incorporated in to the Mayo staging system.16 In addition, attempts at understanding the pathophysiology of AL amyloidosis have suggested differences in organ involvement by monoclonal protein type as well as light chain clone and immunoglobulin light chain variable gene use. Patients with κ light chain amyloidosis are more likely to present with hepatic involvement, and those with an immunoglobulin M (IgM) monoclonal protein more frequently present with neurological and lung involvement.17-19 The increased incidence of λ light chain amyloidosis compared with κ is also well reported; however, this has not impacted our approach to staging or treatment.20 Herein, we report the association of light chain type on outcomes in patients with AL amyloidosis treated with ASCT at the Mayo Clinic.
Patients and methods
After approval by the Mayo Clinic Institutional Review Board, data were reviewed on all patients with biopsy-proven systemic AL amyloidosis who underwent autologous stem cell transplant between 1 October 2002 and 31 August 2016. We identified 2 cohorts based on the light chain of amyloidosis, λ and κ. The type of light chain was distinguished by the best available method of typing and data existing at the time of diagnosis. The techniques used included proteomic analysis with mass spectrometry as well as identifying light chain restriction of the underlying plasma cell clone through serum protein electrophoresis, immunoglobulin-free light chain assay, bone marrow biopsy evaluation for light chain restricted clonal plasma cells, and tissue biopsy assessed by immunohistochemistry for light chain expression. Where available, preference was given to subtyping by proteomic analysis using mass spectrometry.
For bone marrow plasma cells (BMPCs), the highest estimate on the aspiration and biopsy was used. Organ involvement and hematological response were assessed according to consensus criteria.21-22 To identify the number of organs involved, heart, kidney, liver, neurological (peripheral nerve or autonomic), and other (soft tissue and gastrointestinal tract) were included as involved or not involved. After an initial analysis, we identified a higher pretransplant dFLC in the κ group compared with the λ group. To ensure this difference did not unduly skew the data with regard to risk stratification, we stratified patients according to both the Mayo 2004 (dFLC not part of staging) and the Mayo 2012 (dFLC included) staging systems.5,23
Patients were selected for ASCT using available criteria at the time of transplant. Patients were mobilized, conditioned, and transplanted according to previously published institutional protocols.24 Response was measured at ∼100 days post-ASCT according to updated consensus criteria. Overall response rate (ORR) was defined as a partial response (PR) or greater. Hematologic progression was defined according to consensus guidelines for reporting of clinical trials in AL amyloidosis.25
Statistical analysis was performed on JMP software (SAS, Cary, NC). Patient and disease-related factors between the 2 cohorts were compared using the χ2 test for categorical variables, and the Wilcoxon signed rank test for continuous variables. Survival analysis was performed using the Kaplan-Meier method. Overall survival (OS) was calculated from day 0 of bone marrow transplant to death from any cause. Treatment-related mortality (TRM) was defined as death from any cause within 100 days of ASCT. The Cox proportional hazards model was used to assess for predictors of OS. The variables included in the univariate analyses were age, sex, number of organs involved, BMPCs, Mayo stage 2004 and 2012, conditioning dose, pretransplantation chemotherapy, time period of transplant (prior to 2010 vs 2010 onwards), and light chain subtype. Variables reaching P < .1 were included in the multivariate analysis. We created 2 multivariate models incorporating the 2004 and 2012 Mayo stages, respectively.
Results
Between 1 October 2002 and 31 August 2016, 557 consecutive patients with AL amyloidosis underwent ASCT at the Mayo Clinic in Rochester. Of these, 73% (n = 404) had λ light chain and 27% (n = 153) had κ light chain AL amyloidosis. Table 1 outlines the baseline characteristics for each group. Median age and the proportion of men in the 2 cohorts were not significantly different. Although more patients with λ had cardiac involvement, this was not statistically significant (λ 49% vs κ 41%, P = .15). Patients with λ light chain amyloidosis had a higher rate of renal involvement and neurological involvement (λ 69% vs κ 57%, P = .02 and λ 16% vs κ 9%, P = .03, respectively). Patients with κ light chain amyloidosis had more hepatic involvement (λ 7% vs κ 18%, P = .0003). The median creatinine was slightly higher in the κ group (λ 1.0 mg/dL vs κ 1.1 mg/dL, P = .002). In patients with renal involvement, the degree of proteinuria as measured by 24-hour urine protein excretion was significantly higher in the λ cohort (λ 5.7 g vs κ 4.4 g, P = .016). Among patients with cardiac involvement, the NT-proBNP was not significantly different between the 2 cohorts (λ 1669 pg/mL vs κ 2071 pg/mL, P = .07). Patients with κ light chain amyloidosis had a higher pretransplant light chain level assessed by the dFLC (median dFLC 11.5 mg/dL for λ vs median dFLC 21.3 mg/dL for κ, P = .0049). More patients in the λ cohort had early Mayo stage 2012 compared with the κ cohort (47% stage I for λ vs 36% stage I for κ, P = .03). This is expected given the higher dFLC in the κ group, a key component of Mayo stage 2012.
Baseline characteristics
| Characteristic . | λ (n = 404) . | κ (n = 153) . | P . |
|---|---|---|---|
| Age, median (IQR), y | 59 (53-65) | 59 (53-64) | .83 |
| Male, % | 62 | 64 | .69 |
| Organs involved, n (%) | |||
| Cardiac | 198 (49) | 64 (41) | .15 |
| Renal | 277 (69) | 88 (57) | .02 |
| Hepatic | 29 (7) | 28 (18) | .0003 |
| Neurologic | 66 (16) | 14 (9) | .03 |
| Other | 92 (23) | 46 (30) | .08 |
| >2 organs involved | 57 (14) | 20 (13) | .89 |
| BMPCs, median (IQR), % | 8 (5-13) | 8 (5-15) | .84 |
| BMPCs ≥10%, n (%) | 164 (41) | 61 (40) | 1 |
| Monoclonal protein, n (%) | |||
| IgG | 139 (34) | 53 (35) | 1 |
| IgA | 47 (12) | 5 (3) | .0017 |
| IgM | 14 (3) | 12 (8) | .04 |
| IgD | 5 (1) | 1 (<1) | 1 |
| Light chain only | 199 (49) | 82 (54) | .39 |
| Creatinine, median (IQR), mg/dL | 1.0 (0.9-1.2) | 1.1 (0.9-1.5) | .0022 |
| ALP, median (IQR), U/L | 81 (65-110) | 89 (69-134) | .01 |
| NT-Pro BNP, median (IQR), pg/mL | 499 (157-1903) | 514 (163-2119) | .79 |
| Troponin T, median (IQR), ng/mL | 0.01 (0.01-0.02) | 0.01 (0.01-0.03) | .65 |
| dFLC, median (IQR), ng/mL | 11.5 (5.2-33.8) | 21.3 (5.9-65.2) | .0049 |
| dFLC >5 mg/dL, n (%) | 272 (76) | 104 (78) | .81 |
| dFLC >18 mg/dL, n (%) | 138 (36) | 70 (52) | .0076 |
| 24-h urine protein (IQR), g | 3.4 (0.2-7.2) | 0.96 (0.2-5.4) | .0026 |
| Mayo stage 2004, n (%) | .88 | ||
| I | 227 (66) | 87 (67) | |
| II | 59 (17) | 20 (15) | |
| III | 60 (17) | 24 (18) | |
| Missing | 58 | 22 | |
| Mayo stage 2012, n (%) | .16 | ||
| I | 159 (47) | 46 (36) | .03 |
| II | 95 (28) | 47 (36) | |
| III | 51 (15) | 22 (17) | |
| IV | 36 (10) | 14 (11) | |
| Missing | 63 | 24 |
| Characteristic . | λ (n = 404) . | κ (n = 153) . | P . |
|---|---|---|---|
| Age, median (IQR), y | 59 (53-65) | 59 (53-64) | .83 |
| Male, % | 62 | 64 | .69 |
| Organs involved, n (%) | |||
| Cardiac | 198 (49) | 64 (41) | .15 |
| Renal | 277 (69) | 88 (57) | .02 |
| Hepatic | 29 (7) | 28 (18) | .0003 |
| Neurologic | 66 (16) | 14 (9) | .03 |
| Other | 92 (23) | 46 (30) | .08 |
| >2 organs involved | 57 (14) | 20 (13) | .89 |
| BMPCs, median (IQR), % | 8 (5-13) | 8 (5-15) | .84 |
| BMPCs ≥10%, n (%) | 164 (41) | 61 (40) | 1 |
| Monoclonal protein, n (%) | |||
| IgG | 139 (34) | 53 (35) | 1 |
| IgA | 47 (12) | 5 (3) | .0017 |
| IgM | 14 (3) | 12 (8) | .04 |
| IgD | 5 (1) | 1 (<1) | 1 |
| Light chain only | 199 (49) | 82 (54) | .39 |
| Creatinine, median (IQR), mg/dL | 1.0 (0.9-1.2) | 1.1 (0.9-1.5) | .0022 |
| ALP, median (IQR), U/L | 81 (65-110) | 89 (69-134) | .01 |
| NT-Pro BNP, median (IQR), pg/mL | 499 (157-1903) | 514 (163-2119) | .79 |
| Troponin T, median (IQR), ng/mL | 0.01 (0.01-0.02) | 0.01 (0.01-0.03) | .65 |
| dFLC, median (IQR), ng/mL | 11.5 (5.2-33.8) | 21.3 (5.9-65.2) | .0049 |
| dFLC >5 mg/dL, n (%) | 272 (76) | 104 (78) | .81 |
| dFLC >18 mg/dL, n (%) | 138 (36) | 70 (52) | .0076 |
| 24-h urine protein (IQR), g | 3.4 (0.2-7.2) | 0.96 (0.2-5.4) | .0026 |
| Mayo stage 2004, n (%) | .88 | ||
| I | 227 (66) | 87 (67) | |
| II | 59 (17) | 20 (15) | |
| III | 60 (17) | 24 (18) | |
| Missing | 58 | 22 | |
| Mayo stage 2012, n (%) | .16 | ||
| I | 159 (47) | 46 (36) | .03 |
| II | 95 (28) | 47 (36) | |
| III | 51 (15) | 22 (17) | |
| IV | 36 (10) | 14 (11) | |
| Missing | 63 | 24 |
Only results for patients with renal involvement are included in the analysis.
ALP, alkaline phosphatase; IQR, interquartile range.
Melphalan was the preferred conditioning regimen with patients receiving full intensity conditioning with melphalan 200 mg/m2 (n = 390) or reduced intensity with melphalan <200 mg/m2 (n = 170, 86% of patients receiving reduced intensity received 140 mg/m2). Reduced intensity melphalan was given for patients ≥70 years of age or those with a creatinine level >1.8 mg/dL. The rates of patients receiving reduced intensity conditioning were not different between the 2 cohorts (λ 28% vs κ 30%, P = .52). Six patients (4%) received conditioning with carmustine, etoposide, cytarabine, and melphalan, all of whom had IgM amyloidosis with bone marrow revealing clonal lymphoplasmacytic cells. The majority of patients were transplanted within 6 months of diagnosis in both cohorts (λ 75% vs κ 62%, P = .0032). More patients in the κ light chain cohort were transplanted later than 6 months postdiagnosis (38%), and this likely reflects the increased number of patients in the κ cohort that received pretransplantation chemotherapy (λ untreated 62% vs κ untreated 46%, P = .0015). The types of agents used as treatment prior to transplantation for the 2 groups are summarized in Table 2.
Treatment characteristics
| Treatment . | λ (n = 404) . | κ (n = 153) . | P . |
|---|---|---|---|
| Conditioning regimen, n (%) | .52 | ||
| Melphalan 200 mg/m2 | 289 (72) | 101 (66) | |
| Melphalan <200 mg/m2 | 115 (28) | 55 (30) | |
| Other | 0 | 6 (4) | |
| Timing of transplant from diagnosis, n (%) | .0061 | ||
| <6 mo | 303 (75) | 91 (62) | .0032 |
| 6-12 mo | 62 (15) | 41 (27) | |
| >12 mo | 39 (10) | 17 (11) | |
| Pretransplantation chemotherapy, n (%) | .0018 | ||
| Untreated | 249 (62) | 71 (46) | .0015 |
| Corticosteroid only | 38 (10) | 18 (12) | |
| Melphalan based | 25 (6) | 5 (3) | |
| IMiD based | 26 (6) | 10 (6) | |
| Bortezomib based | 58 (14) | 44 (29) | |
| Other | 9 (2) | 5 (3) |
| Treatment . | λ (n = 404) . | κ (n = 153) . | P . |
|---|---|---|---|
| Conditioning regimen, n (%) | .52 | ||
| Melphalan 200 mg/m2 | 289 (72) | 101 (66) | |
| Melphalan <200 mg/m2 | 115 (28) | 55 (30) | |
| Other | 0 | 6 (4) | |
| Timing of transplant from diagnosis, n (%) | .0061 | ||
| <6 mo | 303 (75) | 91 (62) | .0032 |
| 6-12 mo | 62 (15) | 41 (27) | |
| >12 mo | 39 (10) | 17 (11) | |
| Pretransplantation chemotherapy, n (%) | .0018 | ||
| Untreated | 249 (62) | 71 (46) | .0015 |
| Corticosteroid only | 38 (10) | 18 (12) | |
| Melphalan based | 25 (6) | 5 (3) | |
| IMiD based | 26 (6) | 10 (6) | |
| Bortezomib based | 58 (14) | 44 (29) | |
| Other | 9 (2) | 5 (3) |
IMiD, immunomodulatory drug.
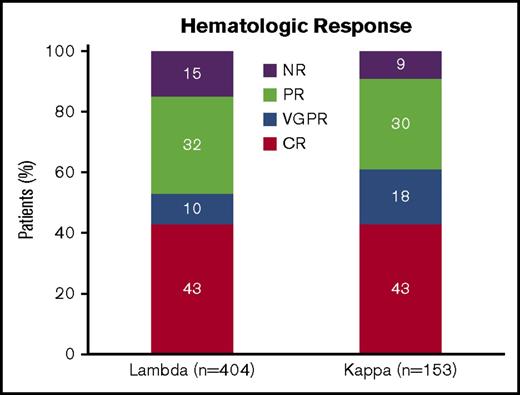
Figure 1 highlights the hematological response in both cohorts. The rate of CR was identical between the 2 groups (43%). ORR rate was not significantly different between the 2 groups (λ 85% vs κ 91%, P = .12). There was a trend toward improvement in hematologic response with chemotherapy prior to transplantation (pretreated CR 45%, VGPR 15%, PR 31%, and NR 9% vs untreated CR 42%, VGPR 10%, PR 32%, and NR 16%, P = .047). However, treatment with chemotherapy prior to transplantation was not associated with an improved ORR when the λ and κ cohorts were assessed individually (λ: pretreated ORR 89% vs untreated ORR 83%, P = .14 and κ: pretreated ORR 95% vs untreated ORR 86%, P = .09). Melphalan conditioning dose predicted rates of CR in the λ cohort (melphalan 200 mg/m2 50% CR vs melphalan <200 mg/m2 25% CR, P < .0001) but not in the κ cohort (melphalan 200 mg/m2 43% CR vs melphalan <200 mg/m2 49% CR, P = .59).
Hematologic response by light chain type. CR, complete response; NR, no response; VGPR, very good partial response.
Hematologic response by light chain type. CR, complete response; NR, no response; VGPR, very good partial response.
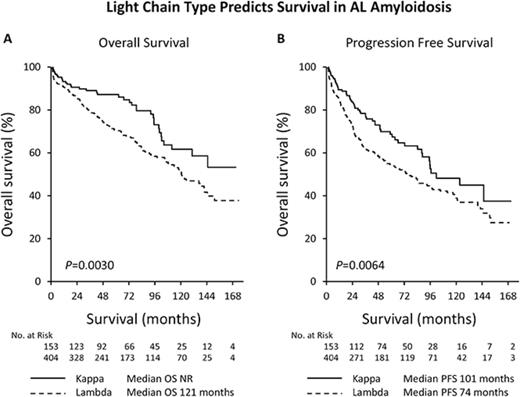
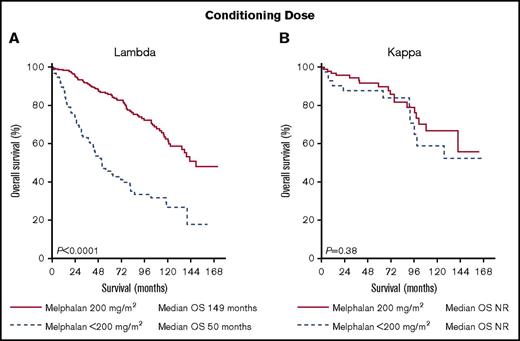
After a median follow-up of 71 months among survivors, patients with κ light chain amyloidosis had a better PFS and OS (PFS: λ 74 months vs κ 101 months, P = .0064 and OS: λ 121 months vs κ not reached, P = .003) (Figure 2). Chemotherapy prior to transplantation did not significantly improve OS (λ: median OS pretreated 121 months vs untreated 120 months, P = .95 and κ median OS pretreated 131 months vs untreated not reached, P = .30). BMPCs ≥10% was associated with a significantly worse survival in the λ cohort (median OS BMPCs ≥10% 107 months vs BMPCs <10% 137 months, P = .02) but not in the κ cohort (median OS BMPCs ≥10% 104 months vs BMPCs <10% not reached, P = .05). Conditioning dose has previously been reported as a significant predictor of survival with those receiving reduced intensity conditioning having worse outcomes.26-27 In our data, full intensity conditioning with melphalan 200 mg/m2 was associated with a significantly longer OS only in the λ cohort (λ: median OS 146 months for melphalan 200 mg/m2 vs 45 months for melphalan <200 mg/m2, P < .0001 and κ: median OS not reached for both groups, P = .33). We noted a significant difference in 100-day all-cause mortality in the λ group depending on conditioning dose (3.8% for melphalan 200 mg/m2 vs 13.9% for melphalan <200 mg/m2, P = .0006). To account for this, we performed a landmark survival analysis from day 100 post-ASCT. Conditioning dose remained a significant predictor of survival in the λ cohort with no impact on survival in the κ cohort (λ: median OS 149 months for melphalan 200 mg/m2 vs 50 months for melphalan <200 mg/m2, P < .0001 and κ: median OS not reached for both groups, P = .38) (Figure 3).
Landmark survival analysis 100 days post–stem cell transplant by light chain type and conditioning dose. (A) λ light chain OS by conditioning dose. (B) κ light chain OS by conditioning dose. NR, not reached.
Landmark survival analysis 100 days post–stem cell transplant by light chain type and conditioning dose. (A) λ light chain OS by conditioning dose. (B) κ light chain OS by conditioning dose. NR, not reached.
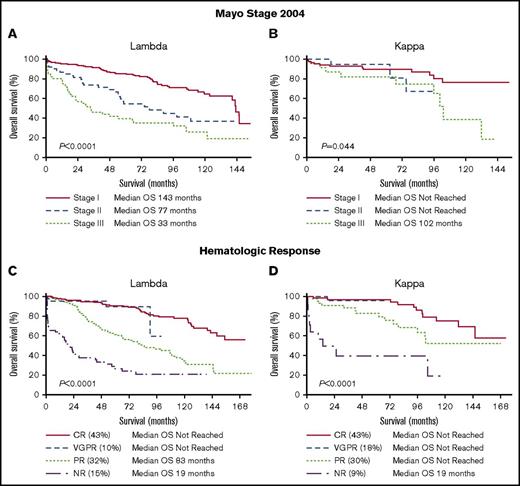
Mayo stage and hematologic response are factors that strongly predict outcomes in AL amyloidosis.28-29 Mayo stage 2004 was a powerful predictor of survival in the λ cohort (median OS of 143 months stage I vs 77 months stage II vs 33 months stage III, P < .0001) but less discriminatory in the κ cohort (median OS not reached for stage I and II and 102 months for stage III, P = .044) (Figure 4A-B). Mayo stage 2012 predicted survival in the λ cohort (median OS of 146 months stage I vs 142 months stage II vs 54 months stage III vs 22 months stage IV, P < .0001) but not in the κ cohort (median OS not reached for stage I, II, III and 102 months for stage IV, P = .19) (supplemental Figure 1). Hematologic response was associated with survival in both cohorts (Figure 4C-D). When survival between λ and κ cohorts was compared according to response, there was a statistically significant difference only in those patients achieving a PR (CR: median OS not reached for λ and κ, P = .47; VGPR: median OS not reached for λ and κ, P = .56; PR: median OS 83 months for λ vs not reached for κ, P = .02 and NR: median OS 19 months for both λ and κ, P = .52).
OS according to Mayo stage 2004 and hematologic response. (A) OS of the λ cohort by Mayo stage 2004. (B) OS of the κ cohort by Mayo stage 2004. (C) OS of the λ cohort by hematologic response. (D) OS of the κ cohort by hematologic response.
OS according to Mayo stage 2004 and hematologic response. (A) OS of the λ cohort by Mayo stage 2004. (B) OS of the κ cohort by Mayo stage 2004. (C) OS of the λ cohort by hematologic response. (D) OS of the κ cohort by hematologic response.
All-cause mortality at 100 days (TRM) for the whole cohort was 5.7% (n = 32). The rates of early mortality were not significantly different between the 2 cohorts (6.7% for λ vs 3.3% for κ, P = .1). We assessed the impact of Mayo stage, conditioning dose, and organ involvement (>2 organs) on TRM. In the λ cohort, Mayo stage 2012 (TRM 1.9% stage I vs 9.5% stage II vs 9.8% stage III vs 19.4% stage IV, P = .001), Mayo stage 2004 (TRM 3.9% stage I vs 8.5% stage II vs 16.6% stage III, P = .006), conditioning dose (see above), and organ involvement (TRM 5.2% ≤2 organs involved vs 15.8% for >2 organs involved, P = .0083) were all predictive of TRM. In the κ cohort, TRM was not significantly affected by Mayo stage 2012 (TRM 2.1% stage I vs 4.3% stage II vs 4.6% stage III vs 0% stage IV, P = .72), Mayo stage 2004 (TRM 3.4% stage I vs 0% stage II vs 4.2% stage III, P = .50), conditioning dose (see above), or organ involvement (TRM 2.3% ≤2 organs involved vs 10% for >2 organs involved, P = .13).
Subgroup analysis of treatment naive patients
We recognize including patients who received chemotherapy prior to transplantation may confound the data. To account for this, we performed a subgroup analysis of outcomes and prognostic factors by light chain type in the cohort of patients who received no chemotherapy prior to transplant. A total of 320 previously untreated patients were included in the subgroup analysis, 249 (78%) with λ and 71 (22%) with κ light chain type AL amyloidosis. Patients with κ light chain amyloidosis had better PFS and OS (PFS: λ 69 months vs κ 123 months, P = .058 and OS: λ 120 months vs κ not reached, P = .0078), although the PFS difference did not meet statistical significance. Mayo stage 2004 predicted survival in the λ cohort (median OS of 142 months stage I vs 55 months stage II vs 29 months stage III, P < .0001) but not in the κ cohort (median OS not reached for stage I, II and 100 months for stage III, P = .32). Similarly, the Mayo stage 2012 also predicted survival in the λ cohort (median OS of 146 months stage I vs 109 months stage II vs 58 months stage III vs 20 months stage IV, P < .0001) but not in the κ cohort (median OS not reached for stage I, II, III and 100 months for stage IV, P = .41). Finally, conditioning dose after a landmark survival analysis from day 100 post-ASCT was a significant predictor of survival in the λ cohort with no impact on survival in the κ cohort (λ: median OS 142 months for melphalan 200 mg/m2 vs 43months for melphalan <200 mg/m2, P < .0001 and κ: median OS not reached for both groups, P = .37).
Multivariate analysis for whole cohort
On univariate analysis, factors that significantly predicted survival included age >65, male sex, BMPCs ≥10%, >2 organs involved, time period of transplant, Mayo stage 2012, Mayo stage 2004, conditioning dose, and light chain subtype. These factors were used in our Cox proportional hazards model. Independent predictors of survival after multivariate analysis were male sex, BMPCs ≥10%, time period of transplant, Mayo stage, conditioning dose, and light chain subtype. When the 2012 Mayo stage was incorporated in the model instead of the 2004 staging system, male sex and time period of transplant were no longer significant (Table 3).
Multivariate model
| Multivariate model A . | Multivariate model B . | ||||
|---|---|---|---|---|---|
| Parameter . | Risk ratio (95% CI) . | P . | Parameter . | Risk ratio (95% CI) . | P . |
| λ Light chain | 2.3 (1.5-3.7) | .0001 | λ Light chain | 2.1 (1.4-3.4) | .0004 |
| Mayo stage 2012 III/IV vs I/II | 2.5 (1.7-3.8) | <.0001 | Mayo stage 2004 II/III vs I | 2.1 (1.4-3.0) | <.0001 |
| Conditioning dose 200 vs <200 mg/m2 | 0.4 (0.3-0.5) | <.0001 | Conditioning dose 200 vs <200 mg/m2 | 0.4 (0.3-0.6) | <.0001 |
| BMPCs ≥10% | 1.5 (1.1-2.1) | .015 | BMPCs ≥10% | 1.6 (1.2-2.2) | .005 |
| ASCT date <2010 vs ≥2010 | 1.5 (0.99-2.3) | .06 | ASCT date <2010 vs ≥2010 | 1.6 (1.0-2.4) | .03 |
| >2 organs involved | 0.8 (0.5-1.3) | .37 | >2 organs involved | 0.8 (0.5-1.3) | .44 |
| Male sex | 1.4 (0.99-2.0) | .06 | Male sex | 1.4 (1.0-2.0) | .048 |
| Age ≥65 y | 1.1 (0.7-1.5) | .72 | Age ≥65 y | 1.1 (0.7-1.6) | .66 |
| Multivariate model A . | Multivariate model B . | ||||
|---|---|---|---|---|---|
| Parameter . | Risk ratio (95% CI) . | P . | Parameter . | Risk ratio (95% CI) . | P . |
| λ Light chain | 2.3 (1.5-3.7) | .0001 | λ Light chain | 2.1 (1.4-3.4) | .0004 |
| Mayo stage 2012 III/IV vs I/II | 2.5 (1.7-3.8) | <.0001 | Mayo stage 2004 II/III vs I | 2.1 (1.4-3.0) | <.0001 |
| Conditioning dose 200 vs <200 mg/m2 | 0.4 (0.3-0.5) | <.0001 | Conditioning dose 200 vs <200 mg/m2 | 0.4 (0.3-0.6) | <.0001 |
| BMPCs ≥10% | 1.5 (1.1-2.1) | .015 | BMPCs ≥10% | 1.6 (1.2-2.2) | .005 |
| ASCT date <2010 vs ≥2010 | 1.5 (0.99-2.3) | .06 | ASCT date <2010 vs ≥2010 | 1.6 (1.0-2.4) | .03 |
| >2 organs involved | 0.8 (0.5-1.3) | .37 | >2 organs involved | 0.8 (0.5-1.3) | .44 |
| Male sex | 1.4 (0.99-2.0) | .06 | Male sex | 1.4 (1.0-2.0) | .048 |
| Age ≥65 y | 1.1 (0.7-1.5) | .72 | Age ≥65 y | 1.1 (0.7-1.6) | .66 |
CI, confidence interval.
Discussion
Data regarding the importance of light chain type in AL amyloidosis are limited. Studies have suggested variability in clinical presentation and response to therapy by light chain subtype.16,30 Our study identifies light chain subtype as an independent predictor of survival in patients undergoing stem cell transplantation for systemic AL amyloidosis, showing that those with λ subtype have worse outcomes. We also highlight important differences in the clinical and biologic features with respect to organ involvement and degree of light chain production between the 2 subtypes. What was particularly notable is the different way in which the 2 types of light chain amyloidosis behave with respect to the traditional prognostic markers in patients with AL amyloidosis. The pretransplantation variables of Mayo stage, number of organs involved, and conditioning dose are important predictors of outcome in patients receiving stem cell transplantation for amyloidosis. In our data, these variables remain prognostic only in the λ cohort.
There were significant differences in the types of organ involved depending on light chain subtype. This likely reflects a difference in the underlying biology of the type of light chains, with previous reports highlighting the importance of immunoglobulin light chain variable gene use and tropism of organ involvement in patients with AL amyloidosis.19,31
Patients with κ light chain amyloidosis had a higher pretransplant dFLC. This may to some extent reflect the reported propensity of the immunoglobulin free light chain assay to overestimate κ light chain levels more than λ.32-33 However, the degree of difference detected between the 2 cohorts we believe is not entirely accounted for by analytical error of the assay and rather reflects more proliferative disease in this cohort. The higher baseline dFLC level in the κ cohort also explains the reason more patients in this cohort had a Mayo stage 2012 of greater than I compared with the λ cohort. This may in part account for inability of the 2012 staging system to prognosticate with respect to survival in the κ cohort. However, our analysis of the 2004 staging system revealed it to be a strong prognostic factor in the λ cohort while continuing to be less discriminatory in the κ cohort. Although patients in the κ cohort were more likely to be treated with chemotherapy prior to transplantation, pretreatment with chemotherapy was not associated with improved survival. The λ cohort had more renal involvement and higher 24-hour urine protein excretion, and neither of these variables predicted survival.
Despite a worse OS and PFS, the λ group had a similar response rate to ASCT compared with the κ cohort. It was particularly notable, however, on analysis of survival by light chain type and response category that the primary difference in survival between the 2 light chain cohorts was in those achieving a PR. This suggests that those with a deep response (≥VGPR) do well irrespective of light chain type and likewise those that do not respond have a poor prognosis irrespective of light chain type. However, in patients who achieve a PR, the λ cohort has a poor prognosis compared with κ and may identify a population that benefits most from consolidation therapy posttransplantation in an effort to deepen responses beyond PR. Attaining deep responses in the treatment of AL amyloidosis is critical given the role of the amyloidogenic light chain in the development of organ dysfunction. We have also identified that conditioning dose is a strong predictor of attaining CR in the λ cohort. Given this finding, dose reduction of melphalan conditioning regimen should be avoided in patients with λ light chain amyloidosis, particularly with the increasing availability of novel agents as alternatives to ASCT.
Although TRM was not significantly different between the 2 cohorts, we have identified particular subgroups of patients within the λ cohort that appear to have higher risk: those with a higher Mayo stage, those with a number of organs involved, and patients receiving reduced intensity conditioning. These factors need consideration when assessing patients for eligibility for ASCT.
Our results need to be viewed in the context of the retrospective nature and long time period of our study. Diagnostic techniques and treatment practices have evolved over the time period of our study. Given light chain type was central to our study, we only included patients who were transplanted after the introduction of the immunoglobulin free light chain assay to our clinical practice. Nevertheless, amyloid typing has evolved from histological and immunohistochemical methods to mass spectrometry over the years, and the methods for typing our patients were not uniform.
In summary, we have shown that light chain type is an independent prognostic marker in patients with AL amyloidosis receiving stem cell transplant and predicts the relative likelihood of organ involvement. In addition, we have shown that the known prognostic factors appear to be less relevant in those with κ type compared with λ. This challenges our traditional paradigm of considering these 2 types as identical diseases that are risk stratified according to other features such as organ involvement or Mayo stage. The relevance of dose of melphalan to response and outcomes in only the λ cohort also raises the question of potential differences in response to other agents based on light chain type and needs further evaluation.
The full-text version of this article contains a data supplement.
Authorship
Contribution: M.H.S. designed the study, analyzed the data, wrote the first draft, and approved the final version of the manuscript; M.A.A. assisted in data collection, analyzed the data, revised the manuscript critically, and approved the final version; E.M., F.K.B., R.W., M.Q.L., A.D., D.D., N.L., W.I.G., S.K.K., P.K., T.V.K., and W.J.H. performed patient management, revised the manuscript critically, and approved the final version of the manuscript; and M.A.G. designed the study, analyzed the data, wrote the first draft, approved the final version of the manuscript, and performed patient management.
Conflict-of-interest disclosure: M.A.G. received consultancy from Millenium and honoraria from Celgene, Millenium, Onyx, Novartis, SmithKline, Prothena, Ionis, and Amgen. S.K.K. received consultancy from Celgene, Millennium, Onyx, Janssen, and BMS and research funding from Celgene, Millennium, Novartis, Onyx, AbbVie, Janssen, and BMS. M.Q.L. received research funding from Celgene. D.D. received research funding from Karyopharm Therapeutics, Amgen, and Millenium Pharmaceuticals. P.K. received research funding from Takeda, Celgene, and Amgen. A.D. received research funding from Celgene, Millennium, Pfizer, and Janssen and a travel grant from Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Morie A. Gertz, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gertz.morie@mayo.edu.