Background
Hodgkin lymphoma (HL) has been an exemplar for many advances in cancer care. It has remained at the vanguard in terms of recent successes that use targeted monoclonal antibodies, including those that deliver conjugated cytotoxic agents (eg, brentuximab vedotin [BV]) and those that enhance endogenous antitumor immunity through manipulation of immune regulatory checkpoints (eg, the programmed cell death protein 1:programmed death-ligand 1 [PD-1:PD-L1] axis and the CTLA-4:B7 axis). It is the development of these antibody therapies, in particular, that leads to the need to re-evaluate established treatment paradigms. To address the question of whether all patients with HL who relapse after autologous stem cell transplantation (ASCT) should be considered for allogeneic stem cell transplantation (alloSCT), it is helpful to briefly consider the positioning of alloSCT before the advent of these therapies. This will provide the platform for debating whether our views should be changed in the context of available outcome data from the newer agents.
The unmet clinical need for treatment for patients who relapse after ASCT is well described. A study of outcomes in 756 relapsed patients receiving ASCT before 2007 demonstrated a median post progression survival of 1.3 years1 ; the majority of patients had poor long-term outcomes, with approximately 10% surviving at 10 years. The utility of alloSCT in this cohort of patients, the majority of whom remain responsive to some form of salvage therapy, has remained controversial. Prohibitive non-relapse mortality (NRM) rates were greatly improved after the introduction of reduced-intensity conditioning, which also facilitated the demonstration of a clinically relevant graft-versus-HL effect.2 With follow-up now exceeding 15 years, the curative potential of alloSCT is established.3 Although representing a selected subgroup, such series indicate overall survival (OS) of 65% (95% confidence interval, 47% to 82%) and progression-free survival (PFS) of 43% (95% confidence interval, 23% to 64%) at 4 years.4 In the absence of prospective comparative trials, several retrospective studies suggested that survival outcomes after failure of ASCT were improved in those deemed potentially suitable for alloSCT who had an HLA-compatible donor and underwent transplantation vs those who did not,5-7 with a significant advantage (P < .001) for both OS and PFS in a donor vs no donor comparison of 185 patients.6 Age itself is rarely prohibitive for patients who are considered for alloSCT, because modern alloSCT platforms support transplantation for patients with a similar age range to that used for ASCT, and most relapses after ASCT will occur relatively early. Potential donor sources have been expanded to include both cord blood and haploidentical donors, which means that virtually all patients now have an HLA-compatible donor.8 There will still be some patients, however, who are not deemed appropriate for alloSCT, either through their own choice or because of comorbidities. These issues do not preclude consideration of alloSCT but merely inform the consideration process.
The evolving impact of BV
The remarkable single-agent activity of BV in 102 patients who relapsed after ASCT added to the controversy of whether responding patients should still be considered for alloSCT.9 Those with stable disease or partial responses had very poor durability of response (median PFS, 5.8 and 6.9 months, respectively), suggesting that early consolidative alloSCT might be the best option, whereas some of those who achieved a complete response (CR) seemed to have more durable responses. With the caveat that this group with ongoing CR included only 13 of 34 patients who had achieved CR (of whom 4 were consolidated with alloSCT), it was suggested that these patients should not undergo alloSCT, particularly because emergent data suggested that re-treatment with BV might be an option. The published re-treatment data in HL remains relatively modest (n = 20).10 Key conclusions are that it is feasible without significant toxicity and with reasonable response rates (overall response rate, 60%; CR rate [mixture of computed tomography and positron emission tomography], 30%). Another point worth noting is that there is no apparent plateau in PFS, which suggests that these second responses are less durable and that consolidation should be considered. This issue is highly relevant when we consider the current usage of BV in the overall treatment pathway. Patients in the pivotal study were BV naïve,9 but this is unlikely to be the case with patients we are now seeing in the clinic. Many will have received BV either as part of their salvage therapy or as maintenance after ASCT (according to National Comprehensive Cancer Network and American Society for Blood and Marrow Transplantation guidelines in high-risk patients),11-12 and BV may become established as part of first-line therapy on the basis of improvement in modified PFS in the ECHELON-1 study. We would therefore be considering alloSCT in the context of re-treatment if not progression on BV therapy. In either case, there is no compelling argument for not considering alloSCT in this setting in the absence of other novel therapies. Of note, the administration of BV before alloSCT does not seem to have an adverse impact on transplantation outcomes.13 The final decision on appropriateness would then depend on patient and donor-specific issues that map to the risk of transplantation and projected outcomes.
PD-1:PD-L1 axis inhibitors
The introduction of PD-1:PD-L1 axis inhibitors necessitates re-evaluation of our treatment strategies. These agents will likely move quickly up the treatment pathway, making the considerations outlined for BV also relevant. Currently, however, relatively few patients will have been exposed to PD-1:PD-L1 axis inhibitors by the time they relapse after ASCT. The 2 agents associated with the most mature clinical data are both anti-PD-1 antibodies (nivolumab and pembrolizumab). Single-agent activity is impressive in both BV-exposed (overall response, 66% to 71%; CR, 9% to 22%) and BV-naïve patients.14-16 Thus, it could be argued that we should treat all patients who do not achieve a metabolic complete response (mCR) after BV therapy with 1 of these agents, and that we should also defer alloSCT in those who achieve mCR to BV in favor of using anti-PD-1 antibodies to salvage those who subsequently relapse. To understand whether we should consider alloSCT for consolidation of response, we need to revisit the arguments that were presented for BV. This largely depends on a balance between how many patients would be spared an unnecessary alloSCT with its attendant risk of mortality and morbidity and how many patients would subsequently relapse and not regain sufficient disease control to allow alloSCT or would develop preclusive comorbidities as a result of the additional therapy. It also depends on whether alloSCT outcomes are negatively influenced by prior treatment with these agents. The latter is particularly important if we are going to recommend such therapies in patients who have achieved sufficient response to most recent salvage to be considered good candidates for alloSCT.
To inform these decisions, we need to consider which patients would be good candidates for alloSCT and the impact of PD-1 inhibitors on transplantation outcomes. As noted earlier, the majority of responses to BV manifest as either stable disease or partial response. Do these responses necessarily require further consolidation before alloSCT? The question remains controversial, but I believe the answer is no. The presence of residual fluorodeoxyglucose-avid disease before ASCT has a significant impact on survival outcomes, but its impact in alloSCT seems to be far more modest in those with chemotherapy-sensitive disease.4 Indeed, the number of lines of prior therapy becomes an important predictor of NRM after alloSCT, adversely impacting survival outcomes. Could PD-1 inhibitors adversely affect alloSCT outcomes? Although the issue is contentious, the answer is quite possibly yes, largely through their impact on the incidence of more severe forms of acute graft-versus-host disease (GVHD) or hepatic sinusoidal obstruction syndrome (SOS). In a relatively small retrospective study of patients undergoing alloSCT after PD-1 blockade, the 1-year cumulative incidences of grade 3 to 4 acute GVHD was 23% and 8% for SOS.17 These rates were felt to be in excess of expected incidence rates. Although these data are far from definitive, they align well with the experience of using PD-1 inhibitors for relapse after alloSCT, in which high response rates are documented along with the frequent occurrence of GVHD (1 study of 31 patients reported 8 deaths (26%) after the onset of new acute GVHD).18 For this reason, I believe that patients should be considered for alloSCT if they achieve sufficient response to prior salvage, rather than routinely receiving PD-1 blockade to upgrade the depth of response.
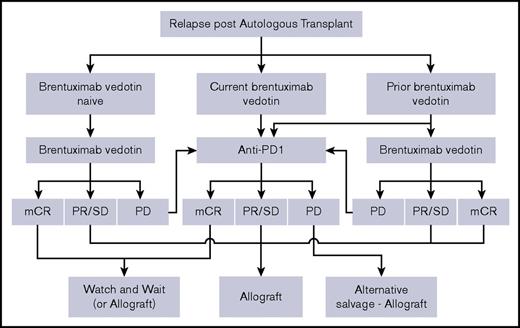
The argument becomes somewhat different if the intent is to defer alloSCT indefinitely. Because the vast majority of responses to PD-1 inhibitors are only partial, we might expect a gradual loss of response over time, even in patients with ongoing therapy. Although follow-up is relatively short, that is the emergent pattern. With either nivolumab or pembrolizumab, approximately half of patients lose response by 12 to 15 months.14-16 Longer follow-up is clearly required to define the size of the population that gains more durable disease control, but this is likely to be a minority. Because patients with disease progression will require alternative salvage therapy before they can be considered for alloSCT (and the efficacy and toxicity of such salvage is uncertain), and because alloSCT outcomes may be prejudiced in patients who have received anti-PD-1 therapy close to the time of transplant, deferring consideration for alloSCT will clearly have an adverse impact on some patients. This is, however, balanced by the good quality of life that can be achieved with PD-1 inhibitors and deferral of NRM risk. Avoidance of alloSCT would undoubtedly be preferable if it were possible, and a proportion of patients may achieve this. In this respect, the decision to proceed to alloSCT is an individualized one. Our current difficulty is that we cannot prospectively identify which patients should or should not proceed to alloSCT. Furthermore, given the intrinsic delays in identifying appropriate donors, we do need to consider such issues in advance. Therefore, I believe that, given current uncertainties, we should at least still consider alloSCT for any patient who relapses after ASCT. A potential algorithm to aid clinical decision-making is provided in Figure 1.
Simplified management algorithm for patients who relapse after ASCT. The schema outlines a suggested approach to these patients, stratified according to prior or current exposure to brentuximab vedotin. Other options in terms of salvage chemotherapy-radiotherapy exist but have been omitted to reduce complexity. It is recognized that the decision to proceed to alloSCT is an individualized one that will be influenced by other clinical considerations, donor options, and patient views. Given that survival outcomes with T-cell–depleted alloSCT are no worse, and indeed may even be better than those with T-replete regimens,19 and additional concerns regarding GVHD risk after anti-PD-1 agents, use of a T-cell–depleted platform may be preferable after anti-PD-1 therapy. PD, progressive disease; PR, partial response; SD, stable disease.
Simplified management algorithm for patients who relapse after ASCT. The schema outlines a suggested approach to these patients, stratified according to prior or current exposure to brentuximab vedotin. Other options in terms of salvage chemotherapy-radiotherapy exist but have been omitted to reduce complexity. It is recognized that the decision to proceed to alloSCT is an individualized one that will be influenced by other clinical considerations, donor options, and patient views. Given that survival outcomes with T-cell–depleted alloSCT are no worse, and indeed may even be better than those with T-replete regimens,19 and additional concerns regarding GVHD risk after anti-PD-1 agents, use of a T-cell–depleted platform may be preferable after anti-PD-1 therapy. PD, progressive disease; PR, partial response; SD, stable disease.
Authorship
Contribution: K.S.P. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Karl S. Peggs, University College London Cancer Institute, Paul O’Gorman Building, 72 Huntley St, London WC1E 6BT, United Kingdom; e-mail: k.peggs@cancer.ucl.ac.uk.