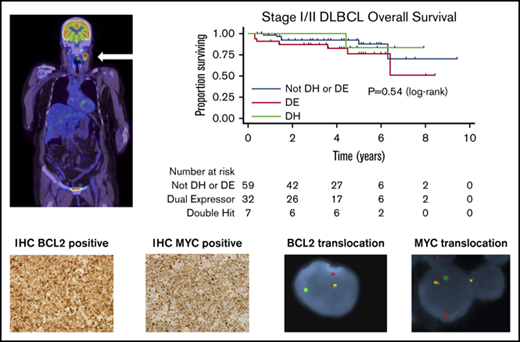
Key Points
In early-stage DLBCL, COO and DE/DH status may not confer an inferior prognosis.
Stage I/II DLBCL has an excellent outcome when treated with R-CHOP–like therapy ± radiation, with 4-year PFS and OS rates of 85% and 88%.
Abstract
In advanced-stage diffuse large B-cell lymphoma (DLBCL), the presence of an activated B-cell phenotype or a non–germinal center (GCB) phenotype, coexpression of MYC and BCL2 by immunohistochemistry, and the cooccurrence of MYC and BCL2 or BCL6 rearrangements are associated with inferior outcomes. It is unclear whether these variables remain prognostic in stage I/II patients. In this retrospective study, we evaluated the prognostic impact of cell of origin (COO), as well as dual-expressor (DE) status and molecular double-hit (DH) status, in stage I/II DLBCL by positron emission tomography with computed tomography (PET-CT). A total of 211 patients treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone)–like regimens, with or without radiotherapy, was included. The median follow-up in the entire cohort was 4 years (range, 0.4-9.4), with estimated 4-year progression-free survival (PFS) and overall survival (OS) rates of 85% (95% confidence interval [CI], 79-89) and 88% (95% CI, 83-92), respectively. By univariable analysis, DE (PFS: hazard ratio [HR], 1.27; 95% CI, 0.58-2.81, P = .55 and OS: HR, 1.40; 95% CI, 0.60-3.30; P = .44), DH (PFS: HR, 1.21; 95% CI, 0.27-5.31; P = .80 and OS: HR, 0.61; 95% CI, 0.08-4.73; P = .64), and non-GCB status (PFS: HR, 1.59; 95% CI, 0.83-3.03; P = .16 and OS: HR, 1.80; 95% CI, 0.89-3.67; P = .10) were associated with poorer outcomes. In patients with PET-CT–defined stage I/II DLBCL treated with R-CHOP–like therapy, with or without radiation, COO and DE and DH status were not significantly associated with inferior PFS or OS.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma worldwide. Up to 30% of patients present with stage I/II disease when positron emission tomography with computed tomography (PET-CT) is used for staging.1-3 Two major subtypes of DLBCL have been defined by gene-expression profiling (GEP): activated B-cell like (ABC) and germinal center B-cell like (GCB).4 Subsequently, the use of immunohistochemistry (IHC)-based algorithms have been shown to correctly assign cell of origin (COO) with ∼80% concordance with GEP.5-7
Among DLBCL patients, COO has been shown to influence overall prognosis, with the ABC (or non-GCB) group experiencing inferior outcomes when treated with standard R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemoimmunotherapy in some studies.5,8-11 In contrast, a recent retrospective analysis by Kumar et al found that non-GCB COO (defined by Hans algorithm) did not have prognostic impact among 87 patients with limited-stage DLBCL treated with R-CHOP and radiation therapy (RT) at Memorial Sloan Kettering Cancer Center.12 Other investigators have suggested that part of the adverse prognostic impact observed in patients with ABC DLBCL may be attributable to the greater frequency of cases with overexpression of MYC and BCL2 (so-called “dual expressors” [DEs]) within this subgroup.13,14 It also remains unclear whether the negative prognostic impact of DE status is retained in stage I/II disease.
Tumors with morphologic features of DLBCL bearing rearrangements in MYC and BCL2 and/or BCL6 represent a unique biologic entity that is characterized by aggressive clinical behavior, tendency toward extranodal and central nervous system involvement, and poor responses to R-CHOP chemoimmunotherapy.15 In the 2016 revision of the World Health Organization classification, such cases now fall under a new category: high grade B-cell lymphoma with rearrangements in MYC and BCL2 and/or BCL6 – double or triple hit (HGBL-DH/TH). Intensified chemotherapy regimens and targeted therapies have been proposed as potential strategies to improve cure rates among these biologically defined high-risk patients.16-18 However, widespread adoption can increase treatment toxicity and healthcare expenditure and may be unnecessary in a subset of patients with favorable outcomes.19,20 Data from 2 retrospective series suggest that, among the small number of patients with HGBL-DH/TH and stage I/II disease, outcomes may be comparable to stage I/II DLBCL.21,22 Because of potential selection bias among retrospective series published to date, the true incidence of HGBL-DH/TH among patients with DLBCL morphology and stage I/II disease remains unknown.
We hypothesized that, among patients with DLBCL morphology and PET-CT–defined stage I/II disease treated with R-CHOP–like regimens, with or without RT, non-GCB COO, DE status, and/or HGBL-DH/TH may not impact prognosis.
Methods
We performed a retrospective review of medical records and lymphoma registries at Sir Charles Gairdner Hospital, Hollywood Private Hospital, Princess Alexandra Hospital, British Columbia Centre for Lymphoid Cancer, Aalborg University Hospital, and Nottingham University Hospital to identify patients with PET-CT–defined stage I/II DLBCL treated with R-CHOP, with or without RT, who were diagnosed between January 2002 and December 2013. Patients with dose-intensified chemotherapy were excluded. Eligible patients had a morphologic diagnosis of DLBCL. Patients with evidence of histologic transformation from indolent lymphoma, follicular lymphoma grade 3B, and posttransplant lymphoproliferative disorder were excluded.
CD10, BCL6, and MUM1 IHC were used to assign COO using the Hans algorithm.5 IHC staining for MYC expression (positive ≥40%) and BCL2 expression (positive ≥50%) was used to define DE status. Fluorescence in situ hybridization (FISH) testing for rearrangements in MYC and BCL2 and/or BCL6 defined double-hit (DH) and triple-hit (TH) cases. Methods used for the selection of cases on which to perform FISH, at the time of diagnosis and retrospectively, differed between sites; some centers tested all cases, whereas others limited testing to those with MYC IHC positivity. Local IHC and FISH results were included in the analysis. IHC cutoffs were standardized among sites. We attempted to retrieve original diagnostic material to complete the missing IHC and FISH testing; however, this was not possible for all cases as a result of insufficient or missing material, refusal from hospitals to release the samples, and patients declining consent for further testing on archival tissue. PET-CT imaging was interpreted locally and based on individual practices. There was no central review.
The primary end point of the study was to determine the impact of COO and DE and DH status on progression-free survival (PFS) and overall survival (OS). Secondary end points included the evaluation of other prognostic variables and treatment modalities on PFS and OS. Differences in patient characteristics among groups (DE, DH, and non-DE/DH) were analyzed using Fisher’s exact test. OS was defined as the time from the date of diagnosis until death from any cause, and PFS was defined as the time from diagnosis until relapse/progression or death; both were calculated using the Kaplan-Meier method, as were 4-year OS and PFS.23 Differences in OS/PFS were compared using log-rank tests, and associations between prognostic factors and outcomes were analyzed using Cox proportional hazards model. Variables with P < .1 on univariable analysis and primary outcome measures were included in the multivariable analysis, with P < .05 considered significant. Cumulative incidence was calculated using inverse Kaplan-Meier methodology.
Results
We identified 211 patients with PET-CT–defined stage I/II DLBCL who met inclusion criteria. For the purpose of the primary analysis, patients were divided into 3 groups: DH (irrespective of DE status [n = 7]), DE (not DH) (n = 33), and “other” (cases who did not fulfill either criteria [n = 171]). No TH cases were identified. Their characteristics are summarized in Table 1. Cases found to be both DE and DH were assigned to the DH group. Of the “other” group, 59 (35%) had sufficient IHC/FISH data to assign them as non-DE/DH. In the entire cohort, 175 cases (83%) had sufficient information to assess DE status; 37 (21%) were classified as DE, and 4 were concurrently DH, leaving 33 in the DE (not DH) group. One DH case had insufficient IHC to assess for DE status, and 106 cases (50%) had adequate FISH results to assign DH status, which was present in 7 (7%) cases. DH status was determined retrospectively in all 7 cases and not at the time of diagnosis; 3 of 7 DH cases were of non-GCB COO, of which 2 bore rearrangements in MYC and BCL6. In 192 cases (91%), there were sufficient IHC data to assign COO: 116 (60%) GCB and 76 (40%) non-GCB. The median age was 62 years (range, 19-89), and 51% were female. In total, 98% of patients received standard R-CHOP chemotherapy as first-line treatment, with the remainder receiving “R-CHOP–like” regimens: rituximab, cyclophosphamide, vincristine, etoposide, and prednisolone (n = 1), R-CHOP de-escalated to rituximab, cyclophosphamide, vincristine, and prednisolone (n = 2), and R-CHOP changed to rituximab, cyclophosphamide, vincristine, etoposide, and prednisolone (n = 2). The median number of chemotherapy cycles was 6 (1-2 cycles [n = 7], 3-4 cycles [n = 80], 5-6 cycles [n = 117], 7-8 cycles [n = 7]). Forty-one percent (n = 87) of patients received RT with a median of 30 Gy (range, 18-45) over 15 fractions (range, 5-30). Of the patients who received RT, 64% received abbreviated chemotherapy (<6 cycles). None of the patients received treatment intensification with strategies such as dose-adjusted etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin, and rituximab (daEPOCH-R); rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine; cyclophosphamide, vincristine, doxorubicin, methotrexate, ifosfamide, etoposide, cytarabine, and rituximab; or consolidative autologous stem cell transplant.
Patient characteristics by DE status, DH status, or other
| Characteristic . | Other* (N = 171) . | DE (N = 33)† . | DH (N = 7) . | P . |
|---|---|---|---|---|
| Age | ||||
| Median (range), y | 62 (19-89) | 62 (35-84) | 65 (44-77) | |
| >60 y | 96 (56) | 19 (58) | 4 (57) | 1.000 |
| Sex | ||||
| Male | 89 (52) | 11 (33) | 3 (43) | .128 |
| Stage at diagnosis | ||||
| I | 97 (57) | 26 (79) | 3 (43) | .030 |
| II | 74 (43) | 7 (21) | 4 (57) | |
| Poor performance status | ||||
| ECOG >1 | 24 (14) | 4 (12) | 0 (0) | .831 |
| LDH > ULN | 44 (31) | 10 (37) | 3 (50) | .418 |
| Extranodal sites–any | 91 (53) | 21 (64) | 3 (43) | .458 |
| >1 | 5 (3) | 0 (0) | 0 (0) | |
| Supradiaphragmatic | 103 (60) | 22 (67) | 3 (43) | .517 |
| Ki67, median (range) | 80 (15-100) | 82 (25-95) | 85 (40-90) | .337 |
| Bulk >7.5 cm | 33 (21) | 5 (18) | 4 (57) | .075 |
| COO | ||||
| GCB | 99 (65) | 13 (39) | 4 (57) | .019 |
| Non-GCB | 53 (35) | 20 (61) | 3 (43) | |
| Stage-modified IPI | ||||
| Low (0-1 point) | 71 (51) | 19 (73) | 3 (50) | .180 |
| Intermediate (2 points) | 41 (30) | 3 (12) | 1 (17) | |
| High (3-4 points) | 27 (19) | 4 (15) | 2 (33) |
| Characteristic . | Other* (N = 171) . | DE (N = 33)† . | DH (N = 7) . | P . |
|---|---|---|---|---|
| Age | ||||
| Median (range), y | 62 (19-89) | 62 (35-84) | 65 (44-77) | |
| >60 y | 96 (56) | 19 (58) | 4 (57) | 1.000 |
| Sex | ||||
| Male | 89 (52) | 11 (33) | 3 (43) | .128 |
| Stage at diagnosis | ||||
| I | 97 (57) | 26 (79) | 3 (43) | .030 |
| II | 74 (43) | 7 (21) | 4 (57) | |
| Poor performance status | ||||
| ECOG >1 | 24 (14) | 4 (12) | 0 (0) | .831 |
| LDH > ULN | 44 (31) | 10 (37) | 3 (50) | .418 |
| Extranodal sites–any | 91 (53) | 21 (64) | 3 (43) | .458 |
| >1 | 5 (3) | 0 (0) | 0 (0) | |
| Supradiaphragmatic | 103 (60) | 22 (67) | 3 (43) | .517 |
| Ki67, median (range) | 80 (15-100) | 82 (25-95) | 85 (40-90) | .337 |
| Bulk >7.5 cm | 33 (21) | 5 (18) | 4 (57) | .075 |
| COO | ||||
| GCB | 99 (65) | 13 (39) | 4 (57) | .019 |
| Non-GCB | 53 (35) | 20 (61) | 3 (43) | |
| Stage-modified IPI | ||||
| Low (0-1 point) | 71 (51) | 19 (73) | 3 (50) | .180 |
| Intermediate (2 points) | 41 (30) | 3 (12) | 1 (17) | |
| High (3-4 points) | 27 (19) | 4 (15) | 2 (33) |
Unless otherwise noted, all data are n (%). Bold indicates statistically significant results.
ECOG, Eastern Cooperative Oncology Group; IPI, International Prognostic Index; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Sufficient IHC/FISH in 59 cases for full DH/DE assessment.
Excludes DH cases.
Of the patients who had an end-of-treatment (EOT) PET-CT assessment (n = 192), 94% achieved a complete response (CR) (defined according to local practice), 4% achieved a partial response (PR), and 2% had progressive disease. On analysis, there was a statistically significant superior outcome in patients who received >3 vs ≤3 cycles of chemotherapy (PFS: hazard ratio [HR], 0.28; 95% confidence interval [CI], 0.15-0.53; P < .001 and OS: HR, 0.25; 95% CI, 0.13-0.50; P < .001); however, there was no difference in outcome between those who received chemotherapy only vs combined modality therapy (PFS: HR, 1.23; 95% CI, 0.66-2.30; P = .51 and OS: HR, 1.09; 95% CI, 0.55-2.14; P = .81). Most, but not all, patients who received <6 cycles of chemotherapy received concurrent RT (61%). In this group, there also was no difference between those who received chemotherapy only vs combined modality therapy (PFS: HR, 1.28; 95% CI, 0.54-3.05 and OS: HR, 1.06; 95% CI, 0.43-2.62; P = .90) (Table 2).
EOT response
| . | DE (N = 32) . | DH (N = 6) . | Other (N = 160) . |
|---|---|---|---|
| CR | 28 | 5 | 147 |
| PR | 2 | 0 | 6 |
| PD | 0 | 1 | 3 |
| Dead before assessment | 2 | 0 | 4 |
| . | DE (N = 32) . | DH (N = 6) . | Other (N = 160) . |
|---|---|---|---|
| CR | 28 | 5 | 147 |
| PR | 2 | 0 | 6 |
| PD | 0 | 1 | 3 |
| Dead before assessment | 2 | 0 | 4 |
PD, progressive disease.
All patients with adequate IHC and FISH results to allow categorization of disease as DH, DE, or non-DH/DE were included in survival analyses. The median follow-up in the entire cohort was 4 years (range 0.4-9.4), with estimated 4-year PFS and OS rates of 85% (95% CI, 79-89) and 88% (95% CI, 83-92), respectively. Patients achieving a CR on EOT assessment (n = 180/199) had a 4-year PFS of 91% (95% CI, 86-95) and OS of 93% (95% CI, 88-96) compared with those who had residual PET positivity (n = 12/199): 31% (95% CI, 8-58) and 62% (95% CI, 26-84), respectively (PFS: P ≤ .001; OS: P = .003). There was no difference in 4-year PFS and OS between those who received chemotherapy only vs combined modality therapy (PFS: 87% vs 91%, P = .51; OS: 90% vs 86%, P = .81).
The baseline characteristics that differed among the DE, DH, and “other” groups were COO and stage. There was a nonsignificant trend toward a higher proportion of patients with bulky disease (defined as >7.5 cm) in the DH group (P = .075, DH 57%, DE 18%, and other 21%) (Table 1).
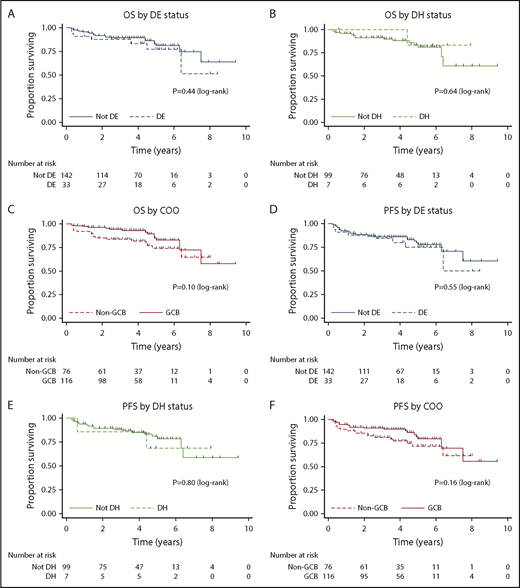
Univariable analysis of candidate prognostic factors for PFS and OS is provided in Table 3. Only cases with adequate IHC or FISH to assign DE or DH status, respectively, were included in the analysis. DE status (PFS: HR, 1.27; 95% CI, 0.58-2.81, P = .55 and OS: HR, 1.40; 95% CI, 0.60-3.30, P = .44; Figure 1A), DH status (PFS: HR, 1.21; 95% CI, 0.27-5.31; P = .80 and OS: HR, 0.61; 95% CI, 0.08-4.73; P = .64; Figure 1B), and non-GCB status (PFS: HR, 1.59; 95% CI, 0.83-3.03; P = .16 and OS: HR, 1.80; 95% CI, 0.89-3.67; P = .10; Figure 1C) did not affect outcomes. Age > 60 years, increased serum lactate dehydrogenase, and stage II disease were adversely prognostic for PFS and OS, whereas bulk disease was adversely prognostic for PFS only. BCL-2 expression by IHC exhibited a trend toward an adverse prognosis for PFS.
Univariable and multivariable analysis
| Candidate factor . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| PFS . | OS . | PFS . | OS . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Age >60 y | 4.53 (1.99-10.28) | <.001 | 7.50 (2.63-21.35) | <.001 | 2.51 (0.51-12.42) | .259 | ||
| Male | 0.72 (0.39-1.34) | .302 | 0.68 (0.34-1.34) | .268 | ||||
| Elevated serum LDH | 2.21 (1.11-4.38) | .023 | 2.43 (1.14-5.19) | .014 | 0.98 (0.20-4.86) | .976 | 1.54 (0.18-12.98) | .690 |
| Stage (II vs I) | 2.60 (1.39-4.90) | .003 | 2.35 (1.19-4.63) | .014 | 0.75 (0.16-3.62) | .723 | 0.32 (0.04-2.85) | .304 |
| Extranodal sites | 1.07 (0.57-2.00) | .831 | 1.16 (0.59-2.31) | .660 | ||||
| Site (supra- vs infradiaphragmatic) | 1.01 (0.53-1.90) | .98 | 1.09 (0.55-2.18) | .800 | ||||
| Ki67 | 0.85 (0.44-1.64) | .622 | 0.88 (0.44-1.79) | .730 | ||||
| Bulk disease | 2.10 (1.06-4.16) | .033 | 2.05 (0.97- 4.32) | .06 | 3.67 (1.00-13.60) | .050 | 3.69 (0.67-20.33) | .134 |
| COO | 0.63 (0.33-1.20) | .159 | 0.55 (0.27-1.13) | .103 | 0.94 (0.18-4.90) | .937 | 0.58 (0.05-6.22) | .652 |
| DH | 1.21 (0.27-5.31) | .804 | 0.61 (0.08-4.73) | .639 | 1.34 (0.18-10.62) | .753 | 0.68 (0.04-12.58) | .792 |
| DE | 1.27 (0.58-2.81) | .552 | 1.40 (0.60-3.30) | .439 | 1.58 (0.35-7.24) | .555 | 1.92 (0.25-15.04) | .533 |
| Stage-modified IPI (n = 92) | ||||||||
| Low risk | ||||||||
| Intermediate risk | 2.95 (1.22-7.15) | .020 | 2.57 (0.93-7.11) | .063 | ||||
| High risk | 5.37 (2.29-12.62) | <.001 | 6.19 (2.43-15.77) | <.001 | ||||
| Candidate factor . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| PFS . | OS . | PFS . | OS . | |||||
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Age >60 y | 4.53 (1.99-10.28) | <.001 | 7.50 (2.63-21.35) | <.001 | 2.51 (0.51-12.42) | .259 | ||
| Male | 0.72 (0.39-1.34) | .302 | 0.68 (0.34-1.34) | .268 | ||||
| Elevated serum LDH | 2.21 (1.11-4.38) | .023 | 2.43 (1.14-5.19) | .014 | 0.98 (0.20-4.86) | .976 | 1.54 (0.18-12.98) | .690 |
| Stage (II vs I) | 2.60 (1.39-4.90) | .003 | 2.35 (1.19-4.63) | .014 | 0.75 (0.16-3.62) | .723 | 0.32 (0.04-2.85) | .304 |
| Extranodal sites | 1.07 (0.57-2.00) | .831 | 1.16 (0.59-2.31) | .660 | ||||
| Site (supra- vs infradiaphragmatic) | 1.01 (0.53-1.90) | .98 | 1.09 (0.55-2.18) | .800 | ||||
| Ki67 | 0.85 (0.44-1.64) | .622 | 0.88 (0.44-1.79) | .730 | ||||
| Bulk disease | 2.10 (1.06-4.16) | .033 | 2.05 (0.97- 4.32) | .06 | 3.67 (1.00-13.60) | .050 | 3.69 (0.67-20.33) | .134 |
| COO | 0.63 (0.33-1.20) | .159 | 0.55 (0.27-1.13) | .103 | 0.94 (0.18-4.90) | .937 | 0.58 (0.05-6.22) | .652 |
| DH | 1.21 (0.27-5.31) | .804 | 0.61 (0.08-4.73) | .639 | 1.34 (0.18-10.62) | .753 | 0.68 (0.04-12.58) | .792 |
| DE | 1.27 (0.58-2.81) | .552 | 1.40 (0.60-3.30) | .439 | 1.58 (0.35-7.24) | .555 | 1.92 (0.25-15.04) | .533 |
| Stage-modified IPI (n = 92) | ||||||||
| Low risk | ||||||||
| Intermediate risk | 2.95 (1.22-7.15) | .020 | 2.57 (0.93-7.11) | .063 | ||||
| High risk | 5.37 (2.29-12.62) | <.001 | 6.19 (2.43-15.77) | <.001 | ||||
Bold indicates statistically significant results.
OS and PFS by DE status, DH status, and COO. Kaplan-Meier curves for OS by DE status (A), OS by DH status (B), OS by COO (C), PFS by DE status (D), PFS by DH status (E), and PFS by COO (F).
OS and PFS by DE status, DH status, and COO. Kaplan-Meier curves for OS by DE status (A), OS by DH status (B), OS by COO (C), PFS by DE status (D), PFS by DH status (E), and PFS by COO (F).
Multivariable analysis did not show any association between non-GCB COO, DE status, DH status, or IHC BCL-2 expression and PFS or OS. The only candidate factor that retained significance on multivariable analysis was tumor bulk >7.5 cm for PFS (HR, 5.10; 95% CI, 1.18-22.12; P = .03). There was no impact on OS (P = .13). Of the 42 patients with tumor bulk >7.5 cm, 11 (26%) received <6 cycles of chemoimmunotherapy, and 21 (50%) received consolidative RT. Of those who had EOT imaging, 32 (89%) patients with bulk disease achieved a CR at the EOT, of whom 16 (50%) received RT. Of the patients with bulk disease achieving CR, 26 patients remained in remission at the time of follow-up, 2 experienced disease relapse and died of lymphoma, 1 died of a nonhematological malignancy, 1 died of renal failure, and 1 experienced lymphoma relapse but underwent second-line therapy and remains in remission. Among this group, there was no impact of RT on PFS or OS (P = .48). Of the 4 (11%) patients with bulk disease who achieved a less than CR at EOT, 1 received RT, and 3 did not. Although 1 patient experienced subsequent disease progression and died, the remaining 3 patients did not progress and were alive at the time of final follow-up. Of the patients who did not have EOT response assessment, 2 patients died of sepsis before PET-CT; 1 patient displayed PET-CT positivity (Deauville score ≥ 4) at the end of chemotherapy, had RT but subsequently died of lymphoma; 1 patient achieved a PR on interim PET-CT and died of cardiac complications; 1 patient had a CR on interim PET-CT, did not progress, and was alive at the time of follow-up; and the remaining patient had no imaging response assessment and died of cardiac complications. Multivariable analysis is summarized in Table 3.
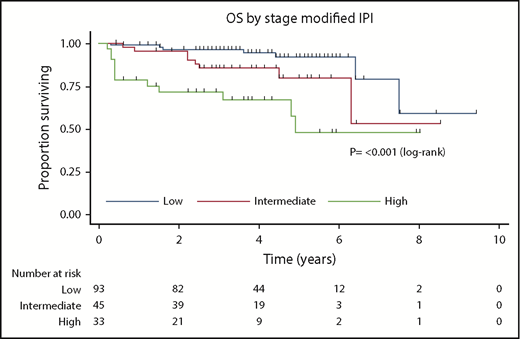
Next, the prognostic utility of the stage-modified International Prognostic Index24 was tested. This showed that patients with low, intermediate, and high risk had 4-year OS of 95%, 86%, and 67%, respectively (P ≤ .001; Figure 2).
Kaplan-Meier curve for stage-modified International Prognostic Index for OS.
Discussion
In this international observational cohort of 211 patients with PET-CT stage I/II DLBCL treated with R-CHOP–like therapy, with or without RT, biological factors known to adversely impact outcomes in DLBCL patients unselected for stage, such as COO and DE and DH status, were not associated with an inferior PFS or OS.
In multiple studies of patients with DLBCL unselected for stage, investigators have demonstrated inferior outcomes in ABC DLBCL (defined by GEP) in the pre- and postrituximab era.4,8,9,25 Although the use of IHC algorithms, such as the Hans algorithm, has been recommended in the latest revision of the World Health Organization classification,26 studies examining the prognostic impact of non-GCB COO using this algorithm have yielded conflicting results.16,27,28 In the present series, 40% were non-GCB phenotype, and this was not prognostic for PFS or OS. A similar finding was demonstrated by Kumar et al in a smaller cohort of 87 patients.12
In several large studies, investigators have shown that DE status confers an inferior outcome in DLBCL cohorts unselected for stage.13,14 DH status in patients with DLBCL morphology was associated with an extremely poor prognosis in early studies.29,30 A large multicenter retrospective analysis of 311 patients with HGBL-DH treated with induction chemotherapy regimens of varied intensity demonstrated poor outcomes, with median PFS and OS of 10.9 and 21.9 months, respectively.21 Similarly, Oki et al reported a 2‐year event‐free survival rate of 33% in 129 patients with HGBL-DH/TH treated at MD Anderson Cancer Center.22 However, data from large prospective studies suggest that these older series may potentially overestimate the adverse prognostic impact as a result of selection bias. In the largest study ever performed in newly diagnosed DLBCL, the phase III GOYA study, patients were randomized to receive obinutuzumab or rituximab plus 6 or 8 cycles of CHOP.31 Sehn et al reported predefined exploratory biomarker analyses of a subset of patients in the study with evaluable pretreatment biopsies.32 DE cases (151 [42%] of the evaluable cohort) had inferior outcomes relative to the non-DE group, with 3-year PFS of 63% and 76%, respectively. DH cases accounted for only 20 patients (4%) in the cohort evaluable by FISH, and their 3-year PFS was 55%, inferior to DE and non-DH patients but better than suggested by previous series, which may be explained, in part, by selection bias. By multivariable analysis, DH status conferred a greater adverse prognostic impact than DE status (HR, 2.11; 95% CI, 1.03-4.32). IHC BCL-2 positivity was seen in 178 (49%) cases. They had an inferior 3-year PFS of 63% compared with 78% seen with the IHC BCL-2− cases (BCL-2 expression < 50%). By multivariable analysis, BCL-2 positivity conferred a greater prognostic impact than DE status but less than that seen for DH status (HR, 1.72; 95% CI, 1.05-2.82).
Compared with the GOYA population (in which 76% of patients were stage III/IV), in the present series of exclusively stage I/II DLBCL, DE, DH, and IHC BCL-2+ cases accounted for 33 of 175 (19%), 7 of 106 (7%), and 128 of 187 (68%) cases with adequate data, respectively. Although the proportion of DH cases was comparable, the proportion of DE cases was lower and the proportion of IHC BCL-2+ cases was higher.32-34 Patients had favorable outcomes, regardless of DE, DH, and BCL-2 status, using R-CHOP (or similar) regimens, with or without RT. Although our relatively modest cohort size limits our ability to draw definitive conclusions, particularly for the DH patients (n = 7), these data suggest that intensified chemoimmunotherapy regimens (such as daEPOCH-R) may not be required for stage I/II HGBL-DH patients. Stage I/II HGBL-DH patients in other retrospective series21,22,35 had favorable outcomes, consistent with our findings.
These data are important to place in the context of modifications to frontline therapy being explored to improve outcomes in high-risk subgroups of patients with DLBCL. Nowakowski et al found that the addition of lenalidomide to R-CHOP appeared to overcome the adverse impact of the ABC phenotype.36 Two prospective phase 3 randomized studies comparing R-CHOP with R-CHOP + lenalidomide (NCT02285062, NCT01122472) in patients with treatment-naive DLBCL are ongoing. Investigators from Denmark and the MD Anderson Cancer Center37,38 have suggested that the use of intensified regimens (R-CHOEP or daEPOCH-R, respectively) may overcome the negative prognostic impact of DE status among patients with DLBCL. Retrospective analyses suggest that chemotherapy intensification may improve outcomes in this DH group compared with standard R-CHOP chemoimmunotherapy.21,22 In the Alliance 50303 study, investigators randomized patients with treatment-naive DLBCL to receive R-CHOP or da-EPOCH.19 No difference in PFS or OS was observed; however, at the time of writing, the biomarker analysis (including impact of daEPOCH on DE and DH subgroups) has not been reported. Given that the study was not powered to detect differences in outcome for these subgroups, it is unlikely that this study will definitively answer the question of whether intensification is of benefit. More recently, the phase 2 CAVALLI study and HOVON trial have found that the addition of venetoclax and lenalidomide, respectively, to R-CHOP may improve outcomes in DH lymphoma.17,18 Even if these approaches prove beneficial in high-risk molecular subgroups of DLBCL, such as non-GCB COO, DE, or DH, the additional cost and toxicity of these regimens may not be justified in patients with stage I/II disease.39
Although overall outcomes for stage I/II DLBCL are superior to III/IV, the pattern of late disease relapses observed in the former raise the possibility that limited-stage DLBCL is a biologically distinct entity. In the Southwest Oncology Group Study S8736, investigators randomized patients with stage I/II DLBCL to 3 cycles of CHOP + RT or 8 cycles of CHOP.40 This cohort was evaluated against results from a phase II study in which patients received 3 cycles of R-CHOP + RT. After a median of 17 years of follow-up, there was a continuous pattern of treatment failure without apparent PFS plateau, regardless of rituximab or RT use. Two large analyses exploring the genetic landscape of DLBCL did not compare early and advanced stages of disease, thus leaving this question wholly unanswered.41,42
There are several limitations to this study. First, the study design was retrospective and subject to potential sources of bias inherent to this methodology, including missing data and nonuniform follow-up and treatment. The local practices for performing FISH prospectively and retrospectively differed among sites, which may have contributed to biases in data availability and interpretation. The decision to include patients treated with combined modality therapy and chemoimmunotherapy was based on the weight of published evidence showing no difference in outcomes in the rituximab era.43,44 Although these studies are mostly retrospective, Lamy et al demonstrated, in a prospective randomized study of nonbulky stage I/II DLBCL, that patients who achieved a negative interim positron emission tomography could be managed with no RT without compromising disease control.45 Second, in this study we used the Hans algorithm to assign COO. Although concordance is high with the gold standard of GEP, ∼20% of cases will still be misclassified.7 We chose this approach for pragmatic reasons; because of the low cost, widespread availability of, and familiarity with IHC, as well as the lack of impact on therapeutic decision making (at present), the IHC algorithms remain the most commonly used method worldwide. The use of more sophisticated methods for determining COO, such as GEP and NanoString, remain largely confined to large academic centers or research laboratories. Third, there was no central pathology review leading to the potential for increased interobserver variability; however, the pathologists at the centers included are highly experienced with an academic interest in lymphoma diagnosis and are considered local and national reference laboratories in their respective jurisdictions. Lastly, it is important to emphasize that the low number of DH patients makes it difficult to draw definitive conclusions regarding this subset. Exploring the prognostic impact of COO and DE and DH status in larger prospectively treated datasets is required to confirm these findings.
In patients with PET-CT–defined stage I/II DLBCL treated with R-CHOP–like therapy, with or without RT, COO, DE, and DH status were not associated with inferior PFS or OS.
Acknowledgment
T.E.-G. thanks Hans Erik Johnsen (Aalborg University Hospital).
Authorship
Contribution: A.B. designed research, performed research, collected data, analyzed and interpreted data, performed statistical analyses, and wrote the manuscript; M.A. and M.S.E. performed research and prepared the manuscript; M.B., J.B., M.K.G., T.E.-G., and D.V. performed research, collected data, and prepared the manuscript; C.v.V., P.F., S.B., and V.S. performed research; C.G. collected data and prepared the manuscript; L.H.S., J.M.C., D.W.S., and K.J.S. prepared the manuscript; M.S.W. and B.A. collected data; C.Y.C. designed research, analyzed and interpreted data, performed statistical analyses, and wrote manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.B. has received travel expenses from Amgen and Bristol-Myers Squibb. M.A. has received honoraria from Roche. M.B. has received travel expenses from Roche and Takeda; has acted as a consult/advisor for Janssen; has received research funding from Celgene, Roche, and AbbVie; and has received honoraria from Takeda. C.v.V. has received travel expenses from Takeda. C.G. has received travel expenses from Novo Nordisk. L.H.S. has acted as an advisor for and/or has received honoraria from Janssen, AbbVie, Celgene, Seattle Genetics, Amgen, and Roche/Genentech. B.A. has acted as an advisor for Janssen and has received travel expenses from Amgen. J.B. has been employed by P95. J.M.C. has received research funding from Cephalon, Bayer Healthcare, and Merck and has received research funding from NanoString Technologies, Amgen, Bayer, Bristol-Myers Squibb, Roche, Genentech, Janssen, Eli Lilly and Company, Seattle Genetics, Takeda, and F. Hoffmann-La Roche. D.W.S. has acted as a consult/advisor for Celgene and Janssen; has received research funding from Roche/Genentech, Janssen, and NanoString Technologies; and has received travel expenses from Celgene. M.K.G. has acted as a consultant/advisor for Gilead Sciences, Merck, Janssen, Celgene, and Bristol-Myers Squibb; has received honoraria from Gilead Sciences, Takeda, Amgen, Merck, and Janssen; and has received research funding from Gilead Sciences, Celgene, and Bristol-Myers Squibb. K.J.S. has acted as an advisor and received honoraria from Bristol-Myers Squibb, Merck, SeaGen, Verastem, and AbbVie; has received honoraria from Takeda; and has received research funding from Roche. T.E.-G. has been employed by Roche; has acted as an advisor for Roche (no fee); and has received travel expenses from Roche and Takeda. D.V. has acted as a consultant/advisor for and has received honoraria from Roche, Celgene, Seattle Genetics, Lundbeck, Janssen, AstraZeneca, Gilead Sciences, and AbbVie and has received travel expenses and received research funding from Roche. C.Y.C. has acted as a consultant/advisor for Roche, Janssen, Takeda, MSD, Gilead Sciences, Bristol-Myers Squibb, and AstraZeneca; has received research funding from Celgene, Roche, and AbbVie; has received honoraria from Roche, Janssen, Takeda, MSD, Gilead Sciences, Bristol-Myers Squibb, and AstraZeneca; and has received travel expenses from Roche and Amgen. The remaining authors declare no competing financial interests.
Correspondence: Chan Yoon Cheah, Department of Haematology, Sir Charles Gairdner Hospital, Hospital Ave, Nedlands, WA 6009, Australia; e-mail: chan.cheah@health.wa.gov.au.