Key Points
VWF and FVIII levels after desmopressin, which mimic hemostatic response, are associated with the bleeding phenotype of type 1 VWD patients.
Variability in VWF and FVIII response to hemostatic challenges may partly explain heterogeneity in bleeding phenotype of VWD patients.
Abstract
The bleeding phenotype of patients with type 1 von Willebrand disease (VWD) is very heterogeneous. We hypothesized that this heterogeneity may partly be explained by variability in response of von Willebrand factor (VWF) and factor VIII (FVIII) levels to stress during hemostatic challenges. We therefore investigated whether VWF and FVIII levels after administration of desmopressin, which mimic in vivo hemostatic response during hemostatic challenges, explain the heterogeneity in bleeding phenotype of patients with type 1 VWD. We performed a retrospective cohort study in 122 patients with type 1 VWD. All patients received a test dose of desmopressin shortly after diagnosis. Patients’ mean age was 47 ± 14 years, and the mean Tosetto bleeding score was 10 ± 7. Higher FVIII activity during the complete time course after desmopressin administration (1, 3, and 5-6 hours), and higher VWF and FVIII levels combined at 3 hours after desmopressin administration, were associated with a lower bleeding score: β = –0.9 (–1.7; −0.1) and β = –1.2 (–1.9; −0.5), respectively, adjusted for age, sex, body mass index (BMI), and comorbidities. Patients with FVIII activity in the highest quartile 3 hours after desmopressin administration had a much lower bleeding score compared with patients in the other 3 quartiles (β = –5.1 [–8.2; −2.0]) and also had a lower chance of an abnormal bleeding score (odds ratio = 0.2 [0.1-0.5]), both adjusted for age, sex, BMI, and comorbidities. In conclusion, VWF and FVIII levels after desmopressin administration, which mimic hemostatic response to hemostatic challenges, are associated with the bleeding phenotype of patients with type 1 VWD. This may partly explain the variability in bleeding phenotype of these patients.
Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder.1 VWD is characterized by mucocutaneous bleeding such as menorrhagia, epistaxis, and gum bleeds.1-4 Patients with type 1 VWD have reduced von Willebrand factor (VWF) levels, whereas patients with type 2 VWD have an abnormal function of VWF, and patients with type 3 VWD have an absence of VWF.1,5,6
The bleeding phenotype of patients with type 1 VWD is very heterogeneous.1 Some determinants that are associated with the bleeding phenotype of type 1 VWD patients are age, sex, VWF and FVIII levels, presence of comorbidities, body mass index (BMI), VWF gene mutations, and some single-nucleotide polymorphisms outside the VWF gene.2,7-10 Part of the heterogeneity in bleeding phenotype may be due to differences in hemostatic response during hemostatic challenges. It is well known that certain circumstances, including stress, exercise, and surgery, are associated with an increase in both VWF and FVIII, a so-called hemostatic response.7,8,11-15 Patients who have a strong increase in VWF and FVIII levels during a hemostatic challenge may have less frequent or less severe bleeding episodes compared with patients with a smaller increase in VWF and FVIII levels.
The response to hemostatic challenges can be investigated by evaluating VWF and FVIII levels after exposure to desmopressin.16 Desmopressin stimulates the release of endogenous VWF from Weibel-Palade bodies of the endothelial cells into the circulation, and thus represents an individual’s potential to release endogenous VWF into the blood during hemostatic challenges.1 It is therefore assumed that patients who increase well in VWF and FVIII levels after desmopressin administration may also increase well in VWF and FVIII levels during hemostatic challenges.16 It was recently shown in a study of hemophilia A carriers that individuals with abnormal bleeding scores had a lower FVIII response to desmopressin compared with those with normal bleeding scores.16 To the best of our knowledge, no studies have yet been conducted to investigate whether VWF and FVIII levels after desmopressin administration explain the variability in bleeding phenotype of patients with type 1 VWD.
Thus, the aim of the current study was to investigate whether VWF and FVIII levels after desmopressin administration, which mimic in vivo hemostatic response to hemostatic challenges, explain the variability in bleeding phenotype of patients with type 1 VWD.
Methods
Patients
In this retrospective single-center cohort study, we included patients with type 1 VWD of all ages who were diagnosed and/or treated in the Erasmus University Medical Center after 1 January 1990. All patients had a hemorrhagic diathesis or family history of VWD and historically lowest VWF antigen (VWF:Ag) and/or VWF Ristocetin Cofactor activity ≤0.30 IU/mL with a VWF activity/VWF:Ag ratio >0.60. Patients who did not undergo desmopressin administration or did not have a bleeding score assessment were excluded. All patients participated in the Willebrand in the Netherlands (WiN) study, for which approval was obtained from our institutional review board.2,3,7,17 No additional approval was required for the retrospective analyses of the data that are presented in the current article.
Desmopressin test dose
Patients with type 1 VWD routinely received a test dose of desmopressin to evaluate whether they responded sufficiently to this medication.18 Desmopressin was, in most patients, administered intravenously at a dosage of 0.3 μg/kg in 50 mL of sodium chloride 0.9% infused in 30 minutes, or intranasally at a total dose of 300 µg.18 All patients received the test dose of desmopressin in the same institution. Venous blood samples were routinely obtained before and 1, 3, and 5 to 6 hours after desmopressin administration.
Assessment methods
VWF:Ag, VWF activity (measured by using the monoclonal antibody assay [VWF:Ab]), VWF collagen binding (VWF:CB), and FVIII activity (FVIII:C) were measured centrally before and after desmopressin administration for routine diagnostics in the same laboratory. The data were retrospectively obtained from the electronic patient files.
In short, VWF:Ag was measured with an in-house enzyme-linked immunosorbent assay using polyclonal rabbit anti-human VWF antibodies and horseradish peroxidase–conjugated anti-human VWF antibodies (DakoCytomation, Glostrup, Denmark) for detection. VWF:CB was also measured with an in-house enzyme-linked immunosorbent assay, for which collagen type 1 (MilliporeSigma, Burlington, MA) was used for capture and horseradish peroxidase–conjugated anti-human VWF antibodies (DakoCytomation) for detection. We measured VWF:Ab with a latex immune assay on an automated coagulometer with monoclonal antibodies against the GP1bα binding site of VWF (HemosIL VWF activity; Instrumentation Laboratory BV, Breda, The Netherlands).19 FVIII:C was measured by using a one-stage clotting assay (TriniCLOT; bioMérieux, Marcy l’Etoile, France). The laboratory measurements have been reported in detail previously.2,7
Furthermore, all patients also participated in the WiN study.3,7,17 A self-administered Tosetto bleeding score, BMI, and presence of comorbidities were assessed at inclusion in the WiN study. In the bleeding score assessment, we did not score for bleeding in case of prophylactic treatment.20 VWF and FVIII levels were measured centrally at the Erasmus University Medical Center.21 VWF propeptide (VWFpp) was measured at the Leiden University Medical Center as previously described.21 The assessment methods of the WiN study have been described in detail elsewhere.2,3,7
Statistical methods
Categorical data are presented as frequencies and proportions. Continuous data are presented as median and interquartile range or mean ± standard deviation. Outliers were excluded based on z scores <3 or >3. Continuous variables were compared between 2 groups by using an independent sample Student t test (central limit theorem).
We used generalized estimating equation (GEE) analysis with maximum likelihood estimation and Wald χ2 hypothesis testing to analyze the association between VWF and FVIII levels during the complete time course (1, 3, and 5-6 hours) after desmopressin administration and the bleeding score. In this analysis, we included levels (eg, VWF:Ag) at 1, 3, and 5 to 6 hours as a repeated measurement in a single model, taking into account the intrapatient correlation. GEE analysis was also used to assess the combined association between VWF and FVIII levels measured at one specific time point and the bleeding phenotype. VWF:Ag, VWF:Ab, VWF:CB, and FVIII:C at a specific time point (eg, 1 hour after desmopressin administration) were included as repeated measurements in a single model, taking into account the intrapatient correlation. All these analyses were based on previous literature adjusted for age, sex, BMI, and comorbidities.7,8 Comorbidities were defined as diseases that were previously found to be associated with higher VWF and FVIII levels in patients with type 1 VWD: hypertension, diabetes mellitus, cancer, and thyroid dysfunction.7 Outcomes of GEE analyses are presented as unstandardized β with 95% confidence intervals (CIs).
The association between individual VWF and FVIII levels and the total bleeding score was assessed with regression analysis, in which we also adjusted for age, sex, BMI, and comorbidities. Some analyses were also adjusted for baseline VWF and FVIII levels. Outcomes of regression analysis are also presented as unstandardized β with 95% CIs. In addition, linear regression analyses were used to analyze the association between an increased clearance and reduced synthesis of VWF and the bleeding phenotype. Based on previous literature, increased clearance of VWF was defined as a VWFpp/VWF:Ag ratio >2.2, whereas reduced synthesis of VWF was defined as an FVIII:C/VWF:Ag ratio >1.9.21
Furthermore, binary logistic regression analyses were used to analyze whether a normal vs abnormal bleeding score is associated with VWF and FVIII levels or with an increased clearance or reduced synthesis of VWF. An abnormal bleeding score was defined as a bleeding score >3 for men and >5 for women.2 Outcomes of logistic regression analyses are presented as odds ratios (ORs) and 95% CIs. Statistical analyses were performed with SPSS Statistics version 24 (IBM Corporation, Armonk, NY).
Results
We identified 173 eligible patients with type 1 VWD in our treatment center who were previously included in the WiN study. After exclusion of 50 patients who did not receive a desmopressin test dose, and 1 patient who did not have a bleeding score assessment, 122 patients with type 1 VWD were included. Table 1 presents the patient characteristics. The mean age was 47 ± 14 years; most patients were adult (98%), female (66%), and had blood group O (73%). Genetic data on VWF gene were available from 105 patients. In 85 patients, genetic analysis was performed in the patient who was included in this study. In 19 included patients, genetic analysis was performed in a family member with VWD. In 1 patient, it was unknown whether genetic analysis was performed in the patient or in a relative. The median bleeding score was 9 (interquartile range, 5-14; range −2; 29). Desmopressin administration was performed in all patients (on average, 10 ± 4.6 years) before assessment of the Tosetto bleeding score.
Patient characteristics
| Characteristic . | Type 1 VWD (N = 122) . |
|---|---|
| Age, mean ± SD, y | 47 ± 14 |
| Adult, n (%) | 120 (98) |
| Female, n (%) | 80 (66) |
| Blood group O, n (%) | 89 (73) |
| VWF gene mutation, n (%) | 48 (46)* |
| Historically lowest levels, IU/mL | |
| VWF:Ag | 0.33 [0.25-0.41] |
| VWF:Ab | 0.22 [0.14-0.26] |
| VWF:CB | 0.22 [0.16-0.30] |
| FVIII:C | 0.50 [0.38-0.63] |
| Levels immediately before desmopressin administration, IU/mL | |
| VWF:Ag | 0.44 [0.28-0.58] |
| VWF:Ab | 0.32 [0.22-0.52] |
| VWF:CB | 0.33 [0.18-0.62] |
| FVIII:C | 0.61 [0.48-0.86] |
| Bleeding score | 9 [5-14] |
| Characteristic . | Type 1 VWD (N = 122) . |
|---|---|
| Age, mean ± SD, y | 47 ± 14 |
| Adult, n (%) | 120 (98) |
| Female, n (%) | 80 (66) |
| Blood group O, n (%) | 89 (73) |
| VWF gene mutation, n (%) | 48 (46)* |
| Historically lowest levels, IU/mL | |
| VWF:Ag | 0.33 [0.25-0.41] |
| VWF:Ab | 0.22 [0.14-0.26] |
| VWF:CB | 0.22 [0.16-0.30] |
| FVIII:C | 0.50 [0.38-0.63] |
| Levels immediately before desmopressin administration, IU/mL | |
| VWF:Ag | 0.44 [0.28-0.58] |
| VWF:Ab | 0.32 [0.22-0.52] |
| VWF:CB | 0.33 [0.18-0.62] |
| FVIII:C | 0.61 [0.48-0.86] |
| Bleeding score | 9 [5-14] |
Data are presented as median [interquartile range], unless otherwise specified.
Genetic analysis was performed in 105 patients.
Response to test dose of desmopressin
A total of 121 patients had baseline VWF and FVIII levels measured immediately before desmopressin administration; 120 patients had levels measured at 1 hour, 105 patients had levels measured at 3 hours, and 113 patients had levels measured at 5 to 6 hours after desmopressin administration (Figure 1). Median [interquartile range] VWF:Ag levels before desmopressin administration and 1 hour, 3 hours, and 5 to 6 hours after administration were, respectively, 0.44 IU/mL [0.28-0.58], 1.83 IU/mL [1.20-2.54], 1.52 IU/mL [1.07-2.01], and 1.40 IU/mL [0.97-1.75]. Median VWF:Ab levels at these time points were 0.32 IU/mL [0.23-0.52], 1.67 IU/mL [1.10-2.16], 1.43 IU/mL [0.85-1.96], and 1.17 IU/mL [0.74-1.73]; and FVIII:C levels were 0.61 IU/mL [0.48-0.86], 2.76 IU/mL [1.95-3.42], 2.05 IU/mL [1.50-2.66], and 1.68 IU/mL [1.15-2.22].
VWF:Ag levels after a desmopressin test dose in the total population. Data are presented as median with interquartile range.
VWF:Ag levels after a desmopressin test dose in the total population. Data are presented as median with interquartile range.
VWF and FVIII levels after desmopressin administration and bleeding phenotype
Higher FVIII:C during the total time course after desmopressin administration (ie, 1, 3, and 5-6 hours) was associated in the GEE analysis with a lower bleeding score; β = –0.9 change in bleeding score for 1 IU/mL increase in FVIII:C (95% CI, −1.7; −0.1), adjusted for age, sex, BMI, and comorbidities. After additional adjustment for presence or absence of a VWF gene mutation, FVIII:C was still associated with a lower bleeding score: β = –1.1 (–1.9; −0.3). VWF:Ag, VWF:Ab, and VWF:CB during the total time course after desmopressin administration were not associated with the bleeding score: β = 0.3 (–0.7; 1.4), β = –0.2 (–1.2; 0.9), and β = –0.2 (–0.8; 0.5), respectively, all adjusted for age, sex, BMI, and comorbidities.
Combined analysis of VWF and FVIII at each single time point after desmopressin administration revealed that levels at 3 hours postadministration were strongly associated with the bleeding score: β = –1.2 (–1.9; −0.5), adjusted for age, sex, BMI, and comorbidities. After additional adjustment for baseline levels and presence or absence of a VWF gene mutation, VWF and FVIII levels at 3 hours postadministration were still strongly associated with the bleeding score: β = –1.3 (–2.2; −0.5). Baseline VWF and FVIII levels (immediately before desmopressin administration), levels at 1 hour, and levels at 5 to 6 hours after desmopressin administration were not associated with the bleeding score: β = 0.2 (–1.8; 2.3), β = 0.2 (–0.4; 0.8), and β = –0.3 (–1.1; 0.5), respectively, all adjusted for age, sex, BMI, and comorbidities.
Similarly, higher increase of VWF and FVIII levels at 3 hours after desmopressin administration compared with baseline levels, calculated as levels at 3 hours minus baseline levels, was also associated with a lower bleeding score (β = –1.3 [–2.1; −0.5]). Increases in levels at 1 hour and 5 to 6 hours postadministration were not associated with the bleeding score (β = 0.4 [–3.2; 1.1] and β = –0.2 [–1.2; 0.7], respectively), all adjusted for age, sex, BMI, and comorbidities.
Historically lowest VWF and FVIII levels and centrally measured levels were also not associated with the total bleeding score: β = –1.6 (–4.9; 1.8) and β = –0.2 (–2.3; 1.9), respectively, both adjusted for age, sex, BMI, and comorbidities.
Levels at 3 hours after desmopressin administration
FVIII:C at 3 hours after desmopressin administration was associated with the bleeding score (β = –1.5 [−3.0; −0.1]), whereas VWF:Ag (β = –0.1 [−2.1; 1.9]), VWF:Ab (β = –0.5 [−2.4; 1.5]), and VWF:CB (β = –0.7 [−1.9; 0.5]) were not associated with the bleeding score (Figure 2). After adjustment for age, sex, BMI, and comorbidities, FVIII:C was even more strongly associated with the bleeding score: β = –2.3 (–3.9; −0.8). The association between FVIII:C at 3 hours postadministration and the bleeding score also remained after additional adjustment for baseline FVIII:C and presence or absence of a VWF gene mutation: β = –2.6 (–4.4; −0.9).
FVIII:C at 3 hours after desmopressin administration is associated with the bleeding score. Outcomes of linear regression analysis.
FVIII:C at 3 hours after desmopressin administration is associated with the bleeding score. Outcomes of linear regression analysis.
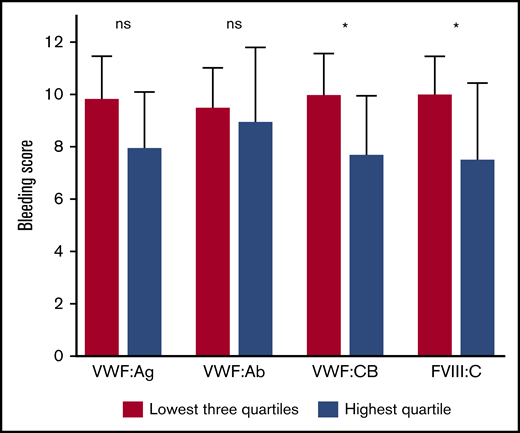
Furthermore, patients with VWF and FVIII levels in the highest quartile 3 hours after desmopressin administration had a lower bleeding score, compared with patients in the other 3 quartiles (Figure 3). The largest difference was again found for FVIII:C: β = –5.1 (–8.2; −2.0), adjusted for age, sex, BMI, and comorbidities. This association remained nearly unchanged after additional adjustment for baseline FVIII:C and presence or absence of a VWF gene mutation: β = –5.5 (–8.9; −2.1). Patients with VWF:CB in the highest quartile also had a significantly lower bleeding score, whereas VWF:Ag in the highest quartile tended to be associated with a lower bleeding score: β = –3.2 (–6.4; −0.1) and β = –2.6 (–5.7; 0.4), respectively, both adjusted for age, sex, BMI, and comorbidities.
Patients with VWF and FVIII levels in the highest quartile 3 hours after desmopressin have a much lower bleeding score. Data are presented as median with interquartile range. *P < .05. ns, not significant.
Patients with VWF and FVIII levels in the highest quartile 3 hours after desmopressin have a much lower bleeding score. Data are presented as median with interquartile range. *P < .05. ns, not significant.
Moreover, patients with FVIII:C in the highest quartile at 3 hours after desmopressin administration had a lower chance of an abnormal bleeding score compared with patients in the other quartiles: OR = 0.2 (0.1-0.5), adjusted for age, sex, BMI, and comorbidities. Additional adjustment for baseline FVIII:C and presence or absence of a VWF gene mutation even decreased the chance of an abnormal bleeding score for patients with FVIII:C in the highest quartile at 3 hours after desmopressin administration: OR = 0.1 (0.0-0.4).
Desmopressin response criteria
When we applied the response criteria by Castaman et al22 on VWF:Ab and FVIII:C levels 1 hour after desmopressin administration, we found that only 3 patients (2.4%) did not have a complete response. Because of the small number, it was statistically impossible to associate this outcome with the bleeding phenotype. When we applied the response criteria on VWF:Ab and FVIII:C levels 3 hours after desmopressin administration, we found that 92 patients had a complete response, 4 patients had a partial response, and 9 patients had no response. Patients who did not respond to desmopressin seemed to have a high bleeding score (13.0 ± 8.0), followed by patients with a partial response (10.3 ± 3.2) and a good response (9.0 ± 6.7). Although the effect sizes suggested an association between desmopressin response and bleeding score, it was not statistically significant because of the small number of patients with a partial response and good response (P = .089).
Pathophysiology of VWD, VWF levels after desmopressin administration, and the bleeding phenotype
Patients with an increased clearance of VWF, which was defined as a VWFpp/VWF:Ag ratio >2.2, had lower VWF:Ag (β = –0.3 [−0.6; −0.1]), VWF:Ab (β = –0.5 [−0.7; −0.2]), VWF:CB (β = –0.5 [−1.0; −0.1]), and FVIII:C (β = –0.7 [−1.0; −0.4]) at 3 hours after desmopressin administration compared with other patients with type 1 VWD, all adjusted for age, sex, BMI, and comorbidities (Figure 4). These patients also had higher bleeding scores compared with other patients with type 1 VWD: β = 2.7 (0.1; 5.4), adjusted for age, sex, BMI, and comorbidities. Even after additional adjustment for baseline VWF:Ag, VWF:Ab, VWF:CB, and FVIII:C, and presence or absence of a VWF gene mutation, this association remained: β = 3.4 (0.5; 6.7). Patients with an increased clearance of VWF also more often had an abnormal bleeding score compared with other patients with type 1 VWD: OR = 3.2 (1.2; 8.8), adjusted for age, sex, BMI, and comorbidities.
Increased clearance of VWF is associated with lower VWF and FVIII levels at 3 hours after desmopressin administration and a higher bleeding score. Data are presented as median with interquartile range. *P < .05. **P < .001. All analyses are adjusted for age, sex, BMI, and comorbidities. Increased clearance of VWF is defined as a VWFpp/VWF:Ag ratio >2.2.
Increased clearance of VWF is associated with lower VWF and FVIII levels at 3 hours after desmopressin administration and a higher bleeding score. Data are presented as median with interquartile range. *P < .05. **P < .001. All analyses are adjusted for age, sex, BMI, and comorbidities. Increased clearance of VWF is defined as a VWFpp/VWF:Ag ratio >2.2.
Patients with a reduced synthesis of VWF, defined as an FVIII:C/VWF:Ag ratio >1.9, did not have a different bleeding score or a difference in incidence of abnormal bleeding score compared with other patients with type 1 VWD: β = –0.9 (–3.8; 2.1) and OR = 1.2 (0.5; 3.2), respectively, both adjusted for age, sex, BMI, and comorbidities.
Discussion
In this cohort study, we found that VWF and FVIII levels after a test dose of desmopressin were associated with the bleeding phenotype of patients with type 1 VWD. Patients with lower VWF and FVIII levels after desmopressin administration had a higher bleeding score compared with patients who had higher levels after desmopressin administration. Because VWF and FVIII levels after desmopressin administration mimic in vivo hemostatic response to hemostatic challenges, our results suggest that part of the heterogeneity of bleeding phenotype in patients with type 1 VWD may be explained by the fact that some patients have a higher and sustained increase in VWF and FVIII levels during hemostatic challenges and may therefore have fewer or less severe bleeding episodes; other patients with type 1 VWD, however, do not have sufficient increases during hemostatic challenges and may therefore have more frequent or more severe bleeding episodes.
Higher VWF and FVIII levels after desmopressin administration were associated with a milder bleeding phenotype in patients with type 1 VWD. In accordance, a recent study in hemophilia carriers showed that participants with an abnormal bleeding score had a lower FVIII response to desmopressin compared with participants with a normal bleeding score.16 Our current study and the previous analysis in hemophilia carriers show the important, underrated, role of hemostatic response to hemostatic challenges in patients with bleeding disorders. Individuals with comparable baseline hemostatic markers may have a different bleeding phenotype due to a difference in hemostatic response. This is strengthened by the fact that in the current study, the association between VWF and FVIII levels after desmopressin administration and the bleeding score persisted, even following adjustment for baseline VWF and FVIII levels. Moreover, historically lowest VWF and FVIII levels, centrally measured levels, and baseline VWF and FVIII levels (measured immediately before desmopressin administration) were not associated with the bleeding phenotype, whereas VWF and FVIII levels after desmopressin administration were inversely associated with the bleeding phenotype. It should be noted that although it was previously found that type 1 VWD patients with VWF and/or FVIII levels <0.10 IU/mL have a higher bleeding score compared with patients with VWF and/or FVIII levels between 0.10 and 0.50 IU/mL or >0.50 IU/mL, in several large cohorts there was no association found between VWF and/or FVIII levels in the range of 0.10 to 0.50 IU/mL and the bleeding score.2,23-26 Therefore, VWF and FVIII levels after desmopressin administration may have an important added value in explaining the variability in bleeding phenotype of patients with type 1 VWD. VWF and FVIII levels after desmopressin administration may also contribute to predicting the chance of future bleeding in patients with type 1 VWD undergoing interventions. However, prospective studies are needed to investigate this theory before it can be applied in clinical practice.
Higher VWF and FVIII levels 3 hours after desmopressin administration were associated with a milder bleeding phenotype, whereas levels at 1 hour and 5 to 6 hours postadministration were not significantly associated. This finding suggests that sustained VWF and FVIII levels after a hemostatic challenge are important to prevent bleeding. We probably did not find a significant association between levels at 5 to 6 hours after desmopressin and the bleeding score, because VWF and FVIII levels were on average lower at 5 to 6 hours after desmopressin (compared to the levels at 3 hours), and therefore the differences in levels at 5 to 6 hours were smaller, causing a lack of power. This was strengthened by our finding that an increased clearance of VWF was associated with the bleeding phenotype of patients. In hemophilia carriers, it was also found that FVIII levels 2 hours after desmopressin administration were associated with the bleeding phenotype, whereas there was no association with levels 1 and 4 hours after desmopressin administration.16 Overall, these results may indicate that a sustained hemostatic response after hemostatic challenge is important to prevent bleeding or to decrease bleeding severity in patients with VWD.
Furthermore, FVIII:C after desmopressin administration was more strongly associated with the bleeding phenotype than VWF levels, suggesting that FVIII:C is more important during hemostatic challenges than VWF levels. In accordance, it has previously been proposed that FVIII is more important than VWF levels in treating bleeding in patients with type 3 VWD and patients with alloantibodies against VWF.2,27-29 Moreover, in patients with severe VWD, the incidence of joint bleeds was more strongly associated with FVIII:C than with VWF levels.30,31 All these results underline the important role of FVIII in the bleeding phenotype of patients with VWD.
Finally, patients with the highest VWF and FVIII levels (highest quartile) 3 hours after desmopressin had a 5-point lower bleeding score, and patients with FVIII:C in the highest quartile had ∼10 times lower chance of presenting with an abnormal bleeding score. These results indicate that a good hemostatic response may have important and clinically relevant consequences on the bleeding phenotype of patients. A good hemostatic response may compensate for the low baseline VWF and FVIII levels in patients with type 1 VWD.
This study is the first to show in a large cohort of patients with type 1 VWD that VWF and FVIII levels after desmopressin administration are associated with the bleeding score, and thereby may partly explain the variability in bleeding phenotype of type 1 VWD patients. A potential limitation is the retrospective study design. However, VWF and FVIII levels after desmopressin administration were well documented in the electronic patient files, and the bleeding score was systematically assessed by using a self-administered bleeding score. Another limitation is the use of a self-administered Tosetto bleeding score, instead of an expert-administered bleeding score. However, we previously showed that the self-administered Tosetto bleeding score yielded scores similar to those that were expert administered.2 We admit that it would have been better if we also had an expert-administered bleeding score, but this was not available in the WiN study cohort. Another potential limitation is the long time period (on average, 10 years) between desmopressin administration and bleeding score assessment. However, it is well known that desmopressin response remains constant over time.15,18,22,32 Furthermore, due to the long follow-up period, VWF and FVIII assays may have changed over time. However, we obtained levels from patient files that were centrally performed with routinely diagnostic assays in the same laboratory, which are highly reliable. In addition, a change in laboratory measurement gives nondifferential information bias (ie, measurement error bias), which leads to wider CIs and therefore may cause dilution of effect (ie, loss of power).33 Because we found highly significant associations, this potential information bias seems negligible. Moreover, for different VWF activity assays, we recently reported that they are generally very comparable to each other in patients with type 1 VWD.34
In conclusion, VWF and FVIII levels after desmopressin administration, which mimic in vivo hemostatic response to hemostatic challenges, are associated with the bleeding phenotype of patients with type 1 VWD. The clinical relevance of this association is highlighted by our findings that patients with the highest VWF and FVIII levels 3 hours after desmopressin administration had a 5-point lower bleeding score, and patients with FVIII:C in the highest quartile had ∼10 times lower chance of presenting with an abnormal bleeding score. These results may partly explain the well-known variability in bleeding phenotype of patients with type 1 VWD.
Acknowledgment
This study was supported (in part) by research funding from the Dutch Hemophilia Foundation (Stichting Haemophilia) and CSL Behring (unrestricted grant).
Authorship
Contribution: F.A. designed the study, collected data, performed statistical analysis, interpreted data, and wrote the manuscript; A.E.M.L. designed the study, collected data, performed statistical analysis, interpreted data, and critically revised the manuscript; J.B. designed the study, interpreted data, and critically revised the manuscript; L.M.S., M.H.C., J.E., M.P.M.d.M., and M.J.H.A.K. interpreted data and critically revised the manuscript; F.W.G.L. conceived of and designed the study, interpreted data, and critically revised the manuscript; and all authors gave their consent to the final version of the manuscript.
Conflict-of-interest disclosure: F.A. received the CSL Behring-professor Heimburger Award 2018 and a travel grant from Sobi. L.M.S. received reimbursement from CSL Behring for attending a symposium. J.B. is an employee of Sobi and has received the CSL Behring-professor Heimburger Award 2016. M.H.C. has received investigator initiated research and/or travel grants from Baxter/Shire/Takeda, Bayer, Pfizer, CSL Behring, Novo Nordisk, Sobi, Nordic Pharma, and Novartis; and is consultant for Bayer and Roche, the fees of which go to the institution. J.E. received research support from CSL Behring and fees for educational activities from Roche, which go to the institution. M.P.M.d.M. has received research support or speaker fees from Siemens, Roche, Stago, and Werfen. M.J.H.A.K. received research support from Bayer, Pfizer, Daiichi Sankyo, and Boehringer Ingelheim. F.W.G.L. received research support from CSL Behring and Shire/Takeda for performing the WiN study; is a consultant for uniQure, Novo Nordisk, and Shire/Takeda, the fees of which go to the institution; and has received a travel grant from Sobi. A.E.M.L. declares no competing financial interests.
Correspondence: Frank W. G. Leebeek, Department of Hematology, Erasmus University Medical Center, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.
References
Author notes
Original data can be obtained by sending an e-mail to the corresponding author (Frank W. G. Leebeek, f.leebeek@erasmusmc.nl).