Abstract
Patients receiving vitamin K antagonists (VKAs) with an international normalized ratio (INR) between 4.5 and 10 are at increased risk of bleeding. We systematically reviewed the literature to evaluate the effectiveness and safety of administering vitamin K in patients receiving VKA therapy with INR between 4.5 and 10 and without bleeding. Medline, Embase, and Cochrane databases were searched for relevant randomized controlled trials in April 2018. Search strategy included terms vitamin K administration and VKA-related terms. Reference lists of relevant studies were reviewed, and experts in the field were contacted for relevant papers. Two investigators independently screened and collected data. Risk ratios (RRs) were calculated, and certainty of the evidence was assessed using Grading of Recommendations Assessment, Development and Evaluation. Six studies (1074 participants) were included in the review and meta-analyses. Pooled estimates indicate a nonsignificant increased risk of mortality (RR = 1.42; 95% confidence interval [CI], 0.62-2.47), bleeding (RR = 2.24; 95% CI, 0.81-7.27), and thromboembolism (RR = 1.29; 95% CI, 0.35-4.78) for vitamin K administration, with moderate certainty of the evidence resulting from serious imprecision as CIs included potential for benefit and harm. Patients receiving vitamin K had a nonsignificant increase in the likelihood of reaching goal INR (1.95; 95% CI, 0.88-4.33), with very low certainty of the evidence resulting from serious risk of bias, inconsistency, and imprecision. Our findings indicate that patients on VKA therapy who have an INR between 4.5 and 10.0 without bleeding are not likely to benefit from vitamin K administration in addition to temporary VKA cessation.
Introduction
Vitamin K antagonists (VKAs) are used to prevent and treat venous thromboembolism (VTE) and to prevent arterial thromboembolism in patients with atrial fibrillation or cardiac disease, including mechanical heart valves.1,2 The international normalized ratio (INR), which measures the intensity of VKA anticoagulant effect, is commonly used to monitor VKA therapy. Despite monitoring and careful dose adjustment, INR values outside the target range are frequently observed.3
High INR values have been associated with increased bleeding risk and therefore, at some threshold, need to be corrected.4-6 Vitamin K has been commonly used to normalize high INR values. VKAs act by inhibiting vitamin K–dependent γ-carboxylation of coagulation factors II, VII, IX, and X. Exogenously administered vitamin K (available commercially as phytonadione) normalizes the INR by providing the necessary substrate to synthesize these coagulation factors.7,8
Two systematic reviews on this topic were published in 2006 and concluded that vitamin K may be effective in reducing elevated INR values. One of these reviews did not pool estimates from individual studies and reported narrative results only.9 The other review pooled individual study results and concluded that oral and IV vitamin K effectively reduce follow-up INRs measured within 24 hours to <4.0 in about three-quarters of patients with excessive VKA anticoagulation. These reviews did not investigate effects on bleeding or other patient-important outcomes.9-12
Clinical practice guideline recommendations regarding the optimal management of patients with excessive VKA anticoagulation have evolved over time. Early guidelines dating back to 1995 recommended subcutaneous administration of vitamin K when rapid reversal is required because of elective surgery or if the INR is between 6.0 and 10.0.13 Later guideline recommendations changed to administering oral vitamin K in these instances.1 As of 2008, guidelines were changed and recommended against the administration of vitamin K for patients with INRs between 4.5 and 10.0 who have no significant bleeding, in addition to withholding the next 1 or 2 doses of VKA with more frequent INR monitoring.14 We aimed to update the literature on this topic by answering the following question for the American Society of Hematology clinical practice guidelines on VTE: In patients receiving VKA presenting with an INR between 4.5 and 10 and without bleeding, should vitamin K in addition to temporary cessation of VKA be used to reverse excessive anticoagulation compared with temporary cessation of VKA alone?
Methods
This systematic review was performed as part of the American Society of Hematology guidelines on VTE, developed in partnership with the McMaster University’s Grading of Recommendations Assessment, Development and Evaluation (GRADE) Centre. Review and meta-analysis methodology followed the Cochrane Handbook,14 with reporting according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.15
Search strategy
MEDLINE (1996 to week 2 of April 2018), EMBASE (1974 to week 2 of April 2018), and the Cochrane Central Register of Controlled Trials (until week 2 of April 2018) were searched. The search strategy consisted of keywords specific to each database and was restricted to randomized controlled trials (RCTs) of human subjects but not restricted by language. The MEDLINE search strategy is provided in supplemental Material 1. Additionally, the reference lists of relevant studies and reviews were reviewed, and clinical experts in the field of anticoagulation management were consulted for additional references.
Study selection
Two reviewers (R.K. and M.L.) independently screened titles, abstracts, and the full text of relevant articles based on prespecified inclusion and exclusion criteria. Disagreements were resolved by consensus and by a third reviewer when needed (R.N.). RCTs were included if they fulfilled the following criteria.
Patients.
Adult patient population (≥18 years of age) using VKAs with a first episode of an elevated INR value (between 4.5 and 10) that required temporary VKA cessation and without bleeding. Patients who required VKA reversal for urgent surgery or because of bleeding were excluded. Studies were included with patients taking VKA for any indication.
Intervention.
Administration of vitamin K (oral, IV, or subcutaneous) at any dose.
Comparison.
Placebo or observation only.
Outcomes.
All-cause mortality, major bleeding, thromboembolism, and proportion of patients reaching goal INR assessed at 24 hours and at 1 week of vitamin K administration.
Study design.
Only RCTs were included.
Data abstraction and quality assessment
One reviewer (M.L.) collected data from each eligible study using a pretested data abstraction form, and data were checked by another reviewer (R.K.) to assess accuracy. Disagreements were resolved by discussion, and by a third reviewer (R.N.) when needed. The data collected included patient characteristics (baseline INR values and VKA indication), vitamin K treatment (dose, route, and frequency of administration), study setting, follow-up, and outcomes. Information on risk of bias was also collected and assessed for each outcome in each included study using the Cochrane risk of bias tool for RCTs.16 Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated by pooling the results from RCTs using the Mantel Haenszel method and the random effects model. Heterogeneity was assessed using the I2 index and was deemed as moderate to high with an I2 > 50%.16 Data were analyzed using RevMan 5.3.
Two reviewers evaluated the certainty of the evidence for each outcome using the GRADE approach.17 The certainty of the evidence was assessed as high, moderate, low, or very low and summarized in a GRADE Evidence Profile.
Results
Search results
A total of 4327 unique citations were identified from the electronic database search and from other sources. Based on title and abstract screening, 3555 citations were excluded. An additional 12 citations were excluded based on full-text screening. Reasons for excluding full-text citations included: inclusion criteria did not restrict INR range to 4.5 to 10.0, VKA was continued in the intervention arm, vitamin K was administered in the control arm, duplicate trial data, not an RCT, or published in abstract only (supplemental Material 2). Six studies were included in this systematic review after nonrelevant citations were excluded. One study18 reported 0 events for the outcomes of interest. This study was not included in the meta-analysis because it did not provide any indication of either the direction or magnitude of the relative treatment effect,16 leaving 5 studies for inclusion in the meta-analyses.
Study characteristics
Study characteristic details are included in Table 1. A total of 1074 participants were included in the 6 studies.18-23 Three studies were single-center studies conducted in Argentina,23 Canada,24 and the United States.18 The remaining 3 studies were multicenter studies conducted in Italy, Canada, and the United States.19-21 Warfarin was used in the 3 studies; acenocoumarol was used in 2 studies.20,23 Baseline INR values ranged from 5.4 to 8.4 in the vitamin K group, and from 5.8 to 8.1 in the control group. The vitamin K groups received oral vitamin K (dose range, 1-2.5 mg).
Characteristics of included studies
| Reference . | Country . | No. of randomized patients . | Baseline INR . | Type of VKA . | VKA indication . | Treatment* . | Follow-up duration, d . |
|---|---|---|---|---|---|---|---|
| Fondevila 200122 | Argentina | 109 | Treatment, 8.4 (6.0-19.6); control, 8.1 (6.0-14.1) | A | NR | Intervention, 1 mg oral vitamin K; comparison: observation | 7 |
| Crowther 200020 | Canada | 92 | Treatment, 5.4 (4.5-9.8); control, 5.9 (4.5-9.8) | W | NR | Intervention, 1 mg oral vitamin K; comparison: placebo | 30 |
| Patel 200017 † | United States | 30 | Treatment, 7.2 (6.0-9.2); control, 7.0 (6.1-9.5) | W | VTE, 27%; AF, 44%; CVD, 7%; LVD, 13%; other, 10% | Intervention, 2.5 mg oral vitamin K; comparison: placebo | Not specified |
| Ageno 200219 | Italy, Canada | 60 | Treatment, 6.2; control, 6.0 | A | VTE, 32%; AF, 67%; stroke prophylaxis, 1% | Intervention, 1 mg oral vitamin K; comparison: observation | 30 |
| Ageno 200518 | Italy, United States | 59 | Treatment, 7.2; control, 7.7 | W | Mechanical heart value, 100% | Intervention, 1 mg oral vitamin K; comparison: observation | 30 |
| Crowther 200921 | Canada, United States, Italy | 724 | Treatment, 6.0 (4.5-9.9); control, 5.8 (4.5-9.5) | W | Thromboembolism treatment or prevention, AF, or artificial heart valve‡ | Intervention, 1.25 mg oral vitamin K; comparison: placebo | 90 |
| Reference . | Country . | No. of randomized patients . | Baseline INR . | Type of VKA . | VKA indication . | Treatment* . | Follow-up duration, d . |
|---|---|---|---|---|---|---|---|
| Fondevila 200122 | Argentina | 109 | Treatment, 8.4 (6.0-19.6); control, 8.1 (6.0-14.1) | A | NR | Intervention, 1 mg oral vitamin K; comparison: observation | 7 |
| Crowther 200020 | Canada | 92 | Treatment, 5.4 (4.5-9.8); control, 5.9 (4.5-9.8) | W | NR | Intervention, 1 mg oral vitamin K; comparison: placebo | 30 |
| Patel 200017 † | United States | 30 | Treatment, 7.2 (6.0-9.2); control, 7.0 (6.1-9.5) | W | VTE, 27%; AF, 44%; CVD, 7%; LVD, 13%; other, 10% | Intervention, 2.5 mg oral vitamin K; comparison: placebo | Not specified |
| Ageno 200219 | Italy, Canada | 60 | Treatment, 6.2; control, 6.0 | A | VTE, 32%; AF, 67%; stroke prophylaxis, 1% | Intervention, 1 mg oral vitamin K; comparison: observation | 30 |
| Ageno 200518 | Italy, United States | 59 | Treatment, 7.2; control, 7.7 | W | Mechanical heart value, 100% | Intervention, 1 mg oral vitamin K; comparison: observation | 30 |
| Crowther 200921 | Canada, United States, Italy | 724 | Treatment, 6.0 (4.5-9.9); control, 5.8 (4.5-9.5) | W | Thromboembolism treatment or prevention, AF, or artificial heart valve‡ | Intervention, 1.25 mg oral vitamin K; comparison: placebo | 90 |
A, acenocoumarol; AF, atrial fibrillation; CVD, cardiovascular disease; LVD, left ventricular dysfunction; NR, not reported; W, warfarin.
All included studies withheld vitamin K antagonist administration in the treatment and control groups.
Study fulfilled the inclusion criteria but reported 0 events and was therefore not included in the meta-analysis.
Patients may have more than 1 indication for VKA.
Risk of bias and synthesis of results
Overall, the risk of bias of the included studies was low. All studies randomly allocated patients using proper sequence generation, and 4 of the studies reported methods of allocation concealment.18,19,21,23,24 Blinding of patients and outcome assessors was reported in 2 studies only.21,24 Incomplete data were limited or properly dealt with across all studies. Forest plots (Figures 1-4) present a summary of the risk of bias for each study.
All-cause mortality. −, low risk of bias; +, high risk of bias; ?, unkown risk of bias; df, degree of freedom; M-H, Mantel Haenszel; trt, treatment.
All-cause mortality. −, low risk of bias; +, high risk of bias; ?, unkown risk of bias; df, degree of freedom; M-H, Mantel Haenszel; trt, treatment.
Subgroup analyses by vitamin K dose and method of vitamin K administration were prespecified, but identified data were inadequate to perform subgroup analyses. Publication bias was prespecified but could not be assessed given the small number of included studies.
All-cause mortality
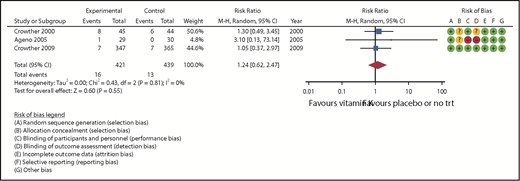
Three studies (860 randomized patients) assessed all-cause mortality during either 30 or 90 days of follow-up (Table 2).19,21,24 The pooled RR was 1.42 (95% CI, 0.62-2.47) in favor of placebo, and no heterogeneity was observed (I2 = 0, Figure 1). The certainty of the evidence, based on the GRADE criteria, was assessed as moderate because of serious imprecision in the pooled estimate.
GRADE evidence table
| Quality assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants (studies) . | Risk bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall quality of evidence . | Study event rates (%) . | RE (95% CI) . | Anticipated absolute effects . | ||
| Temporary cessation of VKA alone . | Temporary cessation of VKA + vitamin K administration . | Risk with temporary cessation of VKA alone . | Risk difference with temporary cessation of VKA + vitamin K administration (range) . | ||||||||
| Mortality (follow-up: 30-90 d; assessed with all-cause mortality) | |||||||||||
| 860 (3 RCTs) | NS | NS | NS | S* | None | ⊕⊕⊕○ Moderate | 13/439 (3.0) | 16/421 (3.8) | RR = 1.24 (0.62-2.47) | 30 per 1000 | 7 more per 1000 (11 fewer-44 more) |
| Major bleeding (follow-up: mean, 90 d; assessed with fatal bleeding or bleeding requiring blood transfusion or admission) | |||||||||||
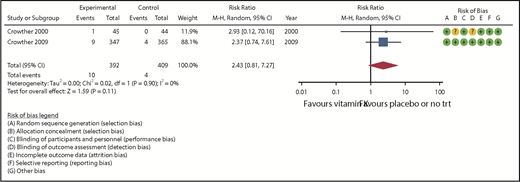
| 801 (2 RCTs) | NS | NS | NS | S* | None | ⊕⊕⊕○ Moderate | 4/409 (1.0) | 10/392 (2.6) | RR = 2.43 (0.81-7.27) | 10 per 1000 | 14 more per 1000 (2 fewer-61 more) |
| Any thromboembolism (follow-up: mean, 90 d; assessed with venous or arterial thromboembolism) | |||||||||||
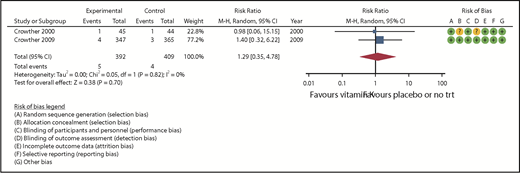
| 801 (2 RCTs) | NS | NS | NS | S* | None | ⊕⊕⊕○ Moderate | 4/409 (1.0) | 5/392 (1.3) | RR = 1.29 (0.35-4.78) | 10 per 1000 | 3 more per 1000 (6 fewer-37 more) |
| Proportion reaching goal INR (follow-up: mean, 1 d; assessed with INR goal ranges: INR, 1.8-3.2; INR, 2.3-4.5; and INR, 2.0-4.0) | |||||||||||
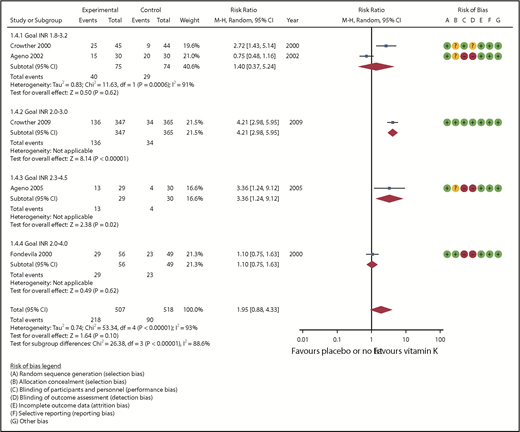
| 1025 (5 RCTs) | S† | S‡ | NS | S* | None | ⊕○○○ Very low | 90/518 (17.4) | 218/507 (43.0) | RR = 1.95 (0.88-4.33) | 174 per 1000 | 165 more per 1000 (21 fewer-579 more) |
| Quality assessment . | Summary of findings . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of participants (studies) . | Risk bias . | Inconsistency . | Indirectness . | Imprecision . | Publication bias . | Overall quality of evidence . | Study event rates (%) . | RE (95% CI) . | Anticipated absolute effects . | ||
| Temporary cessation of VKA alone . | Temporary cessation of VKA + vitamin K administration . | Risk with temporary cessation of VKA alone . | Risk difference with temporary cessation of VKA + vitamin K administration (range) . | ||||||||
| Mortality (follow-up: 30-90 d; assessed with all-cause mortality) | |||||||||||
| 860 (3 RCTs) | NS | NS | NS | S* | None | ⊕⊕⊕○ Moderate | 13/439 (3.0) | 16/421 (3.8) | RR = 1.24 (0.62-2.47) | 30 per 1000 | 7 more per 1000 (11 fewer-44 more) |
| Major bleeding (follow-up: mean, 90 d; assessed with fatal bleeding or bleeding requiring blood transfusion or admission) | |||||||||||
| 801 (2 RCTs) | NS | NS | NS | S* | None | ⊕⊕⊕○ Moderate | 4/409 (1.0) | 10/392 (2.6) | RR = 2.43 (0.81-7.27) | 10 per 1000 | 14 more per 1000 (2 fewer-61 more) |
| Any thromboembolism (follow-up: mean, 90 d; assessed with venous or arterial thromboembolism) | |||||||||||
| 801 (2 RCTs) | NS | NS | NS | S* | None | ⊕⊕⊕○ Moderate | 4/409 (1.0) | 5/392 (1.3) | RR = 1.29 (0.35-4.78) | 10 per 1000 | 3 more per 1000 (6 fewer-37 more) |
| Proportion reaching goal INR (follow-up: mean, 1 d; assessed with INR goal ranges: INR, 1.8-3.2; INR, 2.3-4.5; and INR, 2.0-4.0) | |||||||||||
| 1025 (5 RCTs) | S† | S‡ | NS | S* | None | ⊕○○○ Very low | 90/518 (17.4) | 218/507 (43.0) | RR = 1.95 (0.88-4.33) | 174 per 1000 | 165 more per 1000 (21 fewer-579 more) |
Overall quality of evidence measured according to GRADE criteria.
RE, relative effect; NS, not serious; S, serious.
Lower and upper bounds of 95% CI may lead to different recommendations.
Four of the 5 studies did not blind patients and personnel, or outcome assessors.
I2 = 93%.
Major bleeding
Six studies assessed major bleeding, 4 of which reported 0 events in the treatment and control groups and were not included in the meta-analysis.18-20,23 The remaining 2 studies21,24 (801 randomized patients) reported major bleeding outcomes at 90 days of follow-up and were included in the meta-analysis (Table 2). The pooled RR was 2.43 (95% CI, 0.81-7.27) in favor of placebo with no observed heterogeneity (I2 = 0; Figure 2). The certainty of the evidence was moderate because of serious imprecision of the pooled estimate.
Thromboembolism
Five studies assessed thromboembolism outcomes, 3 of which had 0 events in the treatment and control groups18,20,23 ; therefore only 2 studies were included in the meta-analysis (Table 2). The pooled RR (from 916 randomized patients) was 1.29 (95% CI, 0.35-4.78) in favor of placebo with no observed heterogeneity (I2 = 0, Figure 3). The certainty of the evidence for any thromboembolism was assessed as moderate because of serious imprecision in the pooled estimate.
Proportion of patients who reached goal INR
Five studies (313 randomized patients) provided information on the number of patients who reached goal INR values within 1 day of vitamin K administration (Table 2). The pooled RR of reaching goal INR values was 1.95 (95% CI, 0.88-4.33) in favor of vitamin K. Overall heterogeneity was high (I2 = 93%; Figure 4) and was not explained by the different definitions of goal INR values. The certainty of the evidence was assessed as very low because of serious risk of bias primarily related to lack of blinding, serious inconsistency, and serious imprecision of the pooled estimate. Two studies also reported the number of patients who reached goal INR values after 1 week of vitamin K administration. One study reported a RR of 1.16 (95% CI, 0.67-1.99) for goal INR ranging between 2.0 and 3.0, and a RR of 1.65 (95% CI, 1.04- 2.62) for goal INR ranging between 1.8 and 3.2.20 Another study reported a RR of 0.78 (95% CI, 0.50-1.21) for goal INR ranging between 2.0 and 4.0.23
Discussion
Very low to moderate quality evidence suggests that there is no additional benefit in administering vitamin K in patients who need to temporarily stop VKA because of INR elevation between 4.5 and 10.0 and who are not bleeding. The RCTs were primarily designed to detect a difference in the important surrogate outcome of achieving a targeted INR reduction, but this potential benefit was not significant and may not translate into improved clinical outcomes because the pooled RRs for mortality, major bleeding, and thromboembolism were all consistent with no effect.
The small number and very low to moderate quality of studies included are limitations to our review. Although not statistically significant, our results indicate a trend toward more bleeding when vitamin K is administered, which was an unexpected finding. Given the small number of events and large CIs for these outcomes, it is likely that these results are due to chance alone. Bleeding with or without anticoagulation results from tissue or blood vessel damage or injury. A prospective cohort of 107 nonbleeding patients presenting with an INR >10 found only a single major bleeding event occurred within 7 days of the index INR.25 Although higher INRs are associated with increased risk of bleeding,4-6 there may not be a clinically important difference in INR decline or risk of bleeding over 24 to 72 hours between placebo and vitamin K.
We intended to investigate differences in the effects of vitamin K by dose, route of administration, and type; however, the small number of studies did not permit such subgroup analyses. In terms of type of VKA, 3 of the included studies used warfarin, 2 used acenocoumarol, and none used phenprocoumon. These medications have different pharmacokinetic properties, which may result in different responses to vitamin K and placebo treatment. For example, the half-life of warfarin is close to double that of acenocoumarol.20 The low level of heterogeneity in our results suggests that variations in vitamin K type, dose, or route of administration did not likely influence our observed outcomes. Publication bias could not be assessed because of the small number of studies included. Because publication bias typically causes overly optimistic effect estimates by including more positive studies, it is unlikely that this influenced our conclusions as effects tended to go into the direction of harm.
The most recent systematic review on this topic that attempted to pool individual study data focused on the proportion of patients who reached goal INR in the treatment group and did not provide relative effects or comparison data from a control group.10 There were also several differences compared with our study regarding the inclusion criteria: we identified 1 recent study that was published after the previous systematic review was conducted.21 In addition, we excluded 5 RCTs that the previous systematic review included. These RCTs were excluded because VKA was not temporarily discontinued in the treatment group26 or because they compared different doses or routes of administration for vitamin K, rather than against placebo or observation only.22,27-29 Finally, DeZee et al10 included nonrandomized studies, whereas we focused on RCTs only. We estimate that the risk of bias related to patient selection and confounding in observational studies, among others, would not constitute more trustworthy evidence than that provided by RCTs.
Clinical practice guidelines have recommended since 2008 against the administration of vitamin K for patients with INRs between 4.5 and 10.0 who have no significant bleeding.14 Results from our review support the continued relevance of this recommendation. However, it is not clear to what extent these guidelines have been implemented. In a US survey inquiring about providers’ familiarity and awareness of VKA reversal guidelines, only 29% of physicians and physician assistants were aware of changes in these guidelines.30 In the same study, a retrospective chart review of 51 patients with INRs ranging between 4.5 and 9 showed that 33% were administered vitamin K to reduce INR values. This is worrisome, because overaggressive VKA reversal has been shown to be less cost-effective than withholding VKA alone.31
In conclusion, our results suggest that nonbleeding patients on VKAs with INRs between 4.5 and 10.0 will not likely benefit from vitamin K administration. Consensus guideline recommendations to forego vitamin K administration in this situation remain relevant.
The full-text version of the article contains a data supplement.
Acknowledgments
This systematic review was performed as part of the American Society of Hematology (ASH) guidelines for venous thromboembolism. The entire guideline development process was funded by ASH.
Authorship
Contribution: R.K. contributed to study design, search strategy, study selection, data extraction, statistical analysis, and writing of the report; M.L. contributed to study selection, data extraction, and writing of the report; R.N. contributed to the study design, study selection, statistical analysis, interpretation of results, and writing of the report; and D.M.W., J.A., N.P.C., A.H., W.W., and H.S. contributed to the study design, interpretation of the results, and writing of the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rasha Khatib, Department of Neurology, Northwestern University Feinberg School of Medicine, 633 North St Clair St, Chicago, IL 60611; e-mail: rasha.khatib@phri.ca.