Key Points
Plerixafor-based mobilization is less toxic and more rapid relative to G-CSF, but fewer CD34+ cells are collected.
Despite lower numbers of CD34+ cells mobilized, plerixafor mobilized allografts may be associated with similar clinical outcomes.
Abstract
Plerixafor, a direct antagonist of CXCR4/stromal-derived factor 1, can safely and rapidly mobilize allografts without the use of granulocyte colony-stimulating factor (G-CSF). We conducted a phase 2, multicenter, prospective study of plerixafor-mobilized HLA-identical sibling allografts for allogeneic hematopoietic cell transplantation in recipients with hematological malignancies. Donors (n = 64) were treated with subcutaneous plerixafor (240 µg/kg) and started leukapheresis (LP) 4 hours later. The primary objective was to determine the proportion of donors who were successfully mobilized: defined as collection of ≥2.0 × 106 CD34+ cells per kilogram recipient weight in ≤2 LP sessions. Recipients subsequently received reduced intensity (RIC; n = 33) or myeloablative (MAC; n = 30) conditioning. Sixty-three of 64 (98%) donors achieved the primary objective. The median CD34+ cell dose per kilogram recipient weight collected within 2 days was 4.7 (0.9-9.6). Plerixafor was well tolerated with only grade 1 or 2 drug-related adverse events noted. Bone pain was not observed. Plerixafor-mobilized grafts engrafted promptly. One-year progression-free and overall survivals were 53% (95% confidence interval [CI], 36% to 71%) and 63% (95% CI, 46% to 79%) for MAC and 64% (95% CI, 47% to 79%) and 70% (95% CI, 53% to 84%) for RIC recipients, respectively. Donor toxicity was reduced relative to G-CSF mobilized related donors. This is the first multicenter trial to demonstrate that, as an alternative to G-CSF, plerixafor rapidly and safely mobilizes sufficient numbers of CD34+ cells from matched sibling donors for HCT. Engraftment was prompt, and outcomes in recipients were encouraging. This trial was registered at clinicaltrials.gov as #NCT01696461.
Introduction
The optimal source of donor hematopoietic stem cells (HSC) for hematopoietic cell transplantation (HCT) is controversial. Granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood (G-PB) has replaced bone marrow (BM) as the most common allograft source in adults1 but is associated with donor morbidity and higher rates of chronic graft-versus-host disease (GVHD) compared with BM.2,3 G-PB is the standard graft collected from adult related donors based on past studies showing more rapid engraftment, a shorter hospital stay, and superior overall survival (OS) in certain studies when compared with unmanipulated BM.3
Although the use of G-PB has allowed a sufficient allograft to be obtained without the need for an invasive surgical procedure, donors can experience moderate albeit transient toxicity attributable to G-CSF administration.4 In addition, the standard 4- to 6-day period of G-CSF mobilization causes significant inconvenience. The CXCR4 antagonist plerixafor mobilizes HSC into the peripheral blood faster than G-CSF and has become a standard agent used in combination with G-CSF for HSC mobilization prior to autologous stem cell transplantation.5,6 Two studies from 1 center showed that plerixafor alone given on the day of leukapheresis (LP) without G-CSF could safely and effectively mobilize functional HSC from healthy adult matched related donors for use in allogeneic transplantation after myeloablative conditioning (MAC).7,8 Analyses of allografts from these studies suggest both quantitative and qualitative differences relative to G-PB grafts that could impact clinical outcomes in recipients.7,8 To test the generalizability of these single-center observations, we conducted a phase 2 multicenter trial investigating HSC mobilization with single-agent plerixafor (without G-CSF) from matched sibling adult donors for transplantation of patients with hematological malignancies following either MAC or reduced intensity conditioning (RIC). We hypothesized that (1) plerixafor is a less toxic method for HSC mobilization relative to G-CSF for donors and (2) recipient outcomes after a plerixafor mobilized HCT (P-PB) would be similar to those observed after transplantation of G-PB.
Patients and methods
Recipients and donors
Recipients were eligible if 18 years or older, if recipients had a hematological malignancy suitable for HCT, if recipients had a fully HLA-matched sibling donor, and if recipients met other institutional eligibility standards for allogeneic HCT. All recipients were required to have adequate organ function and a Karnofsky performance status ≥70. Underlying diseases included acute myeloid leukemia, myelodysplastic syndrome, chronic myeloid leukemia, non-Hodgkin lymphoma, Hodgkin lymphoma, and chronic lymphocytic leukemia. Patients with acute leukemia were required to have ≤5% blasts in the BM. Patients with prior allogeneic HCT were excluded, and those with prior autologous HCT were only allowed to undergo RIC.
At recipient registration, centers declared that an individual recipient would receive MAC or RIC. Choice of agents for GVHD prophylaxis was at the discretion of the treating institution provided that (1) a calcineurin inhibitor in combination with methotrexate, mycophenolate mofetil, or sirolimus was used; and (2) no monoclonal (eg, alemtuzumab) or polyclonal anti–T-cell antibodies (eg, anti–thymocyte globulin) or any form of ex vivo T-cell depletion was employed.
Donors were eligible if they were 18 to 65 years of age, HLA-matched to their siblings at HLA-A, -B, and -DRB1, satisfied institution standard criteria to serve as a PB donor, and had adequate renal function. Donors were followed with routine complete blood counts at 1 month and 12 months as well as phone contact at 1, 6, and 12 months after LP.
Both recipients and sibling donors gave written informed consent in accordance with the Helsinki protocol on a study approved by the National Marrow Donor Program institutional review board and the institutional review boards at each participating institution. The study was monitored by the National Marrow Donor Program Data Safety and Monitoring Board.
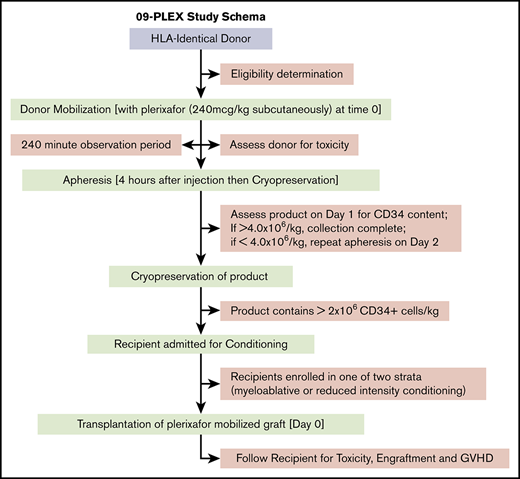
Donor mobilization treatment plan
Donors were treated with subcutaneous plerixafor at a dose of 240 µg/kg (actual weight). Four hours later, large-volume LP (at least 4 times blood volume) was performed per standard institutional methods. The target CD34+ cell dose for the donor allograft was ≥4.0 × 106/kg actual recipient weight. If the target CD34+ cell dose of ≥4.0 × 106/kg actual recipient weight was met after the first LP, no further collections were performed. If the LP product collected following day 1 contained ≥2.0 × 106 CD34+ cells per kilogram actual recipient weight, collection was considered successful; however, the donor returned the following morning, and the same procedures described above were repeated to attempt to achieve the target CD34+ cell dose. If the sum of the 2 collected LP products was ≥2.0 × 106 CD34+ cells per kilogram recipient weight, collection was considered successful and completed. If the sum of the 2 LP products was <2.0 × 106 CD34+ cells per kilogram recipient weight after 2 days, collection was considered unsuccessful, and any further plerixafor administration was at the discretion of the treating center. However, no more than 3 total days of plerixafor were allowed to be administered to each donor. Each LP product was then cryopreserved before the recipient initiated conditioning.
Transplant-related procedures
MAC regimens included 1 of the following 4 combinations: busulfan/fludarabine, busulfan/cyclophosphamide, total body irradiation (TBI)/cyclophosphamide, or TBI/etoposide. RIC regimens included busulfan/fludarabine, melphalan/fludarabine, and fludarabine/cyclophosphamide with dose thresholds determined by the Center for International Blood and Marrow Transplantation Research (CIBMTR) consensus criteria. Conditioning was not initiated until donor allografts had been cryopreserved with cell doses confirmed.
Tapering of immunosuppression was not started until days 90 to 100 after HCT. Supportive care was left to institutional discretion. G-CSF use was mandated for recipients of MAC starting at day +7 and continuing until absolute neutrophil count >1.5 × 109/L for 2 consecutive days. G-CSF use in recipients of RIC was optional.
Transplant-related procedures
Neutrophil engraftment was defined as time from day 0 of HCT to the first of 3 consecutive measurements of absolute neutrophil count ≥0.5 × 109/L. Platelet engraftment was defined as the number of days from day 0 of HCT to the first of 3 consecutive values of platelet count ≥20 × 109/L. Acute GVHD was graded per previously published consensus criteria.9 Chronic GVHD was graded per the National Institutes of Health (NIH) consensus criteria.10 Nonrelapse mortality (NRM) was defined by death from any cause in the absence of disease relapse. OS was defined as from day 0 of HCT to death from any cause. Patients who were alive were censored at the time last seen alive. Progression-free survival (PFS) was defined from day 0 of HCT to relapse, disease progression, or death, whichever occurred first. GVHD-free, relapse-free survival (GRFS) was defined according to published criteria.11
Toxicity assessment
Toxicities were assessed in donors at 30, 60, 120, and 240 minutes after administration of plerixafor on each day of collection and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3. A complete blood count was drawn at 1 and 12 months after LP to confirm normalization, and donors were contacted by telephone follow-up at 1, 6, and 12 months after donation to assess for presence of ongoing toxicities.
Contemporaneous controls
A contemporaneous control group of all adult donors 18 to 65 years of age and donating for the first time following G-CSF–only mobilization of peripheral blood stem cells (PBSCs) enrolled in the Related Donor Safety (RDSafe)12 study (n = 1098) was identified for comparison of donor toxicities and recovery at 1 month after donation.
A contemporaneous control group of recipients of noncryopreserved G-PB grafts consisting of all patients from the CIBMTR registry satisfying the following criteria: undergoing their first allogeneic HCT, 18 years or older, HLA-matched related PBSC recipient, from the same centers that enrolled in this study, and the same diagnoses and conditioning regimens allowed on this study, was selected for comparison of clinical outcomes.
Statistical considerations
This study was a phase 2 multicenter prospective trial. The primary endpoint was the collection of sufficient CD34+ cells using plerixafor as the sole mobilizing agent. A donor was considered successful for this endpoint if ≥2.0 × 106 CD34+ cells per kilogram recipient were collected in no more than 2 LP collections. This minimum CD34+ cell count was considered adequate to promote hematopoietic engraftment based on prior studies transplanting plerixafor mobilized grafts. It was expected that collections from 90% of donors treated with plerixafor would be successful, and this strategy would be considered unacceptable if <75% of donors were successful.
Recipients were classified into 1 of 2 strata, MAC or RIC, based on conditioning regimen planned. Recipient outcomes were analyzed from both strata combined as well as separately for each stratum. There was no statistical comparison of outcomes between the strata. Recipient endpoints were all secondary endpoints and included PFS, OS, NRM, relapse, and cumulative incidences of acute and chronic GVHD, respectively. We also compared GRFS as an unplanned post hoc analysis between study recipients and CIBMTR controls due to increasing interest in this as a surrogate endpoint for survival with good quality of life.
Descriptive statistics were used to summarize characteristics of the study population. Categorical variables were summarized with counts and percentages, whereas medians along with ranges were used for continuous variables. χ2 test and Wilcoxon rank sum test were used to compare categorical variables and continuous variables, respectively, between the donors who received plerixafor and RDSafe controls. The incidence of acute and chronic GVHD, NRM, and relapse was quantified by computing their cumulative incidence probability and an appropriate 95% confidence interval (CI). When evaluating the incidence of acute and chronic GVHD, death was treated as a competing risk. When computing cumulative incidence probability of NRM and relapse, relapse and NRM, respectively, were considered as a competing risk. Kaplan-Meier curve was used to estimate the probability of OS, PFS, and GRFS. Cumulative incidence and survival probabilities between the 2 groups of patients at fixed time points were compared by computing an appropriate z-statistic based on the differences of observed probabilities. A significance level α of 0.05 was used throughout, and all tests are 2 sided. SAS statistical software (SAS Institute, Cary, NC) was used to perform all statistical analyses of clinical data.
Results
Donor and recipient characteristics
Clinical and transplant characteristics of recipients in both MAC (n = 30) and RIC (n = 33) cohorts are shown in Table 1. Donor/recipient pairs were enrolled at 12 different centers. Patients in the RIC arm were generally older and more likely to have a hematopoietic cell transplantation comorbidity index (HCT-CI) score ≥4. Clinical characteristics of all donors are shown in Table 1. The median age of donors was 56 (range 18-65), and the majority were men. One donor was mobilized and leukapheresed, whose recipient never underwent HCT due to disease relapse.
Characteristics of MAC and RIC recipients and donors
| . | MAC . | RIC . | Donors . |
|---|---|---|---|
| Number of recipients | 30 | 33 | 64 |
| Number of centers | 10 | 11 | 12 |
| Age at transplant, median (range), y | 51 (23-62) | 62 (19-69) | 56 (18-65) |
| Sex, n (%) | |||
| Male | 16 (53) | 24 (73) | 41 (64) |
| Female | 14 (47) | 9 (27) | 23 (36) |
| Donor-recipient sex match, n (%) | |||
| Female-male | 6 (20) | 7 (21) | |
| Other | 24 (80) | 26 (79) | |
| Karnofsky performance status, n (%) | |||
| 90-100 | 20 (67) | 15 (45) | |
| ≤80 | 10 (33) | 18 (55) | |
| HCT-CI, n (%) | |||
| 0 | 5 (17) | 9 (27) | |
| 1 | 8 (27) | 6 (18) | |
| 2 | 6 (20) | 2 (6) | |
| 3 | 6 (20) | 4 (12) | |
| ≥4 | 5 (13) | 12 (36) | |
| Recipient CMV serostatus, n (%) | |||
| Negative | 15 (50) | 7 (21) | |
| Positive | 14 (47) | 25 (76) | |
| Inconclusive or unknown | 1 (3) | 1 (3) | |
| Recipient diagnosis, n (%) | |||
| AML | 12 (40) | 11 (33) | |
| ALL | 8 (27) | 2 (6) | |
| CML | 3 (10) | — | |
| MDS | 4 (13) | 11 (33) | |
| Lymphoma | 3 (10) | 9 (27) | |
| Disease risk index | |||
| Low, n (%) | 4 (14) | 5 (17) | |
| Intermediate, n (%) | 19 (66) | 19 (63) | |
| High, n (%) | 3 (10) | 6 (20) | |
| Very high, n (%) | 3 (10) | 0 | |
| Unknown, n | 1 | 3 | |
| Conditioning, n (%) | |||
| Busulfan/cyclophosphamide | 15 (50) | — | |
| Busulfan/fludarabine (MAC) | 5 (17) | — | |
| Cyclophosphamide/TBI | 8 (27) | — | |
| Etoposide/TBI | 2 (7) | — | |
| Melphalan/fludarabine | — | 7 (21) | |
| Busulfan/fludarabine (RIC) | — | 25 (76) | |
| Cyclophosphamide/fludarabine/TBI | — | 1 (3) | |
| GVHD prophylaxis, n (%) | |||
| CNI + MTX based | 24 (80) | 24 (70) | |
| CNI + MMF based | 3 (10) | 5 (15) | |
| CNI + others | — | 3 (9) | |
| CNI alone | 3 (10) | — | |
| Other | — | 1 (3) | |
| Use of post-HCT GCSF, n (%) | |||
| Yes | 22 (73) | 16 (48) | |
| No | 8 (27) | 17 (52) |
| . | MAC . | RIC . | Donors . |
|---|---|---|---|
| Number of recipients | 30 | 33 | 64 |
| Number of centers | 10 | 11 | 12 |
| Age at transplant, median (range), y | 51 (23-62) | 62 (19-69) | 56 (18-65) |
| Sex, n (%) | |||
| Male | 16 (53) | 24 (73) | 41 (64) |
| Female | 14 (47) | 9 (27) | 23 (36) |
| Donor-recipient sex match, n (%) | |||
| Female-male | 6 (20) | 7 (21) | |
| Other | 24 (80) | 26 (79) | |
| Karnofsky performance status, n (%) | |||
| 90-100 | 20 (67) | 15 (45) | |
| ≤80 | 10 (33) | 18 (55) | |
| HCT-CI, n (%) | |||
| 0 | 5 (17) | 9 (27) | |
| 1 | 8 (27) | 6 (18) | |
| 2 | 6 (20) | 2 (6) | |
| 3 | 6 (20) | 4 (12) | |
| ≥4 | 5 (13) | 12 (36) | |
| Recipient CMV serostatus, n (%) | |||
| Negative | 15 (50) | 7 (21) | |
| Positive | 14 (47) | 25 (76) | |
| Inconclusive or unknown | 1 (3) | 1 (3) | |
| Recipient diagnosis, n (%) | |||
| AML | 12 (40) | 11 (33) | |
| ALL | 8 (27) | 2 (6) | |
| CML | 3 (10) | — | |
| MDS | 4 (13) | 11 (33) | |
| Lymphoma | 3 (10) | 9 (27) | |
| Disease risk index | |||
| Low, n (%) | 4 (14) | 5 (17) | |
| Intermediate, n (%) | 19 (66) | 19 (63) | |
| High, n (%) | 3 (10) | 6 (20) | |
| Very high, n (%) | 3 (10) | 0 | |
| Unknown, n | 1 | 3 | |
| Conditioning, n (%) | |||
| Busulfan/cyclophosphamide | 15 (50) | — | |
| Busulfan/fludarabine (MAC) | 5 (17) | — | |
| Cyclophosphamide/TBI | 8 (27) | — | |
| Etoposide/TBI | 2 (7) | — | |
| Melphalan/fludarabine | — | 7 (21) | |
| Busulfan/fludarabine (RIC) | — | 25 (76) | |
| Cyclophosphamide/fludarabine/TBI | — | 1 (3) | |
| GVHD prophylaxis, n (%) | |||
| CNI + MTX based | 24 (80) | 24 (70) | |
| CNI + MMF based | 3 (10) | 5 (15) | |
| CNI + others | — | 3 (9) | |
| CNI alone | 3 (10) | — | |
| Other | — | 1 (3) | |
| Use of post-HCT GCSF, n (%) | |||
| Yes | 22 (73) | 16 (48) | |
| No | 8 (27) | 17 (52) |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CML, chronic myelogenous leukemia; CNI, calcineurin inhibitor; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MTX, methotrexate.
Toxicity in plerixafor mobilized donors
Plerixafor was generally well tolerated, with only grade 1 or 2 treatment-related adverse events noted. Bloating, diarrhea, dizziness, headache, and injection site reaction were the most commonly observed adverse events. Across all days of collection, the maximal grade of plerixafor-related toxicities reported were grade 0 in 30%, grade 1 in 53%, and grade 2 in 16%. One donor experienced a grade 3 vasovagal event at commencement of LP, which was unrelated to plerixafor.
Mobilization efficacy and graft characteristics
The primary endpoint was achieved in 98% of donors, with only 1 donor failing to mobilize ≥2.0 × 106 CD34+ cells per kilogram within 2 LP sessions. The majority achieved the minimum CD34+ dose in 1 LP session (70%), but only 27% achieved the 4.0 × 106 CD34+ cell per kilogram target in 1 LP. After 2 LP sessions, the median CD34+ cell dose collected was 4.7 × 106/kg (Table 2). Of donors, 72% underwent 2 LP sessions, and 5% underwent 3 (all of whom achieved the minimum successful dose after 2 LP sessions). Quantification of specific leukocyte subsets was also performed on plerixafor mobilized grafts. Relative to data we previously published from G-CSF mobilized donors,13 P-PB grafts contained greater numbers of CD3+, CD4+, and CD8+ cells, as noted in prior single-center studies (supplemental Table 1).7,8
Plerixafor donor mobilization characteristics
| Variable . | Median (range) . | n (%) . |
|---|---|---|
| Peripheral blood CD34+ count (×106/µL): day 1 preplerixafor | 2.8 (0.3-28.0) | |
| Peripheral blood CD34+ count (×106/µL): day 1, 240 min postplerixafor | 19.0 (1.7-52.0) | |
| Peripheral blood CD34+ count (×106/µL): day 1, 240 min postplerixafor: fold increase | 6.5 (0.9-26.0) | |
| Total CD34+ cell count/kg in LP product at day 2 | ||
| ≥2.0 × 106/kg recipient weight (yes) | 63 (98) | |
| ≥4.0 × 106/kg recipient weight (yes) | 41 (64) | |
| CD34+ cells collected (×106/kg recipient weight) | ||
| Day 1 (n = 64) | 2.80 (0.30-9.60) | |
| Day 2 (n = 48) | 1.75 (0.50-4.70) | |
| Cumulative total | 4.70 (0.90-9.60) |
| Variable . | Median (range) . | n (%) . |
|---|---|---|
| Peripheral blood CD34+ count (×106/µL): day 1 preplerixafor | 2.8 (0.3-28.0) | |
| Peripheral blood CD34+ count (×106/µL): day 1, 240 min postplerixafor | 19.0 (1.7-52.0) | |
| Peripheral blood CD34+ count (×106/µL): day 1, 240 min postplerixafor: fold increase | 6.5 (0.9-26.0) | |
| Total CD34+ cell count/kg in LP product at day 2 | ||
| ≥2.0 × 106/kg recipient weight (yes) | 63 (98) | |
| ≥4.0 × 106/kg recipient weight (yes) | 41 (64) | |
| CD34+ cells collected (×106/kg recipient weight) | ||
| Day 1 (n = 64) | 2.80 (0.30-9.60) | |
| Day 2 (n = 48) | 1.75 (0.50-4.70) | |
| Cumulative total | 4.70 (0.90-9.60) |
Engraftment and chimerism
At 100 days after HCT, all patients in both the MAC and the RIC cohorts had achieved neutrophil engraftment (Table 3). All patients in the MAC arm and 97% of patients in the RIC arm had achieved platelet engraftment. There were no cases of primary or secondary graft failure. The median time to neutrophil engraftment was 13 and 15 days for MAC and RIC, respectively. The median time to platelet engraftment was 19 and 18 days for MAC and RIC, respectively. Chimerism at serial time points after HCT is displayed in supplemental Table 2. Full donor myeloid chimerism was achieved relatively quickly in both groups (median 100% at day +28 in both RIC and MAC), but conversion to full donor T-cell chimerism appeared to be slower in the RIC patients.
Outcomes after HCT of recipients receiving plerixafor mobilized PBSCs compared with control recipients receiving G-CSF mobilized PBSCs from the CIBMTR
| Outcome . | MAC PLEX (n = 30) . | MAC CIBMTR (n = 99) . | P . | RIC PLEX (n = 33) . | RIC CIBMTR (n = 73) . | P . |
|---|---|---|---|---|---|---|
| Neutrophil engraftment by day +100, % | 100 | 100 | — | 100 | 100 | — |
| Platelet engraftment by day +100, % | 100 | 98 | — | 97 | 94 | .530 |
| Day 100 cumulative incidence of grades 2-4 acute GVHD (range) | 53 (35-71) | 32 (23-42) | .045 | 18 (7-33) | 23 (14-34) | .546 |
| Day 100 cumulative incidence of grades 3-4 acute GVHD (range) | 17 (6-32) | 5 (2-10) | .110 | 3 (0-12) | 7 (2-14) | .369 |
| 1-y cumulative incidence of chronic GVHD (range) | 52 (33-70) | 51 (41-60) | .913 | 39 (23-57) | 40 (29-52) | .933 |
| 1-y cumulative incidence of disease relapse (range) | 30 (15-48) | 35 (26-45) | .586 | 30 (16-47) | 47 (35-58) | .106 |
| 1-y cumulative incidence of NRM (range) | 17 (6-32) | 15 (9-23) | .847 | 6 (1-17) | 7 (2-14) | .879 |
| 1-y progression-free survival (range) | 53 (36-71) | 49 (40-59) | .712 | 64 (47-79) | 47 (35-58) | .095 |
| 1-y OS (range) | 63 (46-79) | 66 (56-75) | .816 | 70 (53-84) | 63 (52-74) | .495 |
| 1-y GRFS (range) | 23 (10-40) | 30 (22-40) | .439 | 39 (24-56) | 29 (19-40) | .289 |
| Outcome . | MAC PLEX (n = 30) . | MAC CIBMTR (n = 99) . | P . | RIC PLEX (n = 33) . | RIC CIBMTR (n = 73) . | P . |
|---|---|---|---|---|---|---|
| Neutrophil engraftment by day +100, % | 100 | 100 | — | 100 | 100 | — |
| Platelet engraftment by day +100, % | 100 | 98 | — | 97 | 94 | .530 |
| Day 100 cumulative incidence of grades 2-4 acute GVHD (range) | 53 (35-71) | 32 (23-42) | .045 | 18 (7-33) | 23 (14-34) | .546 |
| Day 100 cumulative incidence of grades 3-4 acute GVHD (range) | 17 (6-32) | 5 (2-10) | .110 | 3 (0-12) | 7 (2-14) | .369 |
| 1-y cumulative incidence of chronic GVHD (range) | 52 (33-70) | 51 (41-60) | .913 | 39 (23-57) | 40 (29-52) | .933 |
| 1-y cumulative incidence of disease relapse (range) | 30 (15-48) | 35 (26-45) | .586 | 30 (16-47) | 47 (35-58) | .106 |
| 1-y cumulative incidence of NRM (range) | 17 (6-32) | 15 (9-23) | .847 | 6 (1-17) | 7 (2-14) | .879 |
| 1-y progression-free survival (range) | 53 (36-71) | 49 (40-59) | .712 | 64 (47-79) | 47 (35-58) | .095 |
| 1-y OS (range) | 63 (46-79) | 66 (56-75) | .816 | 70 (53-84) | 63 (52-74) | .495 |
| 1-y GRFS (range) | 23 (10-40) | 30 (22-40) | .439 | 39 (24-56) | 29 (19-40) | .289 |
GVHD
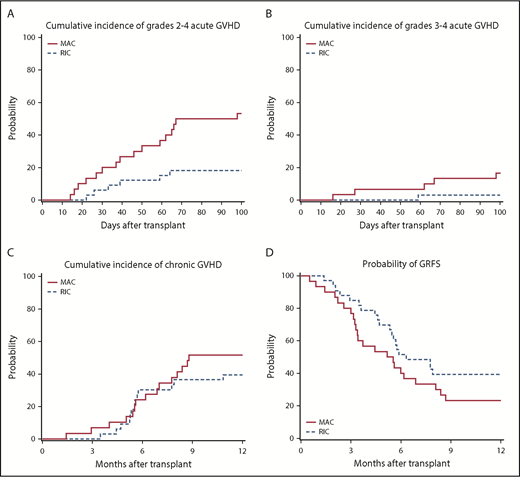
The day +100 cumulative incidence of grades 2 to 4 and 3 to 4 acute GVHD were 53% (95% CI, 35% to 71%) and 17% (95% CI, 6% to 32%), respectively, for patients treated with MAC and 18% (95% CI, 7% to 33%) and 3% (95% CI, 0% to 12%) for RIC-treated patients (Figure 1A-B). The 1-year cumulative incidence of any chronic GVHD for MAC and RIC patients was 52% (95% CI, 33% to 70%) and 39% (95% CI, 23% to 57%), respectively (Figure 1C). The corresponding NIH severity scores for all cGVHD patients were mild (MAC 37%; RIC 31%), moderate (MAC 44%; RIC 31%), and severe (MAC 19%; RIC 38%). We measured 1-year GRFS as an exploratory endpoint post hoc according to previous definition.11 One-year GRFS was 23% (95% CI, 10% to 40%) in MAC recipients and 39% (95% CI, 24% to 56%) in RIC recipients (Table 3; Figure 1D).
GVHD outcomes. (A) Cumulative incidence of grades II-IV acute GVHD. (B) Cumulative incidence of grades III-IV acute GVHD. (C) Cumulative incidence of chronic GVHD. (D) One-year GRFS in RIC and MAC recipients, respectively.
GVHD outcomes. (A) Cumulative incidence of grades II-IV acute GVHD. (B) Cumulative incidence of grades III-IV acute GVHD. (C) Cumulative incidence of chronic GVHD. (D) One-year GRFS in RIC and MAC recipients, respectively.
Relapse, NRM, and survival
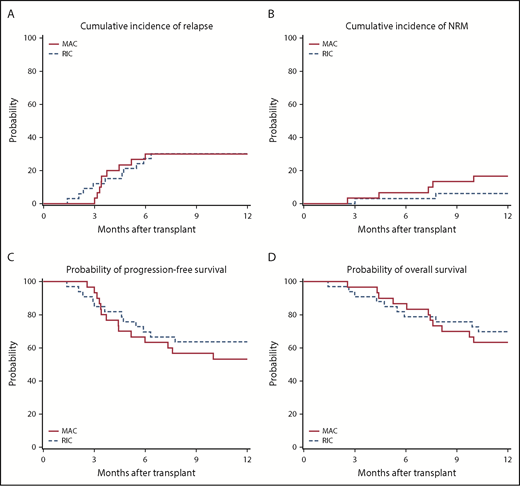
The cumulative incidence of relapse at 1 year was 30% (95% CI, 15% to 48%) after MAC and 30% (95% CI, 16% to 47%) after RIC (Figure 2A). NRM at 1 year was 17% (95% CI, 6% to 32%) after MAC and 6% (95% CI, 1% to 17%) after RIC (Figure 2B). PFS at 1 year was 53% (95% CI, 36% to 71%) after MAC and 64% (95% CI, 47% to 79%) after RIC (Figure 2C). OS at 1 year was 63% (95% CI, 46% to 79%) after MAC and 70% (95% CI, 53% to 84%) after RIC (Figure 2D).
Transplantation outcomes. (A) Cumulative incidence of disease relapse. (B) Cumulative incidence of NRM. (C) Progression-free survival. (D) OS in RIC and MAC recipients, respectively.
Transplantation outcomes. (A) Cumulative incidence of disease relapse. (B) Cumulative incidence of NRM. (C) Progression-free survival. (D) OS in RIC and MAC recipients, respectively.
Immune reconstitution and CMV reactivation
Immune reconstitution was measured by CD3+CD4+ and CD3+CD8+ T cells in the peripheral blood of patients at days 28, 100, 180, and 365 after HCT (supplemental Figure 1A-B). Notably, median CD3+CD4+ cell counts were >200/µL at all times analyzed, including day 28 in both MAC and RIC groups. Nineteen (65%) of the MAC recipients were at risk for cytomegalovirus (CMV) reactivation, and 3 patients (16%) experienced 1 or more CMV reactivations in blood at a median of 43 days after transplantation (range 39-152). Twenty-seven of the 33 (82%) RIC recipients were at risk, and 6 (22%) had at least 1 episode of CMV viremia a median of 57 days (range 35-216) after transplantation.
Comparison with RDSafe donor and CIBMTR recipient controls
In the RDSafe cohort, 725 (66%) donors underwent 1 day of collection; 341 (31%) had 2 days of collection, and 32 (3%) had 3 or more days of cell collection. On the day of collection, the maximum toxicity reported was significantly higher in donors receiving G-CSF with 30% of donors experiencing grade 2 toxicities and 15% experiencing grades 3 to 4 toxicities compared with 16% of plerixafor mobilized donors reporting grade 2 maximum toxicities and 2% reporting grades 3 to 4 toxicities (P < .001) (Table 4). At 1 month after collection, there were no significant differences between plerixafor and G-CSF donors in terms of maximum toxicities reported or overall recovery.
Toxicities experienced by donors on this study and CIBMTR control group
| . | Plerixafor . | RDSafe . | P . |
|---|---|---|---|
| Number of donors | 64 | 1098 | |
| Number of centers | 12 | 42 | |
| Maximum toxicity at collection, n (%) | <.001 | ||
| 0 | 19 (30) | 188 (17) | |
| 1 | 34 (53) | 411 (38) | |
| 2 | 10 (16) | 328 (30) | |
| 3-4 | 1 (2) | 164 (15) | |
| Maximum toxicity at 1 mo, n (%) | .538 | ||
| 0 | 33 (60) | 520 (54) | |
| 1 | 14 (25) | 308 (32) | |
| 2 | 8 (15) | 111 (11) | |
| 3-4 | 0 | 29 (3) | |
| Recovery at 1 mo, n (%) | |||
| Yes | 43 (67) | 590 (62) | .440 |
| No | 21 (33) | 356 (38) | |
| Unknown | 0 | 152 |
| . | Plerixafor . | RDSafe . | P . |
|---|---|---|---|
| Number of donors | 64 | 1098 | |
| Number of centers | 12 | 42 | |
| Maximum toxicity at collection, n (%) | <.001 | ||
| 0 | 19 (30) | 188 (17) | |
| 1 | 34 (53) | 411 (38) | |
| 2 | 10 (16) | 328 (30) | |
| 3-4 | 1 (2) | 164 (15) | |
| Maximum toxicity at 1 mo, n (%) | .538 | ||
| 0 | 33 (60) | 520 (54) | |
| 1 | 14 (25) | 308 (32) | |
| 2 | 8 (15) | 111 (11) | |
| 3-4 | 0 | 29 (3) | |
| Recovery at 1 mo, n (%) | |||
| Yes | 43 (67) | 590 (62) | .440 |
| No | 21 (33) | 356 (38) | |
| Unknown | 0 | 152 |
We compared clinical outcomes in recipients from this study to a matched cohort of CIBMTR controls, stratified by conditioning regimen intensity. There were no significant differences in hematopoietic engraftment, serial chimerism values, cumulative incidence of grades 2 to 4 and 3 to 4 acute GVHD, chronic GVHD, 1-year GRFS, NRM, PFS, or OS (Table 3) with the exception of a significant increase in grade 2 acute GVHD in the MAC recipients (P = .045). Serial immune reconstitution data were not available for control recipients from the CIBMTR.
Discussion
The results of this study suggest that plerixafor-based donor mobilization is a less toxic and faster method to obtain hematopoietic cells for transplantation into patients with hematological malignancies compared with G-PB, and that despite apparent differences in allograft content, important clinical outcomes in recipients appear similar relative to transplantation of G-PB. However, a prospective, randomized controlled trial would be necessary to prove this.
G-CSF–based mobilization of donor peripheral blood stem cells is usually successful, but 15% of donors experience grade 3 to 4 acute toxicity, and >50% of recipients of G-PB develop chronic GVHD3 ; clearly there is room for improvement. The group at Washington University (WashU) has pioneered the use of single-agent plerixafor and demonstrated it can rapidly mobilize cells for transplantation.7,8 However, it is a less potent mobilizer than G-CSF, and two-thirds of donors require 2 days of LP to collect an adequate graft. Efforts to improve the pharmacodynamic response to subcutaneous plerixafor, including dose escalation and IV administration, have been largely unsuccessful.8 As such, we chose to deliver the standard Food and Drug Administration–approved dose of 240 μg/kg administered subcutaneously to donors on this study. Although it is indeed safe and well tolerated, only 27% of our donors achieved the target dose in 1 LP procedure. Thus, although the minimum CD34+ cell dose was achieved after 2 days of LP in 98% of donors, future efforts to reliably reduce collection to a single-day procedure would be desirable.
Potentially important qualitative and quantitative differences comparing P-PB to G-PB could impact clinical outcomes in recipients (supplemental Table 1). Specifically, CD34+ cell doses collected after the first day of LP are lower with P-PB, and G-PB grafts from related donors generally contain higher numbers of CD34+ cells.3 Nevertheless, data from this study and WashU suggest this does not adversely affect the kinetics or completeness of hematopoietic engraftment, perhaps due to improved homing efficiency or other factors.7,8 Conversely, the content of CD3+, CD4+, and CD8+ T cells is greater in plerixafor mobilized allografts. Although the higher T-cell content raises concern that more acute or chronic GVHD may occur, this has not been observed to date in the >100 patients who have received P-PB allografts at WashU. With the exception of possibly more grade 2 acute GVHD in the MAC recipients, we did not observe substantial increases in GVHD on this trial. Data from WashU and another study from the HOVON group show more plasmacytoid dendritic cell (pDC) precursors in P-PB grafts relative to G-PB.8,14 Larger numbers of pDC in BM grafts have been shown to be associated with improved survival and decreased NRM after unrelated donor transplantation.13Nevertheless, the clinical impact of these differences in graft content remains speculative.
Immune reconstitution in our study was generally robust. Notably, the median CD4+ count after transplantation never fell below 200/µL, even in the RIC group with a median age of 62 years. The higher CD4+ content of P-PB allografts relative to G-PB may have played a role in the very low NRM observed. In addition, the higher CD8+ content in P-PB may be beneficial, particularly for RIC patients. Reshef and colleagues recently described the characteristics of G-PB donor grafts that associated with a lower risk of disease relapse and an improvement in OS following RIC-based HCT.15 In an analysis of 200 patients with hematologic malignancies who underwent HCT after RIC, transplantation of grafts containing a high CD8+ T-cell dose (CD8hi) resulted in improved survival due to a reduction in the risk for relapse without a significant increase in GVHD. These CD8hi grafts were collected almost exclusively from younger donors. The odds of finding CD8hi donors correlated inversely with age. In that study, elderly RIC transplant recipients were very unlikely to receive a graft containing the ideal CD8+ cell dose from their older siblings. In our study, all of the allografts from P-PB donors were above the optimal CD8hi level established by Reshef, despite a median age of 56, whereas only 13% of donors over age 50 mobilized a CD8hi graft after G-CSF in their study.15 P-PB may thus be a means to convert an older matched sibling donor into an “optimal” donor relative to prevention of relapse. The 39% GRFS in the RIC recipients on this study was encouraging and suggests that testing this hypothesis in a prospective study may have merit.
Low rates of CMV reactivation following transplantation of P-PB have been observed by the WashU group, hypothetically related to the pDC precursor content.8 We observed that <20% of the at-risk population on this study experienced CMV reactivation, which may be lower than historical controls but would need to be confirmed in a controlled trial.
Although this was a prospective multicenter trial, conclusions are limited by the single-arm design and small sample size even with comparison with contemporaneous control groups from the RDSafe study and CIBMTR, respectively. Although rates of acute and chronic GVHD appeared comparable relative to the CIBMTR control group, assessment of chronic GVHD is fraught with error, and standard grading schemes can often hide the actual severity of disease. In addition, formal assessment of nontraditional endpoints such as cost and quality of life for both donors and recipients was not included, but should be components of future studies comparing methods of PB mobilization.
In conclusion, single-agent subcutaneous plerixafor resulted in safe and rapid CD34+ cell mobilization from healthy HLA-matched related donors. Compared with a contemporaneous control of G-PB donors, plerixafor mobilized donors appeared to experience significantly less grade 2 to 4 toxicities. Grafts mobilized with plerixafor contained a several-fold increase in T and B cells relative to traditional G-CSF mobilized grafts. Recipients of both MAC and RIC regimens experienced rapid and durable engraftment. Within the limits imposed by the size of our study cohort, rates of acute and chronic GVHD, relapse, NRM, PFS, GRFS, and OS were not demonstrably different from those in a contemporaneous control group of recipients of G-PB from the CIBMTR. Definitive conclusions regarding recipient outcomes cannot be reached until a randomized prospective study is performed. Therefore, until we have more effective mobilizing agents capable of reducing the process of collecting an allograft to a 1-day procedure for the majority of donors, G-CSF will remain the standard. Efforts to identify such agents are ongoing, and if successful, should be compared prospectively to G-PB.16
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by Genzyme Corporation, a Sanofi company, in part by National Institutes of Health (NIH), National Cancer Institute (NCI) grant R01 1R01CA188523-01A1 (E.K.W.), and by the Biostatistics and Bioinformatics shared resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the NCI, the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases, a grant/cooperative agreement 1U24HL138660 from NHLBI and NCI, a contract HHSH250201700006C with Health Resources and Services Administration (Department of Health and Human Services), 3 grants (N00014-17-1-2388, N00014-17-1-2850, and N00014-18-1-2045) from the Office of Naval Research, and grants from Adaptive Biotechnologies, *Amgen, Inc, anonymous donation to the Medical College of Wisconsin, Astellas Pharma US, Atara Biotherapeutics, Inc, Be the Match Foundation, *bluebird bio, Inc, *Bristol Myers Squibb Oncology, *Celgene Corporation, *Chimerix, Inc, *CytoSen Therapeutics, Inc, Fred Hutchinson Cancer Research Center, Gamida Cell Ltd, Gilead Sciences, Inc, HistoGenetics, Inc, Immucor, *Incyte Corporation, Janssen Scientific Affairs, LLC, *Jazz Pharmaceuticals, Inc, Karius, Inc, Karyopharm Therapeutics, Inc, *Kite Pharma, Inc, Medac, GmbH, *Mediware, The Medical College of Wisconsin, *Merck & Co, Inc, *Mesoblast, MesoScale Diagnostics, Inc, Millennium, the Takeda Oncology Co, *Miltenyi Biotec, Inc, Mundipharma EDO, National Marrow Donor Program, Novartis Pharmaceuticals Corporation, PCORI, *Pfizer, Inc, *Pharmacyclics, LLC, PIRCHE AG, *Sanofi Genzyme, *Seattle Genetics, Shire, Spectrum Pharmaceuticals, Inc, St. Baldrick’s Foundation, Swedish Orphan Biovitrum, Inc, *Takeda Oncology, and University of Minnesota. *Corporate members.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The views expressed in this article do not reflect the official policy or position of the NIH, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US Government.
Authorship
Contribution: J.P.M., S.M.D., J.L.-R., M.M.H., Y.-B.C., D.M.K., M.H., J.F.D., M.R.L., M.E.H., A.S.A., H.F.F., H.K.D., and E.K.W. conceived and designed the study; S.M.D., J.L.-R., M.M.H., Y.-B.C., M.H., J.F.D., M.R.L., M.E.H., A.S.A., H.F.F., H.K.D., E.K.W., M.C., S.H., and B.L.M. provided study materials and/or patients; R.J.D., S.M.D., J.L.-R., H.K., M.P., M.M.H., Y.-B.C., D.M.K., M.H., J.F.D., M.R.L., M.E.H., B.E.S., A.S.A., H.F.F., H.K.D., E.K.W., M.C., S.H., and B.L.M. collected and assembled data; R.B., J.P.M., S.M.D., J.L.-R., M.M.H., Y.-B.C., D.M.K., B.E.S., M.H., J.F.D., M.R.L., M.E.H., A.S.A., H.F.F., H.K.D., E.K.W., M.C., S.H., and B.L.M. analyzed and interpreted the data; and all authors wrote, had final approval of manuscript, and were accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven M. Devine, Center for International Blood and Marrow Transplant Research (CIBMTR)–Minneapolis, 500 North 5th St, Minneapolis, MN 55401; e-mail: sdevine2@nmdp.org.