Key Points
R2 immunomodulatory treatment in first-line FL can achieve high rates of complete molecular response.
In the RELEVANCE phase 3 study, week 24 complete molecular response was higher with R2 than with R-Chemo.
Abstract
Complete molecular response (CMR) after first-line immunochemotherapy reflects treatment efficacy and may predict prognosis in patients with follicular lymphoma (FL). RELEVANCE is the first phase 3 trial comparing the chemotherapy-free regimen lenalidomide/rituximab (R2) vs rituximab/chemotherapy (R-Chemo) in previously untreated FL patients (ClinicalTrials.gov identifier: NCT01650701). The objective of the minimal residual disease (MRD) analysis was to determine the ability of a chemotherapy-free regimen to induce CMR. Of 440 French patients participating in the Lymphoma Study Association (LYSA) RELEVANCE MRD study, all 222 patients with a BIOMED-2–detectable BCL2-JH translocation at diagnosis were analyzed. MRD was quantified by droplet digital polymerase chain reaction with a sensitivity ≤10−4. At week 24 (end of induction treatment), 98% and 78% of patients achieved CMR in peripheral blood (PB) and bone marrow (BM), respectively. Achievement of CMR (in PB and/or BM) had a significant impact on progression-free survival (PFS), with 3-year PFS of 84% and 55% for patients with CMR and detectable MRD, respectively (P = .015). CMR at week 24 was reached more frequently in the R2 arm (105/117; 90%) than in the R-Chemo arm (70/90; 77%) (P = .022). The poor prognostic value in terms of PFS for the persistence of molecular disease was observed irrespective of treatment arm (interaction test, P = .31). In agreement with the clinical results of the RELEVANCE trial, our results show that R2 immunomodulatory treatment in first-line FL can achieve high rates of CMR.
Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma in western countries.1 In previously untreated FL patients with high tumor burden, standard treatment has been immunochemotherapy eventually followed by maintenance.2,3 Recently, the international phase 3 RELEVANCE trial (ClinicalTrials.gov identifier: NCT01650701) showed that an immunomodulatory regimen combining lenalidomide plus rituximab (R2) was similarly efficacious to immunochemotherapy (rituximab plus chemotherapy [R-Chemo]) in patients with previously untreated advanced FL.4
The clinical history of FL is characterized by recurrent relapses5 that are related to the persistence of tumor cells after treatment, identified as minimal residual disease (MRD), that can eventually be detected in the peripheral blood (PB) and/or bone marrow (BM) using polymerase chain reaction (PCR).6 Other than a few reports that negatively describe the predictive role of MRD in the context of FL,7,8 achieving MRD negativity at the ≤10−4 level after treatment has been primarily associated with improved progression-free survival (PFS) in patients with FL (well discussed by Galimberti et al9 ). Accordingly, measurement of MRD may be regarded as a surrogate marker of treatment efficacy and is deserving of further evaluation in a clinical trial setting.9-16
Here, we report the results of the MRD analysis conducted in the RELEVANCE trial to assess the ability of a chemotherapy-free regimen to induce a molecular response compared with R-Chemo.
Methods
Study design
The RELEVANCE study design has been described in detail (supplemental Figure 1).4 Briefly, the R2 arm consisted of 18 cycles of lenalidomide plus rituximab, followed by rituximab maintenance therapy every 8 weeks for 12 cycles (6 additional doses). The R-Chemo reference arm consisted of the investigator’s choice of 1 of 3 rituximab-based regimens (R-CHOP [rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), R-CVP [rituximab, cyclophosphamide, vincristine, prednisone], or R-B [rituximab, bendamustine]), followed by maintenance with rituximab every 8 weeks for 12 cycles. The primary end points were complete response (confirmed/unconfirmed complete response [CR/CRu]) at 120 weeks and PFS. A sample collection was preplanned for MRD study with no statistical design and conducted in patients treated in France in accordance with ethical guidelines and the Declaration of Helsinki; patients provided written informed consent. Samples from PB and BM were collected at screening and at week 24 (W24), the end of induction for both arms (supplemental Figure 1). Diagnostic PB and BM samples were screened by consensus BIOMED-2 PCR to detect a BCL2/JH translocation.17 In 4 screened patients without detectable BCL2/JH translocation in PB and BM, the translocation was characterized in a lymph node sample.
MRD assessment
In patients with a detectable BCL2/JH translocation, a droplet digital PCR was designed as previously described.18 Briefly, first the BCL2 and JH primer pair capable of amplifying the patient's translocation according to BCL2 breakpoint localization and JH segment involved was identified. As a result, the BCL2 primer was 1 of the multiplexed t(14;18) BIOMED-2 primers, and the JH primer/probe couple was one that was commonly used for immunoglobulin heavy chain gene allele-specific oligonucleotide real-time quantitative PCR.19 All assays (combinations of BCL2/JH primer pairs) reached a sensitivity ≤ 10−4. Results were expressed as a ratio of tumor cells (number of BCL2-JH copies)/total cells analyzed (number of albumin copies/2). A complete molecular response (CMR) is obtained when MRD PCR is negative in BM and/or PB with a sensitivity ≥ 10−4.
Statistical analysis
PFS was measured from randomization into the study to the first observation of documented disease progression or death due to any cause. PFS was censored at the time of last visit, with adequate assessment for patients without disease progression and death. Survival functions were calculated by Kaplan-Meier estimates, and comparison between categories was done using the log-rank test. Characteristics of populations were compared by using the χ2 test or Fisher’s exact test for discrete variables and the Student t test or Mann-Whitney U test for continuous variables. Variables considered for Cox model building included MRD in PB and/or BM and clinical response at W24. Multivariate analyses were performed using Cox proportional-hazards models. Interaction between MRD and study treatment of PFS was tested. P ≤ .05 was considered statistically significant. Statistical analyses used SAS 9.3 software and X-tile 3.6.1 software (Yale University, New Haven, CT).
Results
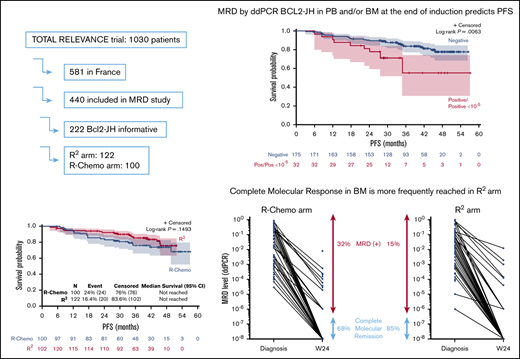
Among 1030 patients who participated in the RELEVANCE trial, including 581 patients treated in France, 440 of those patients were screened for t(BCL2-JH) (226 and 214 patients in the R2 and R-Chemo arms, respectively). Fifty percent (222/440) of patients had a detectable BCL2-JH rearrangement at diagnosis in PB and/or BM and were eligible for MRD study (details about PB and BM sample distribution are given in supplemental Table 1). Compared with the 359 French patients without MRD data, the 222 studied patients had more frequent Ann Arbor stage III-IV disease (96% vs 90%, P = .004), Follicular Lymphoma International Prognostic Index (FLIPI) score > 1 (91% vs 83%, P = .002), and BM involvement (62% vs 48%, P = .003; supplemental Table 2). The same trends were observed when comparisons were expanded to incorporate the 1030 patients included in the trial (supplemental Table 3). Among the 222 patients with detectable BCL2-JH rearrangement, baseline characteristics were well balanced between those in the R2 arm (n = 122) and those in the reference arm (n = 100) (Table 1).
Clinical characteristics of analyzed patients according to treatment arm
| . | R2 (n = 122) . | R-Chemo (n = 100) . | All (N = 222) . | P . |
|---|---|---|---|---|
| Age, median (range), y | 60 (33-78) | 60 (30-89) | 60 (30-89) | .90 |
| Males | 65 (53) | 55 (55) | 120 (54) | .89 |
| ECOG PS | .34 | |||
| 0 | 85 (70) | 63 (63) | 148 (67) | |
| 1 | 33 (27) | 36 (36) | 69 (31) | |
| 2 | 3 (2) | 1 (1) | 4 (2) | |
| Not evaluated | 1 (0.8) | 0 (0) | 1 (0.5) | |
| Ann Arbor stage | .47 | |||
| I-II | 3 (2) | 5 (5) | 8 (4) | |
| III-IV | 119 (98) | 95 (95) | 214 (96) | |
| Nodal mass >6 cm | 58 (47) | 52 (52) | 110 (50) | .59 |
| FLIPI score | .46 | |||
| 0-1 | 13 (11) | 6 (6) | 19 (9) | |
| 2 | 48 (39) | 43 (43) | 91 (41) | |
| 3-5 | 60 (49) | 51 (51) | 111 (50) | |
| Missing data | 1 (0.8) | 0 (0) | 1 (0.5) | |
| FLIPI2 score | .73 | |||
| 0-1 | 34 (28) | 24 (24) | 58 (26) | |
| 2 | 37 (30) | 28 (28) | 65 (29) | |
| 3-5 | 50 (41) | 45 (45) | 95 (43) | |
| Missing data | 1 (0.8) | 3 (3) | 4 (2) | |
| Number of peripheral lymph nodes | .23 | |||
| ≤4 | 53 (43) | 52 (52) | 105 (47) | |
| >4 | 69 (57) | 48 (48) | 117 (53) | |
| β2M, mg/L | .67 | |||
| <3 | 75 (61) | 63 (63) | 138 (62) | |
| ≥3 | 45 (37) | 33 (33) | 78 (35) | |
| Missing data | 2 (2) | 4 (4) | 6 (3) | |
| Elevated LDH (>ULN) | .77 | |||
| No | 85 (70) | 73 (73) | 158 (71) | |
| Yes | 36 (30) | 27 (27) | 63 (28) | |
| Missing data | 1 (0.8) | 0 (0) | 1 (0.5) | |
| BM involvement | .43 | |||
| No | 36 (30) | 39 (39) | 75 (34) | |
| Yes | 79 (65) | 58 (58) | 137 (62) | |
| Unspecified | 3 (2) | 1 (1) | 4 (2) | |
| Not done | 4 (3) | 2 (2) | 6 (3) |
| . | R2 (n = 122) . | R-Chemo (n = 100) . | All (N = 222) . | P . |
|---|---|---|---|---|
| Age, median (range), y | 60 (33-78) | 60 (30-89) | 60 (30-89) | .90 |
| Males | 65 (53) | 55 (55) | 120 (54) | .89 |
| ECOG PS | .34 | |||
| 0 | 85 (70) | 63 (63) | 148 (67) | |
| 1 | 33 (27) | 36 (36) | 69 (31) | |
| 2 | 3 (2) | 1 (1) | 4 (2) | |
| Not evaluated | 1 (0.8) | 0 (0) | 1 (0.5) | |
| Ann Arbor stage | .47 | |||
| I-II | 3 (2) | 5 (5) | 8 (4) | |
| III-IV | 119 (98) | 95 (95) | 214 (96) | |
| Nodal mass >6 cm | 58 (47) | 52 (52) | 110 (50) | .59 |
| FLIPI score | .46 | |||
| 0-1 | 13 (11) | 6 (6) | 19 (9) | |
| 2 | 48 (39) | 43 (43) | 91 (41) | |
| 3-5 | 60 (49) | 51 (51) | 111 (50) | |
| Missing data | 1 (0.8) | 0 (0) | 1 (0.5) | |
| FLIPI2 score | .73 | |||
| 0-1 | 34 (28) | 24 (24) | 58 (26) | |
| 2 | 37 (30) | 28 (28) | 65 (29) | |
| 3-5 | 50 (41) | 45 (45) | 95 (43) | |
| Missing data | 1 (0.8) | 3 (3) | 4 (2) | |
| Number of peripheral lymph nodes | .23 | |||
| ≤4 | 53 (43) | 52 (52) | 105 (47) | |
| >4 | 69 (57) | 48 (48) | 117 (53) | |
| β2M, mg/L | .67 | |||
| <3 | 75 (61) | 63 (63) | 138 (62) | |
| ≥3 | 45 (37) | 33 (33) | 78 (35) | |
| Missing data | 2 (2) | 4 (4) | 6 (3) | |
| Elevated LDH (>ULN) | .77 | |||
| No | 85 (70) | 73 (73) | 158 (71) | |
| Yes | 36 (30) | 27 (27) | 63 (28) | |
| Missing data | 1 (0.8) | 0 (0) | 1 (0.5) | |
| BM involvement | .43 | |||
| No | 36 (30) | 39 (39) | 75 (34) | |
| Yes | 79 (65) | 58 (58) | 137 (62) | |
| Unspecified | 3 (2) | 1 (1) | 4 (2) | |
| Not done | 4 (3) | 2 (2) | 6 (3) |
Unless otherwise indicated, data are n (%).
β2M, β-2 microglobulin; ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; ULN, upper limit of normal.
At diagnosis, considering only patients informative for BCL2-JH rearrangement, 213 of 220 (97%) PB samples and 136 of 139 (98%) BM samples had detectable molecular disease, with a median tumor/total cell ratio of 5.8 × 10−3 and 2.1 × 10−2, respectively. At W24, 207 patients were analyzed for PB alone (n = 74), BM alone (n = 3) or PB and BM (n = 130; supplemental Table 4). CMR was rapidly obtained in PB, with only 5 of 204 (2.5%) patients exhibiting detectable molecular disease at W24. In contrast, 22.5% (30/133) had residual disease in BM. Considering PB and/or BM, 32 of 207 patients were not experiencing a molecular response at W24. When baseline characteristics (sex, age, performance status, Ann Arbor stage, FLIPI, FLIPI2, β2 microglobulin, lactate dehydrogenase level, quantitative value of molecular disease) were compared in patients with or without CMR, only FLIPI2 (66% FLIPI2 3-5 vs 40%, respectively; P = .011) and molecular quantitative values at diagnosis were significantly associated with (+) MRD (median value 10 times higher in patients with W24 [+] MRD; PB = 0.056, BM = 0.16) compared with patients with W24 (−) MRD (PB = 0.0056, BM = 0.016; P =.03 and .02 respectively).
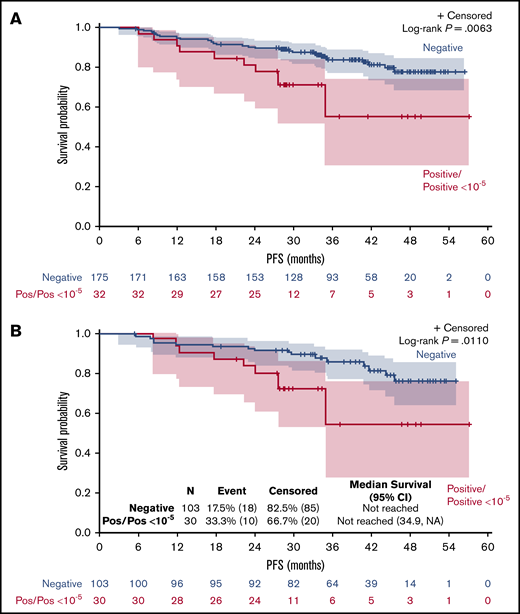
Detection of molecular disease in PB and/or BM at W24 had a significant impact on PFS (P = .0063; Figure 1A), with a 3-year PFS of 84% for patients in CMR vs 55% for patients with detectable MRD (hazard ratio [HR], 2.6; 95% confidence interval [CI], 1.27-5.13; P = .015). The negative prognostic value of (+) MRD when considering BM results only remains significant (P = .011; Figure 1B), with a 3-year PFS of 85% for patients in CMR vs 54% for patients with detectable MRD.
Impact of positive MRD at W24 on PFS. PFS by MRD status at W24 evaluated in PB and/or BM (A) or in BM (B). NA, not applicable; Pos, positive.
Impact of positive MRD at W24 on PFS. PFS by MRD status at W24 evaluated in PB and/or BM (A) or in BM (B). NA, not applicable; Pos, positive.
Of the 32 patients with residual molecular disease detectable in PB or BM, 11 (34%) were clinically considered to be in CR/CRu at W24. Conversely, of the 175 patients in CMR using BM and/or PB, 61 (35%) patients were clinically considered to be in CR/CRu at W24. Of note, 39 patients who were in CMR and would have qualified for CR/Cru based on CT scan measurements were downgraded to partial response after the central review because of missing or unspecified BM reassessment. In a Cox multivariable analysis including clinical response and MRD in PB/BM, only MRD PB/BM positivity was significantly associated with shorter PFS (HR, 2.6; 95% CI, 1.3-5.2; P = .0076) after adjusting on clinical response (HR, 1.8; 95% CI, 0.9-3.6; P = .09 for patients with non-CR/CRu).
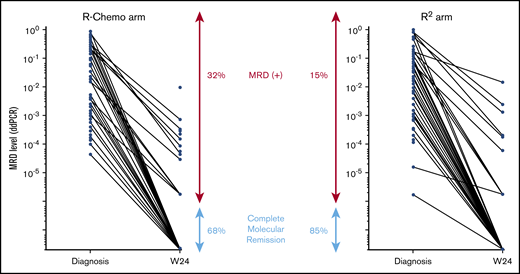
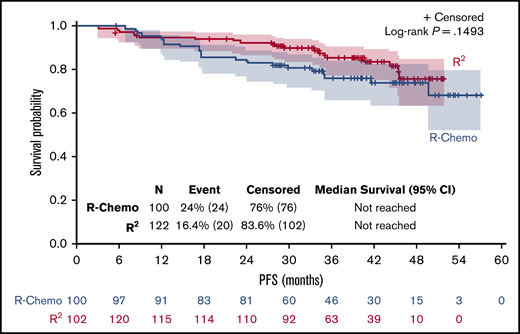
In the R-Chemo arm, all but 1 patient (given R-CVP) received R-CHOP. CMR in PB and/or BM was reached in 105 of 117 (90%) patients in the R2 arm and in 70 of 90 (77%) patients in the R-Chemo arm (P = .022) (results from BM are illustrated in Figure 2). PFS was similar in both arms (Figure 3). Therefore, we looked at the prognostic value of molecular response at W24 in both arms. Persistence of molecular disease was associated with inferior PFS, irrespective of treatment arm (interaction test, P = .31). When evaluated within each treatment group, statistical significance was reached between negative and positive MRD subgroups within the R-Chemo arm (HR, 3.3; 95% CI, 1.2-9.2; P = .02) (supplemental Figure 2A) but not in the R2 arm (HR, 2; 95% CI, 0.6-6.8; P = .27) (supplemental Figure 2B). Similar results were obtained when only BM was taken into account (data not shown).
Molecular disease in BM samples according to treatment arm. MRD level was quantified using droplet digital PCR, and results are expressed as number of tumor cells/number of analyzed cells. Each dot represents a patient sample. The lines connect the diagnostic and W24 samples from the same patient. CMR was defined as negative MRD PCR with a sensitivity ≥10−4.
Molecular disease in BM samples according to treatment arm. MRD level was quantified using droplet digital PCR, and results are expressed as number of tumor cells/number of analyzed cells. Each dot represents a patient sample. The lines connect the diagnostic and W24 samples from the same patient. CMR was defined as negative MRD PCR with a sensitivity ≥10−4.
Discussion
For MRD-evaluable patients from the RELEVANCE phase 3 study, 98% and 78% of patients achieved CMR in PB and BM, respectively, at W24 (end of induction treatment). Achievement of CMR in PB and/or BM correlated with a significant prolongation of 3-year PFS. When comparing molecular response at W24 in the 2 treatment arms, CMR was reached more frequently in the R2 arm (90%) than in the R-Chemo arm (77%).
MRD PB/BM positivity was significantly associated with shorter PFS after adjusting for clinical response. Such a superiority of the predictive value of MRD compared with clinical response has already been observed in relapsed FL,20 as well as in first-line patients.15
Despite a better molecular response observed at W24 in the R2 arm compared with the R-Chemo arm, PFS was similar in both arms. In terms of PFS, the poor prognostic value of the persistence of molecular disease was stronger in the R-Chemo arm than in the R2 arm, most likely because the latter group continued combination therapy beyond W24, whereas the chemotherapy patients were off combination treatment.
Our MRD study has some limitations: first, the population analyzed for MRD response probably had more severe disease than the entire RELEVANCE trial population, in view of the greater prevalence of adverse prognostic factors in patients with detectable clonal markers. This selection of patients with more advanced high-risk disease is a common finding in MRD studies of patients with FL,20 because the probability of detecting BCL2-JH tumor cells in the blood and/or marrow at diagnosis increases with the magnitude of disease spread. However, baseline characteristics were well balanced between the 2 arms and, in this substudy, PFS was similar in both arms, as observed for all RELEVANCE patients,4 reinforcing confidence in the representativeness of our cohort. We feel that showing that there was no difference between the 2 arms in this higher-risk population further underlines the effectiveness of the chemotherapy-free treatment in first-line FL patients needing therapy. Another limitation commonly observed in MRD studies focusing on BCL2-JH translocation14,15,21 is that only 50% of tested patients were informative. This may improve with promising new tools for MRD detection, such as next-generation sequencing, that have been developed over the last several years22,23 and recently used in combination with BCL2-JH translocation in relapsed FL.20
In conclusion, using MRD detected by quantitative BCL2-JH PCR as a surrogate marker to evaluate treatment efficiency in a subset of BCL2-JH+ French patients enrolled in RELEVANCE, we showed that R2 immunomodulatory induction treatment in first-line FL can achieve at least similar high rates of molecular response as R-Chemo after only 6 months of treatment. Longer follow-up is needed to better analyze the prognostic impact of MRD persistence after receiving R2 vs R-Chemo.
Presented in abstract form at the International Conference on Malignant Lymphoma, Lugano, Switzerland, 20 June 2019.
Data sharing requests should be submitted to Marie-Helene Delfau-Larue (marie-helene.delfau@aphp.fr) or Franck Morschhauser (fmorschhauser@gmail.com) and must include a description of the research proposal.
Acknowledgments
The authors thank all patients, families, caregivers, and investigators who participated in the RELEVANCE study, the Lymphoma Academic Research Organization teams for contributing to the management of different aspects of the study, Ichrafe Benmaad and Andréa Cerqueira Do Vale (Creteil Laboratory) for technical assistance, and Catherine Bely for secretarial assistance.
This study was supported by Celgene Corporation and the Lymphoma Academic Research Organization group.
Authorship
Contribution: M.-H.D.-L., G.A.S., and F.M. designed the study, analyzed and interpreted results, and wrote the manuscript; M.-H.D.-L., M.-L.B., A.B.-F., T.F., P.F., H.M., R.-O.C., F.L., G.M.P., R.H., L.Y., H.T., J.-C.E., S.L.G., V.R., P.G., S.G., G.C., and L.X. performed experiments; and all authors were involved in the development and final approval of the submitted manuscript.
Conflict-of-interest disclosure: M.-H.D.-L. has received personal fees for scientific lectures from Amgen, Janssen, and Roche and nonfinancial personal support from Celgene during the conduct of the study. P.F. has received personal fees from Roche and Celgene outside of the submitted work. R.-O.C. has received grants for clinical research, honoraria, and travel expenses from, as well as served on advisory boards for, Roche, Gilead Sciences, and Takeda; honoraria and travel expenses from, as well as served on advisory boards for, Bristol Myers Squibb, MSD, and Celgene; and served on advisory boards for, as well as received honoraria from, AbbVie outside of the submitted work. R.H. has received honoraria or consulting fees from Bristol Myers Squibb, MSD, Gilead Sciences, Kite, Roche, Novartis, Janssen, and Celgene. H.T. has received grants and personal fees from Celgene, personal fees and nonfinancial support from Roche, and personal fees from Karyopharm, AstraZeneca, and Bristol Myers Squibb outside of the submitted work. S.L.G. has acted as a consultant for and served on advisory boards for Roche SAS, Janssen-Cilag, Gilead Sciences/Kite, Novartis, Loxo Oncology, AbbVie, and Servier. V.R. has served on advisory boards for Gilead, Infinity, MSD, Bristol Myers Squibb, Epizyme, NanoString, Incyte, and Roche and has received research funding from argenx outside of the submitted work. G.C. has acted as a consultant for, and received honoraria from, Roche and Celgene and has received honoraria from Sanofi, Gilead Sciences, and Janssen during the conduct of the clinical study. G.A.S. has received grants and personal fees from Celgene and Roche during the conduct of the study and has received honoraria from Janssen, as well as acted as a consultant for, and received honoraria from, Gilead Sciences, Celgene, Novartis, Amgen, Servier, Bristol Myers Squibb, Merck, MorphoSys, Roche, Acerta, and Pfizer outside of the submitted work. F.M. has received nonfinancial support from Celgene during the conduct of the study; has served on advisory boards for Celgene, Roche, and Bristol Myers Squibb; has given scientific lectures for Celgene, Roche, and Janssen; has served on the advisory board for Bristol Myers Squibb; and has acted as a consultant for, served on the advisory board of, and given scientific lectures for Gilead Sciences outside of the submitted work. L.Y. has received grants and personal fees from AbbVie, AstraZeneca, Gilead Sciences, Janssen, and Roche. The remaining authors declare no competing financial interests.
Correspondence: Marie-Helene Delfau-Larue, Département d'Hématologie et Immunologie Biologiques, Hôpitaux Universitaires Henri Mondor, INSERM U955, University UPEC, 51 Ave du marechal Delattre de Tassigny, F-94000 Créteil, France; e-mail: marie-helene.delfau@aphp.fr; and Franck Morschhauser, Département d'Hématologie, EA 7365 Groupe de Recherche sur les formes Injectables et les Technologies Associées, University of Lille, CHU Lille, F-59000 Lille, France, e-mail: franck.morschhauser@chru-lille.fr.
References
Author notes
The full-text version of this article contains a data supplement.