TO THE EDITOR:
Psychosocial stress is increasingly acknowledged for families having children with chronic illness.1 Pediatric chronic illness impacts family structure, parental functioning, and health outcomes of the child.2 Sickle cell disease (SCD) is a chronic disease that can progress to a spectrum of complications, including vaso-occlusive pain crisis and cardiopulmonary dysfunction.3 The disease begins to manifest in early childhood, during which life-threatening conditions can develop rapidly. Chronic disease management, frequent infections, and unpredictable pain episodes cause anxiety and depression in children with SCD.4,5 Families, especially mothers, are a primary resource for stress management and mental health needs.6 The demands of caring for a chronically ill child and the feeling of powerlessness over the child's illness often associate with emotional stress in parents and impact family conflicts.7 Single parenthood, often single motherhood, is more common among families having children with SCD (60%) than the general African-American population (46%).8 Parental distress related to SCD is considerably more difficult to manage for single parents.7,9 The parent–child relationship, in turn, significantly affects a child’s perception of stress in life.5,10 Recent studies have begun to investigate the impact of single parenthood on SCD outcomes.11
Stress has been associated with vaso-occlusive pain episodes in SCD.12 The influence of stress on cardiopulmonary outcomes in SCD, however, is unclear. In adult patients with essential hypertension, the stress hormone adrenaline significantly induces vasoconstriction.13 Moderate mental stress associates with elevated pulmonary artery pressure and pulmonary vascular resistance in adult patients with pulmonary hypertension.14 SCD is a condition with increased prevalence of elevated pulmonary artery pressures in adults and children.15,16 We hypothesized that single parenthood as a psychosocial stressor associates with the risk for cardiopulmonary dysfunction in SCD patients. We assessed this hypothesis in a well-established pediatric cohort.
The Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease cohort prospectively enrolled 510 patients 3 to 20 years of age at 4 US centers and comprehensively followed up 62% of these patients at a median of 22 months in 2006 to 2010.16-18 At the baseline and follow-up visits, the medical history of the patients was recorded on a standard form designed for this study, physical examination was performed, and blood was drawn for laboratory measures. The current home situation was inquired by a single-choice question on the form as (1) 2-parent home, (2) 1-parent home, and (3) foster-parent home. At baseline, only 6 patients lived in a foster-parent home. We therefore focused on patients living in a 1-parent home or a 2-parent home. This study analyzed 355 patients at baseline (discovery) and 131 patients at follow-up (validation) who had sickle cell anemia (SCA) characterized by homozygous HbS mutation. The measuring of tricuspid regurgitation velocity (TRV), nonencouraged 6-minute walk distance (6MW), and oxygen saturation by pulse oximetry was described previously.17 The cutoffs of the outcomes were according to our previous study.17 Among 355 SCA patients at baseline,17 213 (60%) lived with 1 parent and 142 (40%) with 2 parents, consistent with an estimated 60% single parenthood in a previous study.8 Comparisons of relevant demographic and clinical variables are shown in Table 1.
Summary of PUSH baseline covariates shown as number (%) or median (IQR)
| Covariate . | 1-parent . | 2-parent . | P . | ||
|---|---|---|---|---|---|
| Median (IQR) . | n . | Median (IQR) . | n . | ||
| Age, y | 12 (7-16) | 213 | 14 (7-18) | 142 | .049 |
| Female sex, n (%) | 105 (49) | 213 | 66 (46) | 142 | .7 |
| BMI, kg/m2 | 17 (15-21) | 213 | 18 (16-21) | 140 | .033 |
| Systolic blood pressure, mm Hg | 112 (103-119) | 204 | 110 (106-119) | 139 | .7 |
| Diastolic blood pressure, mm Hg | 63 (57-69) | 204 | 64 (59-70) | 139 | .19 |
| Lactate dehydrogenase, U/L | 476 (351-597) | 186 | 437 (324-581) | 124 | .097 |
| Aspartate aminotransferase, U/L | 45 (34-54) | 204 | 45 (32-60) | 137 | .9 |
| Total bilirubin, mg/dL | 2.4 (1.7-3.5) | 203 | 2.7 (2.0-4.9) | 137 | .0089 |
| Reticulocytes, % | 8.8 (6.2-12) | 197 | 9.5 (6.0-13) | 135 | .6 |
| Hemoglobin, g/dL | 8.5 (7.6-9.6) | 206 | 8.8 (7.9-9.8) | 135 | .2 |
| Hematocrit, % | 25 (22-28) | 204 | 25 (22-28) | 136 | .16 |
| Red blood cells, ×106/µL | 2.8 (2.5-3.2) | 205 | 2.8 (2.5-3.3) | 136 | .6 |
| White blood cells, ×103/µL | 11 (8.3-13) | 205 | 11 (8.9-14) | 136 | .10 |
| Platelets, ×103/µL | 401 (336-492) | 205 | 402 (305-507) | 136 | .8 |
| Hydroxyurea, n (%) | 94 (44) | 213 | 61 (43) | 142 | .9 |
| Chronic transfusion, n (%) | 38 (18) | 212 | 27 (19) | 142 | .9 |
| No. of transfusions lifetime | 2 (2-3) | 210 | 2 (2-4) | 142 | .045 |
| No. of hospitalizations in the past 6 mo | 0 (0-1) | 212 | 1 (0-1) | 142 | .12 |
| Covariate . | 1-parent . | 2-parent . | P . | ||
|---|---|---|---|---|---|
| Median (IQR) . | n . | Median (IQR) . | n . | ||
| Age, y | 12 (7-16) | 213 | 14 (7-18) | 142 | .049 |
| Female sex, n (%) | 105 (49) | 213 | 66 (46) | 142 | .7 |
| BMI, kg/m2 | 17 (15-21) | 213 | 18 (16-21) | 140 | .033 |
| Systolic blood pressure, mm Hg | 112 (103-119) | 204 | 110 (106-119) | 139 | .7 |
| Diastolic blood pressure, mm Hg | 63 (57-69) | 204 | 64 (59-70) | 139 | .19 |
| Lactate dehydrogenase, U/L | 476 (351-597) | 186 | 437 (324-581) | 124 | .097 |
| Aspartate aminotransferase, U/L | 45 (34-54) | 204 | 45 (32-60) | 137 | .9 |
| Total bilirubin, mg/dL | 2.4 (1.7-3.5) | 203 | 2.7 (2.0-4.9) | 137 | .0089 |
| Reticulocytes, % | 8.8 (6.2-12) | 197 | 9.5 (6.0-13) | 135 | .6 |
| Hemoglobin, g/dL | 8.5 (7.6-9.6) | 206 | 8.8 (7.9-9.8) | 135 | .2 |
| Hematocrit, % | 25 (22-28) | 204 | 25 (22-28) | 136 | .16 |
| Red blood cells, ×106/µL | 2.8 (2.5-3.2) | 205 | 2.8 (2.5-3.3) | 136 | .6 |
| White blood cells, ×103/µL | 11 (8.3-13) | 205 | 11 (8.9-14) | 136 | .10 |
| Platelets, ×103/µL | 401 (336-492) | 205 | 402 (305-507) | 136 | .8 |
| Hydroxyurea, n (%) | 94 (44) | 213 | 61 (43) | 142 | .9 |
| Chronic transfusion, n (%) | 38 (18) | 212 | 27 (19) | 142 | .9 |
| No. of transfusions lifetime | 2 (2-3) | 210 | 2 (2-4) | 142 | .045 |
| No. of hospitalizations in the past 6 mo | 0 (0-1) | 212 | 1 (0-1) | 142 | .12 |
Bold entries are significant with P < .05. P values are based on Fisher’s exact test or Wilcoxon’s rank sum test for categorical or continuous variable, respectively. Missing values in the data are because of missed records and missed or failed laboratory assays.
IQR, interquartile range; PUSH, Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease.
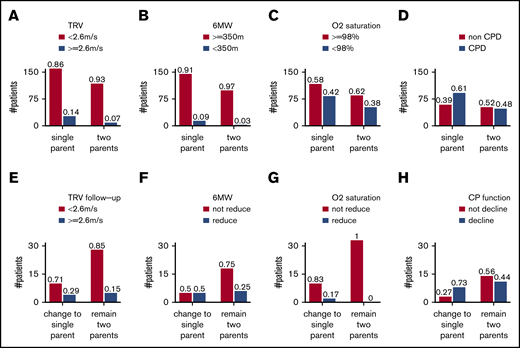
Adjusting for age and sex, single parenthood was associated with elevated estimated pulmonary artery systolic pressure, defined as TRV ≥ 2.6 m/s (odds ratio [OR] = 2.4, 95% confidence interval [CI] = 1.3-5.0, P = .0075, n = 314; Figure 1A). Single parenthood also showed an expected direction of association with reduced exercise capacity in children ≥6 years old, defined as 6MW < 350 m (OR = 3.2, 95% CI = 0.98-14, P = .054, n = 261; Figure 1B) and with O2 saturation < 98% (OR = 1.2, 95% CI = 0.78-2.0, P = .4, n = 337; Figure 1C). A composite outcome of cardiopulmonary dysfunction (CPD), defined as TRV ≥ 2.6 m/s or 6MW < 350 m or oxygen saturation < 98%, was associated with single parenthood (OR = 2.1, 95% CI = 1.2-3.6, P = .0071, n = 252; Figure 1D).
Categorizing patients according to home situation and cardiopulmonary outcomes. Elevated TRV (A), reduced 6MW distance (B), reduced oxygen saturation (C), and CPD (D) at baseline in PUSH and elevated TRV (E), reduced 6MW distance (F), reduced oxygen saturation (G), and decline in cardiopulmonary function (H) at follow-up compared with baseline in PUSH. In each category, the observed numbers of patients were presented. In all panels, the proportions of outcome per home situation are presented above each bar.
Categorizing patients according to home situation and cardiopulmonary outcomes. Elevated TRV (A), reduced 6MW distance (B), reduced oxygen saturation (C), and CPD (D) at baseline in PUSH and elevated TRV (E), reduced 6MW distance (F), reduced oxygen saturation (G), and decline in cardiopulmonary function (H) at follow-up compared with baseline in PUSH. In each category, the observed numbers of patients were presented. In all panels, the proportions of outcome per home situation are presented above each bar.
Intravascular hemolysis, reflected as a hemolytic index derived from serum concentrations of lactate dehydrogenase, aspartate transaminase, and total bilirubin, and percent reticulocytes, is linked to higher risk for CPD in SCD.16 Adjusting for age and sex, the hemolytic index was not different between 1- and 2-parent families (P = .9). Controlling for the hemolysis index, single parenthood was associated with elevated TRV (OR = 2.4, 95% CI = 1.2-5.5, P = .018, n = 266), reduced 6MW (OR = 4.4, 95% CI = 1.1-30, P = .032, n = 220), reduced O2 saturation (OR = 1.7, 95% CI = 1.0-3.0, P = .047, n = 282), and CPD (OR = 3.2, 95% CI = 1.7-6.4, P = .00039, n = 214). This suggests that the increased risk for CPD with single parenthood was not related to more severe hemolysis. High body mass index (BMI) may be a predictor of psychosocial stress in adults.19 The BMI of children from 1-parent homes was lower than from 2-parent homes (Table 1). The association of single parenthood with CPD remained by further controlling for BMI (OR = 3.2, 95% CI = 1.7-6.4, P = .00048, n = 214). The association of single parenthood with CPD also remained by further controlling for hydroxyurea (P = .00051), number of lifetime blood transfusions (P = .00017), a chronic blood transfusion program (P = .00039), and number of hospitalizations in the last 6 months (P = .00062).
To validate these observations, we identified 50 SCA patients living in 2-parent homes at baseline who had information of home situation available at the follow-up visit a median of 22 months later. We compared the cardiopulmonary outcomes of 35 patients staying with both parents and 15 patients whose home situation changed from a 2-parent home to 1-parent home during follow-up (Figure 1E-H). With the sparse data, new single parenthood showed an expected direction of association with elevated TRV at follow-up (controlling for elevated TRV at baseline and follow-up time; OR = 3.0, 95% CI = 0.64-14, P = .16, n = 44; Figure 1E), with decline in exercise capacity defined as a 5% decrease in 6MW at follow-up (OR = 3.0, 95% CI = 0.64-15, P = .16, n = 34; Figure 1F), and with a composite outcome of decline in cardiopulmonary function, defined as TRV ≥ 2.6 m/s at follow-up or 5% decrease in 6MW or O2 saturation (controlling for elevated TRV at baseline and follow-up time, OR = 4.2, 95% CI = 0.90-24, P = .069, n = 34; Figure 1H). As a comparison, we analyzed 81 patients living in a 1-parent home at baseline, among which 54 stayed with a single parent and 27 changed from a 1-parent home to a 2-parent home during follow-up. There was no consistent association of a new 2-parent home with nonelevated TRV at follow-up (OR = 0.88, 96% CI = 0.26-3.0, P = .8, n = 74), with an increase in exercise capacity (OR = 1.4, 95% CI = 0.46-4.4, P = .6, n = 69), or with composite outcome of an improvement in cardiopulmonary function (OR = 0.91, 95% CI = 0.32-2.6, P = .9, n = 67). This may be explained by persisting stress caused by the previous family structure and by the relatively short time of experiencing support from 2 parents.
Family support is crucial for the mental well-being of children and adolescents with SCD.20 Previous studies suggest that living in a single parent household exposes children and adolescents with SCD to more psychosocial stress.9 In the general population, stress contributes to cardiovascular and inflammatory dysfunction by impacting neuroendocrine plasticity.21-23 Adverse experiences predict elevated total peripheral resistance index, diastolic blood pressure, pulse wave velocity, and circulating endothelin-1 in adolescents and young adults.22 Adolescents exposed to chronic stress show heightened cardiovascular reactivity that puts them at risk for subclinical cardiovascular disease.23 Social environment has a direct role in stress development. Households of low socioeconomic status were reported to associate with adolescent vascular reactivity to stress, with the effect being mediated by family conflict.24 Social support has been linked to positive biological profiles and beneficial changes in neuroendocrine, cardiovascular, and immune function.25 Our observations seem to conform to a detrimental effect of stress on cardiopulmonary functions in SCD.
There are a number of limitations to our study. The direct association of single parenthood and greater cardiopulmonary problems can be artificial or overly simplified because of a lack of control for other social determinants of health that could have mediated or moderated this association. Socioeconomic indicators such as household income, access to care, access to nutrition, parent education, insurance status, and neighborhood environment26 can confound single parenthood. Other latent variables can further confound the present analysis. A possible difference may exist between 1- and 2-parent households in their willingness to seek clinical care, thus being enrolled in this study, under the same severity of CPD. Single-parent households may have poorer adherence to hydroxyurea therapy and clinic visits compared with 2-parent households. Our study also lacks detailed information on family structure and dynamics to delineate the various factors related to single parenthood (eg, other adults in the family who may provide support and the exact time of change in home situation that reflects time period of exposure). Furthermore, direct measures of stress level in patients is lacking in this study to confirm the stress difference between 1- and 2-parent households. Validating and incorporating measures of stress and correlation of stress with cardiovascular changes in SCD, with and without pain, would require careful study design to generate rigorous data. In the meantime, health systems may consider allocation of additional resources such as a community network and parenting education program to support single-parent families with children with SCD.
For information on the data, please contact Xu Zhang (zhangxu@uic.edu) or Victor R. Gordeuk (vgordeuk@uic.edu).
Acknowledgments:
The establishment of the Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease cohort was supported in part by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL079912-04, 2 R25-HL03679-08, and 1P30HL107253 (V.R.G.).
Contribution: X.Z. conceived the study, analyzed data, and wrote paper; K.N.K. and L.L.H. provided critical comments and edited paper; C.P.M., A.C., S.R.R., D.S.D., L.L.J., C.S., N.D., G.E., M.A., J.G.T., and S.N. collected data and provided critical comments; V.R.G. designed the Pulmonary Hypertension and the Hypoxic Response in Sickle Cell Disease cohort study, provided critical comments, and edited paper; and all authors participated in writing the manuscript and approved it.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xu Zhang, University of Illinois at Chicago, 820 S Wood St, Chicago, IL 60612; e-mail: zhangxu@uic.edu; and Victor R. Gordeuk, University of Illinois at Chicago, 820 S Wood St, Chicago, IL 60612; e-mail: vgordeuk@uic.edu.